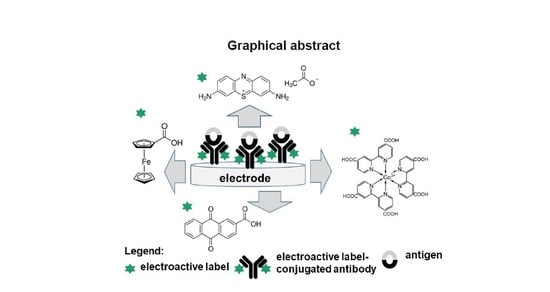
Antibody-Electroactive Probe Conjugates Based Electrochemical Immunosensors
Abstract
:1. Introduction
2. Procedures for Antibody Conjugation with Electroactive Probes
3. Sandwich Immunosensors Based on Antibody-Electroactive Probe Conjugates
4. Direct Signal Immunosensors Based on Antibody-Electroactive Probe Conjugates
5. Immunosensors Based on Multi-Antibody-Electroactive Probe Conjugates Strategy
6. Conclusions and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Thevenot, D.R.; Toth, K.; Durst, R.A.; Wilson, G.S. Electrochemical biosensors: Recommended definitions and classification—(Technical Report). Pure Appl. Chem. 1999, 71, 2333–2348. [Google Scholar] [CrossRef] [Green Version]
- Ricci, F.; Adornetto, G.; Palleschi, G. A review of experimental aspects of electrochemical immunosensors. Electrochim. Acta 2012, 84, 74–83. [Google Scholar] [CrossRef]
- Mahato, K.; Kumar, S.; Srivastava, A.; Maurya, P.K.; Singh, R.; Chandra, P. Chapter 14—Electrochemical immunosensors: Fundamentals and applications in clinical diagnostics. In Handbook of Immunoassay Technologies; Vashist, S.K., Luong, J.H.T., Eds.; Academic Press: Cambridge, MA, USA, 2018; pp. 359–414. [Google Scholar]
- Bahadir, E.B.; Sezginturk, M.K. Applications of electrochemical immunosensors for early clinical diagnostics. Talanta 2015, 132, 162–174. [Google Scholar] [CrossRef] [PubMed]
- Felix, F.S.; Angnes, L. Electrochemical immunosensors—A powerful tool for analytical applications. Biosens. Bioelectron. 2018, 102, 470–478. [Google Scholar] [CrossRef]
- Kokkinos, C.; Economou, A.; Prodromidis, M.I. Electrochemical immunosensors: Critical survey of different architectures and transduction strategies. TrAC-Trends Anal. Chem. 2016, 79, 88–105. [Google Scholar] [CrossRef]
- Iglesias-Mayor, A.; Amor-Gutiérrez, O.; Costa-García, A.; de la Escosura-Muñiz, A. Nanoparticles as emerging labels in electrochemical immunosensors. Sensors 2019, 19, 5137. [Google Scholar] [CrossRef] [Green Version]
- Arya, S.K.; Estrela, P. Recent advances in enhancement strategies for electrochemical ELISA-based immunoassays for cancer biomarker detection. Sensors 2018, 18, 2010. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Ibanez, A.; Chatrathi, M.P. Microchip-based amperometric immunoassays using redox tracers. Electrophoresis 2002, 23, 3744–3749. [Google Scholar] [CrossRef]
- Padeste, C.; Steiger, B.; Grubelnik, A.; Tiefenauer, L. Molecular assembly of redox-conductive ferrocene-streptavidin conjugates—Towards bio-electrochemical devices. Biosens. Bioelectron. 2004, 20, 545–552. [Google Scholar] [CrossRef]
- Padeste, C.; Grubelnik, A.; Tiefenauer, L. Ferrocene-avidin conjugates for bioelectrochemical applications. Biosens. Bioelectron. 2000, 15, 431–438. [Google Scholar] [CrossRef]
- Suzawa, T.; Ikariyama, Y.; Aizawa, M. Multilabeling of ferrocenes to a glucose-oxidase digoxin conjugate for the development of a homogeneous electroenzymatic immunoassay. Anal. Chem. 1994, 66, 3889–3894. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.F.; Zhu, X.; Hu, Z.B.; Wang, J.X.; Zhou, F.M. Voltammetric determination of surface-confined biomolecules with N-(2-ethyl-ferrocene)maleimide. Electroanalysis 2005, 17, 2163–2169. [Google Scholar] [CrossRef]
- Xu, Q.C.; Liu, Z.N.; Fu, J.Y.; Zhao, W.P.; Guo, Y.M.; Sun, X.; Zhang, H.Y. Ratiometric electrochemical aptasensor based on ferrocene and carbon nanofibers for highly specific detection of tetracycline residues. Sci. Rep. 2017, 7, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Seiwert, B.; Karst, U. Ferrocene-based derivatization in analytical chemistry. Anal. Bioanal. Chem. 2008, 390, 181–200. [Google Scholar] [CrossRef] [PubMed]
- Deiss, F.; LaFratta, C.N.; Symer, M.; Blicharz, T.M.; Sojic, N.; Walt, D.R. Multiplexed sandwich immunoassays using electrochemiluminescence imaging resolved at the single bead level. J. Am. Chem. Soc. 2009, 131, 6088–6089. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Habtamu, H.B.; Sentic, M.; Silvestrini, M.; De Leo, L.; Not, T.; Arbault, S.; Manojlovic, D.; Sojic, N.; Ugo, P. A sensitive electrochemiluminescence immunosensor for celiac disease diagnosis based on nanoelectrode ensembles. Anal. Chem. 2015, 87, 12080–12087. [Google Scholar] [CrossRef] [PubMed]
- Liébana, S.; Drago, G.A. Bioconjugation and stabilisation of biomolecules in biosensors. Essays Biochem. 2016, 60, 59–68. [Google Scholar]
- Veloso, A.J.; Chow, A.M.; Ganesh, H.V.S.; Li, N.; Dhar, D.; Wu, D.C.H.; Mikhaylichenko, S.; Brown, I.R.; Kerman, K. Electrochemical immunosensors for effective evaluation of amyloid-beta modulators on oligomeric and fibrillar aggregation processes. Anal. Chem. 2014, 86, 4901–4909. [Google Scholar] [CrossRef]
- Escamilla-Gomez, V.; Campuzano, S.; Pedrero, M.; Pingarron, J.M. Gold screen-printed-based impedimetric immunobiosensors for direct and sensitive Escherichia coli quantisation. Biosens. Bioelectron. 2009, 24, 3365–3371. [Google Scholar] [CrossRef]
- Bae, Y.M.; Oh, B.K.; Lee, W.; Lee, W.H.; Choi, J.W. Study on orientation of immunogrlobulin G on protein G layer. Biosens. Bioelectron. 2005, 21, 103–110. [Google Scholar] [CrossRef]
- Pan, M.F.; Gu, Y.; Yun, Y.G.; Li, M.; Jin, X.C.; Wang, S. Nanomaterials for electrochemical immunosensing. Sensors 2017, 17, 1041. [Google Scholar]
- Cho, I.H.; Lee, J.; Kim, J.; Kang, M.S.; Paik, J.K.; Ku, S.; Cho, H.M.; Irudayaraj, J.; Kim, D.H. Current technologies of electrochemical immunosensors: Perspective on signal amplification. Sensors 2018, 18, 207. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bottari, F.; Oliveri, P.; Ugo, P. Electrochemical immunosensor based on ensemble of nanoelectrodes for immunoglobulin IgY detection: Application to identify hen’s egg yolk in tempera paintings. Biosens. Bioelectron. 2014, 52, 403–410. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Fan, H.F.; Anderson, M.J.; Wright, J.G.; Hua, D.H.; Koehne, J.; Meyyappan, M.; Li, J. Electrochemical activity assay for protease analysis using carbon nanofiber nanoelectrode arrays. Anal. Chem. 2019, 91, 3971–3979. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Singh, N.; Tomar, V.; Chandra, R. A review on electrochemical detection of serotonin based on surface modified electrodes. Biosens. Bioelectron. 2018, 107, 76–93. [Google Scholar] [CrossRef]
- Karimian, N.; Moretto, L.M.; Ugo, P. Nanobiosensing with arrays and ensembles of nanoelectrodes. Sensors 2017, 17, 65. [Google Scholar] [CrossRef]
- Nimse, S.B.; Sonawane, M.D.; Song, K.S.; Kim, T. Biomarker detection technologies and future directions. Analyst 2016, 141, 740–755. [Google Scholar] [CrossRef] [Green Version]
- Jones, A.; Dhanapala, L.; Kankanamage, R.N.T.; Kumar, C.V.; Rusling, J.F. Multiplexed immunosensors and immunoarrays. Anal. Chem. 2020, 92, 345–362. [Google Scholar] [CrossRef]
- Beck, A.; Goetsch, L.; Dumontet, C.; Corvaïa, N. Strategies and challenges for the next generation of antibody–drug conjugates. Nat. Rev. Drug Discov. 2017, 16, 315–337. [Google Scholar] [CrossRef]
- Bernardim, B.; Matos, M.J.; Ferhati, X.; Companon, I.; Guerreiro, A.; Akkapeddi, P.; Burtoloso, A.C.B.; Jimenez-Oses, G.; Corzana, F.; Bernardes, G.J.L. Efficient and irreversible antibody-cysteine bioconjugation using carbonylacrylic reagents. Nat. Protoc. 2019, 14, 86–99. [Google Scholar] [CrossRef]
- McCombs, J.R.; Owen, S.C. Antibody drug conjugates: Design and selection of linker, payload and conjugation chemistry. AAPS J. 2015, 17, 339–351. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kossek, S.; Padeste, C.; Tiefenauer, L. Immobilization of streptavidin for immunosensors on nanostructured surfaces. J. Mol. Recognit. 1996, 9, 485–487. [Google Scholar] [CrossRef]
- Choi, M.J.; Kim, S.Y.; Choi, J.; Paeng, I.R. Labeling digoxin antibody with colloidal gold and ferrocene for its use in a membrane immunostrip and immunosensor. Microchem. J. 1999, 63, 92–99. [Google Scholar] [CrossRef] [Green Version]
- Lim, T.K.; Matsunaga, T. Construction of electrochemical flow immunoassay system using capillary columns and ferrocene conjugated immunoglobulin G for detection of human chorionic gonadotrophin. Biosens. Bioelectron. 2001, 16, 1063–1069. [Google Scholar] [CrossRef]
- Okochi, M.; Ohta, H.; Tanaka, T.; Matsunaga, T. Electrochemical probe for on-chip type flow immunoassay: Immunoglobulin G labeled with ferrocenecarboaldehyde. Biotechnol. Bioeng. 2005, 90, 14–19. [Google Scholar] [CrossRef]
- Tanaka, T.; Tsukube, S.; Izawa, K.; Okochi, M.; Lim, T.-K.; Watanabe, S.; Harada, M.; Matsunaga, T. Electrochemical detection of HbA(1c), a maker for diabetes, using a flow immunoassay system. Biosens. Bioelectron. 2007, 22, 2051–2056. [Google Scholar] [CrossRef]
- Song, Z.J.; Yuan, R.; Chai, Y.Q.; Zhuo, Y.; Jiang, W.; Su, H.L.; Che, X.; Li, J.J. Horseradish peroxidase-functionalized Pt hollow nanospheres and multiple redox probes as trace labels for a sensitive simultaneous multianalyte electrochemical immunoassay. Chem. Commun. 2010, 46, 6750–6752. [Google Scholar] [CrossRef]
- Lu, J.; Jiang, F.; Lu, A.P.; Zhang, G. Linkers having a crucial role in antibody-drug conjugates. Int. J. Mol. Sci. 2016, 17, 561. [Google Scholar] [CrossRef]
- Nath, N.; Godat, B.; Zimprich, C.; Dwight, S.J.; Corona, C.; McDougall, M.; Urh, M. Homogeneous plate based antibody internalization assay using pH sensor fluorescent dye. J. Immunol. Methods 2016, 431, 11–21. [Google Scholar] [CrossRef] [Green Version]
- Nath, N.; Godat, B.; Benink, H.; Urh, M. On-bead antibody-small molecule conjugation using high-capacity magnetic beads. J. Immunol. Methods 2015, 426, 95–103. [Google Scholar] [CrossRef] [Green Version]
- Ambrosi, A.; Castaneda, M.T.; Killard, A.J.; Smyth, M.R.; Alegret, S.; Merkoci, A. Double-codified gold nanolabels for enhanced immunoanalysis. Anal. Chem. 2007, 79, 5232–5240. [Google Scholar] [CrossRef] [PubMed]
- Yuan, L.; Giovanni, M.; Xie, J.P.; Fan, C.H.; Leong, D.T. Ultrasensitive IgG quantification using DNA nano-pyramids. NPG Asia Mater. 2014, 6, e112. [Google Scholar] [CrossRef] [Green Version]
- Chopra, A.; Rawat, S.; Bhalla, V.; Suri, C.R. Point-of-care amperometric testing of diabetic marker (HbA1c) using specific electroactive antibodies. Electroanalysis 2014, 26, 469–472. [Google Scholar] [CrossRef]
- Wang, G.F.; Gang, X.; Zhou, X.; Zhang, G.; Huang, H.; Zhang, X.J.; Wang, L. Electrochemical immunosensor with graphene/gold nanoparticles platform and ferrocene derivatives label. Talanta 2013, 103, 75–80. [Google Scholar] [CrossRef]
- Jampasa, S.; Siangproh, W.; Laocharoensuk, R.; Vilaivan, T.; Chailapakul, O. Electrochemical detection of c-reactive protein based on anthraquinone-labeled antibody using a screen-printed graphene electrode. Talanta 2018, 183, 311–319. [Google Scholar] [CrossRef]
- Sharma, A.; Rao, V.K.; Kamboj, D.V.; Gaur, R.; Shaik, M.; Shrivastava, A.R. Enzyme free detection of staphylococcal enterotoxin B (SEB) using ferrocene carboxylic acid labeled monoclonal antibodies: An electrochemical approach. New J. Chem. 2016, 40, 8334–8341. [Google Scholar] [CrossRef]
- Zhang, S.B.; Zheng, F.; Wu, Z.S.; Shen, G.L.; Yu, R.Q. Highly sensitive electrochemical detection of immunospecies based on combination of Fc label and PPD film/gold nanoparticle amplification. Biosens. Bioelectron. 2008, 24, 129–135. [Google Scholar] [CrossRef]
- Akram, M.; Stuart, M.C.; Wong, D.K.Y. Signal generation at an electrochemical immunosensor via the direct oxidation of an electroactive label. Electroanalysis 2006, 18, 237–246. [Google Scholar] [CrossRef]
- Prabhulkar, S.; Alwarappan, S.; Liu, G.D.; Li, C.Z. Amperometric micro-immunosensor for the detection of tumor biomarker. Biosens. Bioelectron. 2009, 24, 3524–3530. [Google Scholar] [CrossRef]
- Dou, Y.H.; Haswell, S.J.; Greenman, J.; Wadhawan, J. Voltammetric immunoassay for the detection of protein biomarkers. Electroanalysis 2012, 24, 264–272. [Google Scholar] [CrossRef]
- Chen, J.; Yan, F.; Dai, Z.; Ju, H.X. Reagentless amperometric immunosensor for human chorionic gonadotrophin based on direct electrochemistry of horseradish peroxidase. Biosens. Bioelectron. 2005, 21, 330–336. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Yan, F.; Zhang, X.Q.; Yan, Y.T.; Tang, J.H.; Ju, H.X. Disposable reagentless electrochemical immunosensor array based on a biopolymer/sol-gel membrane for simultaneous measurement of several tumor markers. Clin. Chem. 2008, 54, 1481–1488. [Google Scholar] [CrossRef]
- Partington, L.I.; Atkin, S.L.; Kilpatrick, E.S.; Morris, S.H.; Piper, M.; Lawrence, N.S.; Wadhawan, J.D. Electrochemical measurement of antibody-antigen recognition biophysics: Thermodynamics and kinetics of human chorionic gonadotropin (hCG) binding to redox-tagged antibodies. J. Electroanal. Chem. 2018, 819, 533–541. [Google Scholar] [CrossRef]
- Pina, D.G.; Shnyrova, A.V.; Gavilanes, F.; Rodríguez, A.; Leal, F.; Roig, M.G.; Sakharov, I.Y.; Zhadan, G.G.; Villar, E.; Shnyrov, V.L. Thermally induced conformational changes in horseradish peroxidase. Eur. J. Biochem. 2001, 268, 120–126. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Tang, J.H.; Yan, F.; Ju, H.X. A gold nanoparticles/sol-gel composite architecture for encapsulation of immunoconjugate for reagentless electrochemical immunoassay. Biomaterials 2006, 27, 2313–2321. [Google Scholar] [CrossRef]
- Dai, Z.; Yan, F.; Chen, J.; Ju, H.X. Reagentless amperometric immunosensors based on direct electrochemistry of horseradish peroxidase for determination of carcinoma antigen-125. Anal. Chem. 2003, 75, 5429–5434. [Google Scholar] [CrossRef]
- Tan, F.; Yan, F.; Ju, H.X. A designer ormosil gel for preparation of sensitive immunosensor for carcinoembryonic antigen based on simple direct electron transfer. Electrochem. Commun. 2006, 8, 1835–1839. [Google Scholar] [CrossRef]
- Tan, F.; Yan, F.; Ju, H.X. Sensitive reagentless electrochemical immunosensor based on an ormosil sol-gel membrane for human chorionic gonadotrophin. Biosens. Bioelectron. 2007, 22, 2945–2951. [Google Scholar] [CrossRef]
- Lai, W.Q.; Zhuang, J.Y.; Tang, J.; Chen, G.N.; Tang, D.P. One-step electrochemical immunosensing for simultaneous detection of two biomarkers using thionine and ferrocene as distinguishable signal tags. Microchim. Acta 2012, 178, 357–365. [Google Scholar] [CrossRef]
- Yanez-Sedeno, P.; Campuzano, S.; Pingarron, J.M. Multiplexed electrochemical immunosensors for clinical biomarkers. Sensors 2017, 17, 965. [Google Scholar] [CrossRef] [Green Version]
- Pakchin, P.S.; Nakhjavani, S.A.; Saber, R.; Ghanbari, H.; Omidi, Y. Recent advances in simultaneous electrochemical multi-analyte sensing platforms. Trac-Trends Anal. Chem. 2017, 92, 32–41. [Google Scholar] [CrossRef]
- Wu, D.; Guo, A.P.; Guo, Z.K.; Xie, L.L.; Wei, Q.; Du, B. Simultaneous electrochemical detection of cervical cancer markers using reduced graphene oxide-tetraethylene pentamine as electrode materials and distinguishable redox probes as labels. Biosens. Bioelectron. 2014, 54, 634–639. [Google Scholar] [CrossRef] [PubMed]
- Viswanath, K.B.; Krithiga, N.; Jayachitra, A.; Mideen, A.K.S.; Amali, A.J.; Vasantha, V.S. Enzyme-free multiplex detection of pseudomonas aeruginosa and aeromonas hydrophila with ferrocene and thionine-labeled antibodies using ZIF-8/Au NPs as a platform. ACS Omega 2018, 3, 17010–17022. [Google Scholar] [CrossRef] [Green Version]
- Zhu, Q.; Chai, Y.Q.; Yuan, R.; Zhuo, Y.; Han, J.; Li, Y.; Liao, N. Amperometric immunosensor for simultaneous detection of three analytes in one interface using dual functionalized graphene sheets integrated with redox-probes as tracer matrixes. Biosens. Bioelectron. 2013, 43, 440–445. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhong, Z.Y.; Chai, Y.Q.; Song, Z.J.; Zhuo, Y.; Su, H.L.; Liu, S.M.; Wang, D.; Yuan, R. Simultaneous electrochemical immunoassay of three liver cancer biomarkers using distinguishable redox probes as signal tags and gold nanoparticles coated carbon nanotubes as signal enhancers. Chem. Commun. 2012, 48, 537–539. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Q.; Chai, Y.Q.; Yuan, R.; Zhuo, Y. Simultaneous detection of four biomarkers with one sensing surface based on redox probe tagging strategy. Anal. Chim. Acta 2013, 800, 22–28. [Google Scholar] [CrossRef]
- Yang, Z.H.; Zhuo, Y.; Chai, Y.Q.; Yuan, R. High throughput immunosenor based on multi-label strategy and a novel array electrode. Sci. Rep. 2014, 4, 4747. [Google Scholar] [CrossRef] [Green Version]





© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kondzior, M.; Grabowska, I. Antibody-Electroactive Probe Conjugates Based Electrochemical Immunosensors. Sensors 2020, 20, 2014. https://doi.org/10.3390/s20072014
Kondzior M, Grabowska I. Antibody-Electroactive Probe Conjugates Based Electrochemical Immunosensors. Sensors. 2020; 20(7):2014. https://doi.org/10.3390/s20072014
Chicago/Turabian StyleKondzior, Mateusz, and Iwona Grabowska. 2020. "Antibody-Electroactive Probe Conjugates Based Electrochemical Immunosensors" Sensors 20, no. 7: 2014. https://doi.org/10.3390/s20072014
APA StyleKondzior, M., & Grabowska, I. (2020). Antibody-Electroactive Probe Conjugates Based Electrochemical Immunosensors. Sensors, 20(7), 2014. https://doi.org/10.3390/s20072014