Electrochemically Reduced Graphene Oxide-Based Screen-Printed Electrodes for Total Tetracycline Determination by Adsorptive Transfer Stripping Differential Pulse Voltammetry
Abstract
:1. Introduction
2. Material and Methods
2.1. Reagents and Sample
2.2. Instrumentation
2.3. Procedures
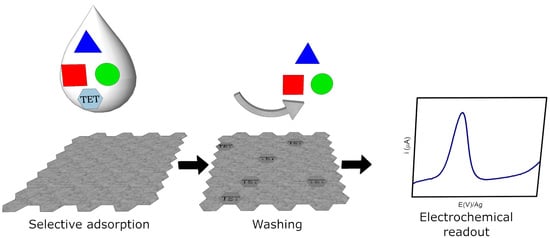
2.3.1. Electrochemical Sensor Fabrication
2.3.2. Electrochemical Measurements
2.3.3. Measurements of Electrode Active Surface by Using a Randles–Sevcik Equation
2.4. Sample Preparation
3. Results and Discussion
3.1. ERGO-SPE Characterization
3.2. Electrochemical Behavior of Tetracyclines at ERGO-SPE by AdTDPV
3.3. Analytical Performance of AdTDPV-ERGO-SPE Sensor for Total Tetracycline Determination and Sample Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AdTDPV | adsorptive transfer stripping differential pulse voltammetry |
| AdSDPV | adsorptive stripping differential pulse voltammetry |
| CTC | chlortetracycline hydrochloride |
| DOX | doxycycline hydrochloride |
| DPV | differential pulse voltammetry |
| ERGO | electrochemically reduced graphene oxide |
| GO | graphene oxide |
| OTC | oxytetracycline hydrochloride |
| SEM | scanning electron microscopy |
| SPE | carbon screen-printed electrode |
| TET | tetracycline hydrochloride |
References
- Garcia, C.D.; Crevillen, A.G.; Escarpa, A. Carbon-Based Nanomaterials in Analytical Chemistry, 1st ed.; Royal Society of Chemistry: Cambridge, UK, 2018; pp. 1–36. [Google Scholar]
- Martín, A.; Escarpa, A. Graphene: The cutting-edge interaction between chemistry and electrochemistry. TrAC Trends Anal. Chem. 2014, 56, 13–26. [Google Scholar] [CrossRef]
- Pérez-López, B.; Merkoçi, A. Carbon nanotubes and graphene in analytical sciences. Microchim. Acta 2012, 179, 1–16. [Google Scholar] [CrossRef]
- Ibrahim, W.A.W.; Nodeh, H.R.; Sanagi, M.M. Graphene-Based Materials as Solid Phase Extraction Sorbent for Trace Metal Ions, Organic Compounds, and Biological Sample Preparation. Crit. Rev. Anal. Chem. 2016, 46, 267–283. [Google Scholar] [CrossRef] [PubMed]
- Sitko, R.; Zawisza, B.; Malicka, E. Graphene as a new sorbent in analytical chemistry. TrAC Trends Anal. Chem. 2013, 51, 33–43. [Google Scholar] [CrossRef]
- Khodaee, N.; Mehdinia, A.; Esfandiarnejad, R.; Jabbari, A. Ultra trace analysis of PAHs by designing simple injection of large amounts of analytes through the sample reconcentration on SPME fiber after magnetic solid phase extraction. Talanta 2016, 147, 59–62. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.J.; Wang, G.N.; Yang, K.; Liu, H.Z.; Wang, J.P. Determination of Tetracyclines in Milk by Graphene-Based Solid-Phase Extraction and High-Performance Liquid Chromatography. Anal. Lett. 2017, 50, 641–650. [Google Scholar] [CrossRef]
- Yan, H.; Sun, N.; Liu, S.; Row, K.H.; Song, Y. Miniaturized graphene-based pipette tip extraction coupled with liquid chromatography for the determination of sulfonamide residues in bovine milk. Food Chem. 2014, 158, 239–244. [Google Scholar] [CrossRef] [PubMed]
- Justino, C.I.L.; Gomes, A.R.; Freitas, A.C.; Duarte, A.C.; Rocha-Santos, T.A.P. Graphene based sensors and biosensors. TrAC Trends Anal. Chem. 2017, 91, 53–66. [Google Scholar] [CrossRef]
- Cinti, S.; Arduini, F. Graphene-based screen-printed electrochemical (bio)sensors and their applications: Efforts and criticisms. Biosens. Bioelectron. 2017, 89, 107–122. [Google Scholar] [CrossRef]
- Taleat, Z.; Khoshroo, A.; Mazloum-Ardakani, M. Screen-printed electrodes for biosensing: A review (2008–2013). Microchim. Acta 2014, 181, 865–891. [Google Scholar] [CrossRef]
- Kushikawa, R.T.; Silva, M.R.; Angelo, A.C.D.; Teixeira, M.F.S. Construction of an electrochemical sensing platform based on platinum nanoparticles supported on carbon for tetracycline determination. Sens. Actuators B Chem. 2016, 228, 207–213. [Google Scholar] [CrossRef] [Green Version]
- Chopra, I.; Roberts, M. Tetracycline Antibiotics: Mode of Action, Applications, Molecular Biology, and Epidemiology of Bacterial Resistance. Microbiol. Mol. Biol. Rev. 2001, 65, 232–260. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pérez-Rodríguez, M.; Pellerano, R.G.; Pezza, L.; Pezza, H.R. An overview of the main foodstuff sample preparation technologies for tetracycline residue determination. Talanta 2018, 182, 1–21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, M.; Jaiswal, S.; Sodhi, K.K.; Shree, P.; Singh, D.K. Antibiotics bioremediation: Perspectives on its ecotoxicity and resistance. Environ. Int. 2019, 124, 448–461. [Google Scholar] [CrossRef]
- Lan, L.; Yao, Y.; Ping, J.; Ying, Y. Recent advances in nanomaterial-based biosensors for antibiotics detection. Biosens. Bioelectron. 2017, 91, 504–514. [Google Scholar] [CrossRef]
- Liu, X.; Huang, D.; Lai, C.; Zeng, G.; Qin, L.; Zhang, C.; Yi, H.; Li, B.; Deng, R.; Liu, S.; et al. Recent advances in sensors for tetracycline antibiotics and their applications. TrAC Trends Anal. Chem. 2018, 109, 260–274. [Google Scholar] [CrossRef]
- Gaudin, V. Advances in biosensor development for the screening of antibiotic residues in food products of animal origin—A comprehensive review. Biosens. Bioelectron. 2017, 90, 363–377. [Google Scholar] [CrossRef]
- Kesavan, S.; Kumar, D.R.; Lee, Y.R.; Shim, J.J. Determination of tetracycline in the presence of major interference in human urine samples using polymelamine/electrochemically reduced graphene oxide modified electrode. Sens. Actuators B Chem. 2017, 241, 455–465. [Google Scholar] [CrossRef]
- Sun, X.; Ji, Z.; Xiong, M.; Chen, W. The Electrochemical Sensor for the Determination of Tetracycline Based on Graphene /L-Cysteine Composite Film. J. Electrochem. Soc. 2017, 164, B107–B112. [Google Scholar] [CrossRef]
- Wong, A.; Scontri, M.; Materon, E.M.; Lanza, M.R.V.; Sotomayor, M.D.P.T. Development and application of an electrochemical sensor modified with multi-walled carbon nanotubes and graphene oxide for the sensitive and selective detection of tetracycline. J. Electroanal. Chem. 2015, 757, 250–257. [Google Scholar] [CrossRef] [Green Version]
- Paleček, E.; Bartošík, M. Electrochemistry of Nucleic Acids. Chem. Rev. 2012, 112, 3427–3481. [Google Scholar] [CrossRef] [PubMed]
- Jampasa, S.; Siangproh, W.; Duangmal, K.; Chailapakul, O. Electrochemically reduced graphene oxide-modified screen-printed carbon electrodes for a simple and highly sensitive electrochemical detection of synthetic colorants in beverages. Talanta 2016, 160, 113–124. [Google Scholar] [CrossRef] [PubMed]
- Hui, K.H.; Ambrosi, A.; Pumera, M.; Bonanni, A. Improving the Analytical Performance of Graphene Oxide towards the Assessment of Polyphenols. Chem. A Eur. J. 2016, 22, 3830–3834. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Xu, J.; Li, Y.; Gao, H.; Guo, J.; Shen, F.; Sun, C. A novel colorimetric aptasensor using cysteamine-stabilized gold nanoparticles as probe for rapid and specific detection of tetracycline in raw milk. Food Control 2015, 54, 7–15. [Google Scholar] [CrossRef]
- Moraes, F.C.; Freitas, R.G.; Pereira, R.; Gorup, L.F.; Cuesta, A.; Pereira, E.C. Coupled electronic and morphologic changes in graphene oxide upon electrochemical reduction. Carbon 2015, 91, 11–19. [Google Scholar] [CrossRef] [Green Version]
- Šalplachta, J.; Horká, M.; Růžička, F.; Šlais, K.; Vykydalová, M.; Kubesová, A.; Holá, V.; Dvořáčková, M. CIEF separation, UV detection, and quantification of ampholytic antibiotics and bacteria from different matrices. Anal. Bioanal. Chem. 2014, 406, 6285–6296. [Google Scholar]
- Sierra, T.; González, M.C.; Moreno, B.; Crevillen, A.G.; Escarpa, A. Total α1-acid glycoprotein determination in serum samples using disposable screen-printed electrodes and osmium (VI) as electrochemical tag. Talanta 2018, 180, 206–210. [Google Scholar] [CrossRef]
- Hou, X.; Wang, X.; Sun, Y.; Wang, Y.; Guo, Y. Graphene oxide for solid-phase extraction of bioactive, phenolic acids. Anal. Bioanal. Chem. 2017, 409, 3541–3549. [Google Scholar] [CrossRef]
- Martín, A.; Hernández-Ferrer, J.; Vázquez, L.; Martínez, M.T.; Escarpa, A. Controlled chemistry of tailored graphene nanoribbons for electrochemistry: A rational approach to optimizing molecule detection. RSC Adv. 2014, 4, 132–139. [Google Scholar] [CrossRef] [Green Version]
- Abellán-Llobregat, A.; Vidal, L.; Rodríguez-Amaro, R.; Berenguer-Murcia, A.; Canals, A.; Morallón, E. Au-IDA microelectrodes modified with Au-doped graphene oxide for the simultaneous determination of uric acid and ascorbic acid in urine samples. Electrochim. Acta 2017, 227, 275–284. [Google Scholar] [CrossRef] [Green Version]







| Electrode | Peak Potential (V) | DPV Peak Height (×108 A) | AdTDPV Peak Height (×108 A) |
|---|---|---|---|
| SPE | 0.58 | 175 | 4.0 |
| ERGO-10 | 0.54 | 23.9 | 131 |
| ERGO-20 | 0.55 | 28.7 | 218 |
| Sample | TET Added (µM) | Found (µM) | Recovery (%) |
|---|---|---|---|
| Milk | 30 | 28 | 95 ± 5 |
| 70 | 71 | 102 ± 21 | |
| River water | 30 | 33 | 110 ± 10 |
| 70 | 66 | 94 ± 9 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lorenzetti, A.S.; Sierra, T.; Domini, C.E.; Lista, A.G.; Crevillen, A.G.; Escarpa, A. Electrochemically Reduced Graphene Oxide-Based Screen-Printed Electrodes for Total Tetracycline Determination by Adsorptive Transfer Stripping Differential Pulse Voltammetry. Sensors 2020, 20, 76. https://doi.org/10.3390/s20010076
Lorenzetti AS, Sierra T, Domini CE, Lista AG, Crevillen AG, Escarpa A. Electrochemically Reduced Graphene Oxide-Based Screen-Printed Electrodes for Total Tetracycline Determination by Adsorptive Transfer Stripping Differential Pulse Voltammetry. Sensors. 2020; 20(1):76. https://doi.org/10.3390/s20010076
Chicago/Turabian StyleLorenzetti, Anabela S., Tania Sierra, Claudia E. Domini, Adriana G. Lista, Agustin G. Crevillen, and Alberto Escarpa. 2020. "Electrochemically Reduced Graphene Oxide-Based Screen-Printed Electrodes for Total Tetracycline Determination by Adsorptive Transfer Stripping Differential Pulse Voltammetry" Sensors 20, no. 1: 76. https://doi.org/10.3390/s20010076
APA StyleLorenzetti, A. S., Sierra, T., Domini, C. E., Lista, A. G., Crevillen, A. G., & Escarpa, A. (2020). Electrochemically Reduced Graphene Oxide-Based Screen-Printed Electrodes for Total Tetracycline Determination by Adsorptive Transfer Stripping Differential Pulse Voltammetry. Sensors, 20(1), 76. https://doi.org/10.3390/s20010076