A Review of Nanocomposite-Modified Electrochemical Sensors for Water Quality Monitoring
Abstract
:1. Introduction
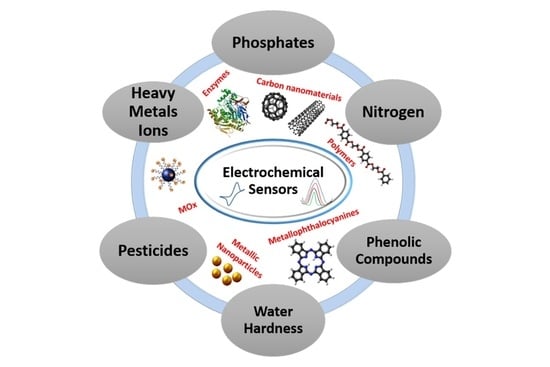
2. Water Contaminants
2.1. Pesticides
2.2. Nitrogen
2.3. Phosphorus
2.4. Water Hardeners
2.5. Water Disinfectant Byproducts
2.6. Emergent Contaminants
3. Electrochemical Sensor for Water Contaminants
3.1. Pesticide Sensors
3.1.1. Sensors Based on Carbon Materials
3.1.2. Sensors Using Molecularly Imprinted Polymers and Their Composites
3.1.3. Biosensors
- (a)
- Enzymatic biosensors
- (b)
- Immunobiosensors
- (c)
- Aptasensors
- (d)
- Biomimetic sensors
3.1.4. Sensors Based on Other Materials
3.2. Water Hardness Ions
3.3. Nitrogen Sensors
3.3.1. Carbon-Based Materials
3.3.2. Metal Nanomaterial
3.3.3. Conducting Polymers
3.4. Phosphorus Sensors
3.4.1. Polymeric Sensors
3.4.2. Carbon Nanomaterials-Based Sensors
3.4.3. Metal and Metal Complex-Based Sensors
3.5. Disinfectants and Byproducts
3.6. Emergent Contaminant Sensors
3.6.1. Carbon-Based Materials for Phenolic Compounds Detection
3.6.2. Metallic Nanomaterials
3.6.3. Polymeric Sensors
3.6.4. Dihydroxybenzene Isomers
- (a)
- Carbon-based hybrid nanocomposites
- (b)
- Carbon material-supported bimetallic composites
- (c)
- Carbon material-supported conducting polymers
3.6.5. Further Emergent Contaminants
4. Conclusions and Perspectives
- A lack of electrochemical sensors for in-situ applications [310].
- Real-time stability and reusability.
- Large-scale and inexpensive fabrication.
Author Contributions
Funding
Conflicts of Interest
References
- More Action Needed to Tackle Mixtures of Chemicals in Europe’s Waters — European Environment Agency. Available online: https://www.eea.europa.eu/highlights/more-action-needed-to-tackle (accessed on 30 March 2021).
- European Waters Getting Cleaner, but Big Challenges Remain — European Environment Agency. Available online: https://www.eea.europa.eu/highlights/european-waters-getting-cleaner-but (accessed on 30 March 2021).
- Hernandez-Vargas, G.; Sosa-Hernández, J.; Saldarriaga-Hernandez, S.; Villalba-Rodríguez, A.; Parra-Saldivar, R.; Iqbal, H. Electrochemical Biosensors: A Solution to Pollution Detection with Reference to Environmental Contaminants. Biosensors 2018, 8, 29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- García-Miranda Ferrari, A.; Carrington, P.; Rowley-Neale, S.J.; Banks, C.E. Recent Advances in Portable Heavy Metal Electrochemical Sensing Platforms. Environ. Sci. Water Res. Technol. 2020, 6, 2676–2690. [Google Scholar] [CrossRef]
- Hernández, F.; Bakker, J.; Bijlsma, L.; de Boer, J.; Botero-Coy, A.M.; Bruinen de Bruin, Y.; Fischer, S.; Hollender, J.; Kasprzyk-Hordern, B.; Lamoree, M.; et al. The Role of Analytical Chemistry in Exposure Science: Focus on the Aquatic Environment. Chemosphere 2019, 222, 564–583. [Google Scholar] [CrossRef] [PubMed]
- Encyclopedia of Applied Electrochemistry; Kreysa, G.; Ota, K.; Savinell, R.F. (Eds.) Springer: New York, NY, USA, 2014; ISBN 978-1-4419-6995-8. [Google Scholar]
- Hanrahan, G.; Patil, D.G.; Wang, J. Electrochemical Sensors for Environmental Monitoring: Design, Development and Applications. J. Environ. Monitor. 2004, 6, 657. [Google Scholar] [CrossRef] [PubMed]
- Kumar, H.; Kumari, N.; Sharma, R. Nanocomposites (Conducting Polymer and Nanoparticles) Based Electrochemical Biosensor for the Detection of Environment Pollutant: Its Issues and Challenges. Environ.Impact Assess. Rev. 2020, 85, 106438. [Google Scholar] [CrossRef]
- Khanmohammadi, A.; Jalili Ghazizadeh, A.; Hashemi, P.; Afkhami, A.; Arduini, F.; Bagheri, H. An Overview to Electrochemical Biosensors and Sensors for the Detection of Environmental Contaminants. J. Iran. Chem. Soc. 2020, 17, 2429–2447. [Google Scholar] [CrossRef]
- Jiang, C.; He, Y.; Liu, Y. Recent Advances in Sensors for Electrochemical Analysis of Nitrate in Food and Environmental Matrices. Analyst 2020, 145, 5400–5413. [Google Scholar] [CrossRef]
- Chen, G.; Wang, X.; Wang, L. Application of Carbon Based Material for the Electrochemical Detection of Heavy Metal Ions in Water Environment. Int. J. Electrochem. Sci. 2020, 4252–4263. [Google Scholar] [CrossRef]
- Waheed, A.; Mansha, M.; Ullah, N. Nanomaterials-Based Electrochemical Detection of Heavy Metals in Water: Current Status, Challenges and Future Direction. Trac. Trends Anal. Chem. 2018, 105, 37–51. [Google Scholar] [CrossRef]
- Ramachandran, R.; Chen, T.-W.; Chen, S.-M.; Baskar, T.; Kannan, R.; Elumalai, P.; Raja, P.; Jeyapragasam, T.; Dinakaran, K.; Gnana kumar, G. peter A Review of the Advanced Developments of Electrochemical Sensors for the Detection of Toxic and Bioactive Molecules. Inorg. Chem. Front. 2019, 6, 3418–3439. [Google Scholar] [CrossRef]
- Wong, A.; Silva, T.; Caetano, F.; Bergamini, M.; Marcolino-Junior, L.; Fatibello-Filho, O.; Janegitz, B. An Overview of Pesticide Monitoring at Environmental Samples Using Carbon Nanotubes-Based Electrochemical Sensors. C 2017, 3, 8. [Google Scholar] [CrossRef] [Green Version]
- Singh, M.; Bhardiya, S.R.; Verma, F.; Rai, V.K.; Rai, A. Graphene-Based Nanomaterials for Fabrication of ‘Pesticide’ Electrochemical Sensors. CGS 2020, 3, 26–40. [Google Scholar] [CrossRef]
- Noori, J.S.; Mortensen, J.; Geto, A. Recent Development on the Electrochemical Detection of Selected Pesticides: A Focused Review. Sensors 2020, 20, 2221. [Google Scholar] [CrossRef] [Green Version]
- European Waters—Assessment of Status and Pressures 2018—European Environment Agency. Available online: https://www.eea.europa.eu/publications/state-of-water (accessed on 30 March 2021).
- MULHERN, G. Updated Surface Water Watch List Adopted by the Commission. Available online: https://ec.europa.eu/jrc/en/science-update/updated-surface-water-watch-list-adopted-commission (accessed on 30 March 2021).
- EUR-Lex - 32020L2184 - EN - EUR-Lex. Available online: https://eur-lex.europa.eu/eli/dir/2020/2184/oj (accessed on 30 March 2021).
- Guidelines for Drinking-Water Quality, 4th Edition, Incorporating the 1st Addendum. Available online: https://www.who.int/publications-detail-redirect/9789241549950 (accessed on 30 March 2021).
- National Primary Drinking Water Regulations|Ground Water and Drinking Water|US EPA. Available online: https://www.epa.gov/ground-water-and-drinking-water/national-primary-drinking-water-regulations (accessed on 9 June 2021).
- Shao, Y.; Chen, Z.; Hollert, H.; Zhou, S.; Deutschmann, B.; Seiler, T.-B. Toxicity of 10 Organic Micropollutants and Their Mixture: Implications for Aquatic Risk Assessment. Sci. Total Environ. 2019, 666, 1273–1282. [Google Scholar] [CrossRef]
- Quesada, H.B.; Baptista, A.T.A.; Cusioli, L.F.; Seibert, D.; de Oliveira Bezerra, C.; Bergamasco, R. Surface Water Pollution by Pharmaceuticals and an Alternative of Removal by Low-Cost Adsorbents: A Review. Chemosphere 2019, 222, 766–780. [Google Scholar] [CrossRef]
- Gruden, R.; Buchholz, A.; Kanoun, O. Electrochemical Analysis of Water and Suds by Impedance Spectroscopy and Cyclic Voltammetry. J. Sens. Sens. Syst. 2014, 3, 133–140. [Google Scholar] [CrossRef] [Green Version]
- Hossain, S.M.Z.; Mansour, N. Biosensors for On-Line Water Quality Monitoring – a Review. Arab J. Basic Appl. Sci. 2019, 26, 502–518. [Google Scholar] [CrossRef]
- Harrison, V.; Mackenzie Ross, S. Anxiety and Depression Following Cumulative Low-Level Exposure to Organophosphate Pesticides. Environ. Res. 2016, 151, 528–536. [Google Scholar] [CrossRef]
- Suarez-Lopez, J.R.; Nguyen, A.; Klas, J.; Gahagan, S.; Checkoway, H.; Lopez-Paredes, D.; Jacobs, D.R.; Noble, M. Associations of Acetylcholinesterase Inhibition Between Pesticide Spray Seasons with Depression and Anxiety Symptoms in Adolescents, and the Role of Sex and Adrenal Hormones on Gender Moderation. Expo. Health 2021, 13, 51–64. [Google Scholar] [CrossRef]
- Panjan, P.; Ohtonen, E.; Tervo, P.; Virtanen, V.; Sesay, A.M. Shelf Life of Enzymatic Electrochemical Sensors. Procedia Technol. 2017, 27, 306–308. [Google Scholar] [CrossRef]
- Panjan, P.; Virtanen, V.; Sesay, A.M. Determination of Stability Characteristics for Electrochemical Biosensors via Thermally Accelerated Ageing. Talanta 2017, 170, 331–336. [Google Scholar] [CrossRef] [PubMed]
- Shinwari, M.W.; Zhitomirsky, D.; Deen, I.A.; Selvaganapathy, P.R.; Deen, M.J.; Landheer, D. Microfabricated Reference Electrodes and Their Biosensing Applications. Sensors 2010, 10, 1679–1715. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Xu, Z.; Morrin, A.; Luo, X. Low Fouling Strategies for Electrochemical Biosensors Targeting Disease Biomarkers. Anal. Methods 2019, 11, 702–711. [Google Scholar] [CrossRef]
- Ayyalusamy, S.; Mishra, S.; Suryanarayanan, V. Promising Post-Consumer PET-Derived Activated Carbon Electrode Material for Non-Enzymatic Electrochemical Determination of Carbofuran Hydrolysate. Sci. Rep. 2018, 8, 13151. [Google Scholar] [CrossRef]
- Hord, N.G.; Tang, Y.; Bryan, N.S. Food Sources of Nitrates and Nitrites: The Physiologic Context for Potential Health Benefits. Am. J. Clin. Nutr. 2009, 90, 1–10. [Google Scholar] [CrossRef]
- Swann, P.F. Environmental Carcinogenesis: Contributions of Basic Research: Carcinogenic Risk from Nitrite, Nitrate and N-Nitrosamines in Food. Proc. R. Soc. Med. 1977, 70, 113–115. [Google Scholar] [CrossRef]
- Nasraoui, S.; Al-Hamry, A.; Anurag, A.; Teixeira, P.R.; Ameur, S.; Paterno, L.G.; Ben Ali, M.; Kanoun, O. Investigation of Laser Induced Graphene Electrodes Modified by MWNT/AuNPs for Detection of Nitrite. In Proceedings of the 2019 16th International Multi-Conference on Systems, Signals & Devices (SSD), Istanbul, Turkey, 21–24 March 2019; pp. 615–620. [Google Scholar]
- Walters, C.L. The Exposure of Humans to Nitrite. Oncology 1980, 37, 289–296. [Google Scholar] [CrossRef]
- Yue, M.; Wang, R.; Cheng, N.; Cong, R.; Gao, W.; Yang, T. ZnCr2S4: Highly Effective Photocatalyst Converting Nitrate into N2 without over-Reduction under Both UV and Pure Visible Light. Sci. Rep. 2016, 6, 30992. [Google Scholar] [CrossRef] [Green Version]
- Li, G.; Xia, Y.; Tian, Y.; Wu, Y.; Liu, J.; He, Q.; Chen, D. Review—Recent Developments on Graphene-Based Electrochemical Sensors toward Nitrite. J. Electrochem. Soc. 2019, 166, B881–B895. [Google Scholar] [CrossRef]
- Ryu, H.; Thompson, D.; Huang, Y.; Li, B.; Lei, Y. Electrochemical Sensors for Nitrogen Species: A Review. Sens. Actuators Rep. 2020, 2, 100022. [Google Scholar] [CrossRef]
- Islam, T.; Hasan, M.M.; Awal, A.; Nurunnabi, M.; Ahammad, A.J.S. Metal Nanoparticles for Electrochemical Sensing: Progress and Challenges in the Clinical Transition of Point-of-Care Testing. Molecules 2020, 25, 5787. [Google Scholar] [CrossRef]
- Sarwar, M.; Leichner, J.; Naja, G.M.; Li, C.-Z. Smart-Phone, Paper-Based Fluorescent Sensor for Ultra-Low Inorganic Phosphate Detection in Environmental Samples. Microsyst. Nanoeng. 2019, 5, 56. [Google Scholar] [CrossRef] [Green Version]
- Nag, A.; Alahi, M.E.E.; Feng, S.; Mukhopadhyay, S.C. IoT-Based Sensing System for Phosphate Detection Using Graphite/PDMS Sensors. Sens. Actuators A Phys. 2019, 286, 43–50. [Google Scholar] [CrossRef]
- Richardson, C.J.; King, R.S.; Qian, S.S.; Vaithiyanathan, P.; Qualls, R.G.; Stow, C.A. Estimating Ecological Thresholds for Phosphorus in the Everglades. Environ. Sci. Technol. 2007, 41, 8084–8091. [Google Scholar] [CrossRef]
- Clinical Aspects of Natural and Added Phosphorus in Foods; Gutiérrez, O.M.; Kalantar-Zadeh, K.; Mehrotra, R. (Eds.) Springer: New York, NY, USA, 2017; ISBN 978-1-4939-6564-9. [Google Scholar]
- Forano, C.; Farhat, H.; Mousty, C. Recent Trends in Electrochemical Detection of Phosphate in Actual Waters. Curr. Opin. Electrochem. 2018, 11, 55–61. [Google Scholar] [CrossRef]
- Lerga, T.M.; O’Sullivan, C.K. Rapid Determination of Total Hardness in Water Using Fluorescent Molecular Aptamer Beacon. Anal. Chim. Acta 2008, 610, 105–111. [Google Scholar] [CrossRef]
- Hardness in Drinking-Water: Background Document for Development of WHO Guidelines for Drinking-Water Quality. Available online: https://apps.who.int/iris/handle/10665/70168 (accessed on 31 March 2021).
- Zhang, W.; Wang, L.; Yang, Y.; Gaskin, P.; Teng, K.S. Recent Advances on Electrochemical Sensors for the Detection of Organic Disinfection Byproducts in Water. ACS Sens. 2019, 4, 1138–1150. [Google Scholar] [CrossRef]
- Richardson, S.D.; Postigo, C. Drinking Water Disinfection By-products. In Emerging Organic Contaminants and Human Health; Barceló, D., Ed.; The Handbook of Environmental Chemistry; Springer: Berlin/Heidelberg, Germany, 2011; Volume 20, pp. 93–137. ISBN 978-3-642-28131-0. [Google Scholar]
- Ghernaout, D.; Ghernaout, B. From Chemical Disinfection to Electrodisinfection: The Obligatory Itinerary? Desalination Water Treat. 2010, 16, 156–175. [Google Scholar] [CrossRef]
- Roberts, M.G.; Singer, P.C.; Obolensky, A. Comparing Total HAA and Total THM Concentrations using ICR Data. J. Am. Water Work. Assoc. 2002, 94, 103–114. [Google Scholar] [CrossRef]
- Dai, N.; Zeng, T.; Mitch, W.A. Predicting N -Nitrosamines: N -Nitrosodiethanolamine as a Significant Component of Total N -Nitrosamines in Recycled Wastewater. Environ. Sci. Technol. Lett. 2015, 2, 54–58. [Google Scholar] [CrossRef]
- Parvez, S.; Rivera-Núñez, Z.; Meyer, A.; Wright, J.M. Temporal Variability in Trihalomethane and Haloacetic Acid Concentrations in Massachusetts Public Drinking Water Systems. Environ. Res. 2011, 111, 499–509. [Google Scholar] [CrossRef] [PubMed]
- Emerging Pollutants in Water and Wastewater. Available online: https://en.unesco.org/emergingpollutantsinwaterandwastewater (accessed on 9 June 2021).
- Rasheed, T.; Bilal, M.; Nabeel, F.; Adeel, M.; Iqbal, H.M.N. Environmentally-Related Contaminants of High Concern: Potential Sources and Analytical Modalities for Detection, Quantification, and Treatment. Environ. Int. 2019, 122, 52–66. [Google Scholar] [CrossRef]
- Gogoi, A.; Mazumder, P.; Tyagi, V.K.; Tushara Chaminda, G.G.; An, A.K.; Kumar, M. Occurrence and Fate of Emerging Contaminants in Water Environment: A Review. Groundw. Sustain. Dev. 2018, 6, 169–180. [Google Scholar] [CrossRef]
- Im, J.; Rizzo, C.B.; de Barros, F.P.J. Resilience of Groundwater Systems in the Presence of Bisphenol A under Uncertainty. Sci. Total Environ. 2020, 727, 138363. [Google Scholar] [CrossRef]
- Ana, K.M.S.; Espino, M.P. Occurrence and Distribution of Hormones and Bisphenol A in Laguna Lake, Philippines. Chemosphere 2020, 256, 127122. [Google Scholar] [CrossRef]
- Fonseca, V.F.; Reis-Santos, P.; Duarte, B.; Cabral, H.N.; Caçador, M.I.; Vaz, N.; Dias, J.M.; Pais, M.P. Roving Pharmacies: Modelling the Dispersion of Pharmaceutical Contamination in Estuaries. Ecol. Indic. 2020, 115, 106437. [Google Scholar] [CrossRef]
- Lecomte, S.; Habauzit, D.; Charlier, T.; Pakdel, F. Emerging Estrogenic Pollutants in the Aquatic Environment and Breast Cancer. Genes 2017, 8, 229. [Google Scholar] [CrossRef] [Green Version]
- Lapworth, D.J.; Baran, N.; Stuart, M.E.; Ward, R.S. Emerging Organic Contaminants in Groundwater: A Review of Sources, Fate and Occurrence. Environ. Pollut. 2012, 163, 287–303. [Google Scholar] [CrossRef] [Green Version]
- Flavio Della Pelle; Dario Compagnone Nanomaterial-Based Sensing and Biosensing of Phenolic Compounds and Related Antioxidant Capacity in Food. Sensors 2018, 18, 462. [CrossRef] [Green Version]
- Pisoschi, A.M.; Cimpeanu, C.; Predoi, G. Electrochemical Methods for Total Antioxidant Capacity and Its Main Contributors Determination: A Review. Open Chem. 2015, 13. [Google Scholar] [CrossRef] [Green Version]
- Yao, D.; Cheng, L.; Wu, Q.; Zhang, G.; Wu, B.; He, Y. Assessment and Prediction of Fishery Water Quality Using Electrochemical Sensor Array Carried by UAV. In Proceedings of the 2019 IEEE International Symposium on Olfaction and Electronic Nose (ISOEN), Fukuoka, Japan, 26–29 May 2019; pp. 1–4. [Google Scholar]
- Lezi, N.; Economou, A. Voltammetric Determination of Neonicotinoid Pesticides at Disposable Screen-Printed Sensors Featuring a Sputtered Bismuth Electrode. Electroanalysis 2015, 27, 2313–2321. [Google Scholar] [CrossRef]
- Al-Qasmi, N.; Hameed, A.; Khan, A.N.; Aslam, M.; Ismail, I.M.I.; Soomro, M.T. Mercury Meniscus on Solid Silver Amalgam Electrode as a Sensitive Electrochemical Sensor for Tetrachlorvinphos. J. Saudi Chem. Soc. 2018, 22, 496–507. [Google Scholar] [CrossRef] [Green Version]
- Gajdár, J.; Barek, J.; Fischer, J. Electrochemical Microcell Based on Silver Solid Amalgam Electrode for Voltammetric Determination of Pesticide Difenzoquat. Sens. Actuators B Chem. 2019, 299, 126931. [Google Scholar] [CrossRef]
- Noori, J.; Dimaki, M.; Mortensen, J.; Svendsen, W. Detection of Glyphosate in Drinking Water: A Fast and Direct Detection Method without Sample Pretreatment. Sensors 2018, 18, 2961. [Google Scholar] [CrossRef] [Green Version]
- Balasubramanian, P.; Balamurugan, T.S.T.; Chen, S.-M.; Chen, T.-W.; Sharmila, G.; Yu, M.-C. One-Step Green Synthesis of Colloidal Gold Nano Particles: A Potential Electrocatalyst towards High Sensitive Electrochemical Detection of Methyl Parathion in Food Samples. J. Taiwan Inst. Chem. Eng. 2018, 87, 83–90. [Google Scholar] [CrossRef]
- Gao, X.; Gao, Y.; Bian, C.; Ma, H.; Liu, H. Electroactive Nanoporous Gold Driven Electrochemical Sensor for the Simultaneous Detection of Carbendazim and Methyl Parathion. Electrochim. Acta 2019, 310, 78–85. [Google Scholar] [CrossRef]
- Shams, N.; Lim, H.N.; Hajian, R.; Yusof, N.A.; Abdullah, J.; Sulaiman, Y.; Ibrahim, I.; Huang, N.M. Electrochemical Sensor Based on Gold Nanoparticles/Ethylenediamine-Reduced Graphene Oxide for Trace Determination of Fenitrothion in Water. RSC Adv. 2016, 6, 89430–89439. [Google Scholar] [CrossRef]
- Shams, N.; Lim, H.N.; Hajian, R.; Yusof, N.A.; Abdullah, J.; Sulaiman, Y.; Ibrahim, I.; Huang, N.M.; Pandikumar, A. A Promising Electrochemical Sensor Based on Au Nanoparticles Decorated Reduced Graphene Oxide for Selective Detection of Herbicide Diuron in Natural Waters. J. Appl. Electrochem. 2016, 46, 655–666. [Google Scholar] [CrossRef]
- Rahmani, T.; Bagheri, H.; Behbahani, M.; Hajian, A.; Afkhami, A. Modified 3D Graphene-Au as a Novel Sensing Layer for Direct and Sensitive Electrochemical Determination of Carbaryl Pesticide in Fruit, Vegetable, and Water Samples. Food Anal. Methods 2018, 11, 3005–3014. [Google Scholar] [CrossRef]
- Hou, X.; Liu, X.; Li, Z.; Zhang, J.; Du, G.; Ran, X.; Yang, L. Electrochemical Determination of Methyl Parathion Based on Pillar[5]Arene@AuNPs@reduced Graphene Oxide Hybrid Nanomaterials. New J. Chem. 2019, 43, 13048–13057. [Google Scholar] [CrossRef]
- Mojtaba, N.J.; Tayadon, F.; Bagheri, H. A New Electrochemical Sensor Based on an Au-Pd/Reduced Graphene Oxide Nanocomposite for Determination of Parathion. Int. J. Environ. Anal. Chem. 2020, 100, 1101–1117. [Google Scholar] [CrossRef]
- Dong, J.; Wang, X.; Qiao, F.; Liu, P.; Ai, S. Highly Sensitive Electrochemical Stripping Analysis of Methyl Parathion at MWCNTs–CeO2–Au Nanocomposite Modified Electrode. Sens. Actuators B Chem. 2013, 186, 774–780. [Google Scholar] [CrossRef]
- Fu, X.-C.; Zhang, J.; Tao, Y.-Y.; Wu, J.; Xie, C.-G.; Kong, L.-T. Three-Dimensional Mono-6-Thio-β-Cyclodextrin Covalently Functionalized Gold Nanoparticle/Single-Wall Carbon Nanotube Hybrids for Highly Sensitive and Selective Electrochemical Determination of Methyl Parathion. Electrochim. Acta 2015, 153, 12–18. [Google Scholar] [CrossRef]
- Abbaci, A.; Azzouz, N.; Bouznit, Y. Development and Validation of a New Sensor for Methomyl Detection. Anal. Methods 2013, 5, 3663. [Google Scholar] [CrossRef]
- Rathnakumar, S.S.; Noluthando, K.; Kulandaiswamy, A.J.; Rayappan, J.B.B.; Kasinathan, K.; Kennedy, J.; Maaza, M. Stalling Behaviour of Chloride Ions: A Non-Enzymatic Electrochemical Detection of α-Endosulfan Using CuO Interface. Sens. Actuators B Chem. 2019, 293, 100–106. [Google Scholar] [CrossRef]
- Ghodsi, J.; Rafati, A.A. A Voltammetric Sensor for Diazinon Pesticide Based on Electrode Modified with TiO2 Nanoparticles Covered Multi Walled Carbon Nanotube Nanocomposite. J. Electroanal. Chem. 2017, 807, 1–9. [Google Scholar] [CrossRef]
- Tian, X.; Liu, L.; Li, Y.; Yang, C.; Zhou, Z.; Nie, Y.; Wang, Y. Nonenzymatic Electrochemical Sensor Based on CuO-TiO2 for Sensitive and Selective Detection of Methyl Parathion Pesticide in Ground Water. Sens. Actuators B Chem. 2018, 256, 135–142. [Google Scholar] [CrossRef]
- Khairy, M.; Ayoub, H.A.; Banks, C.E. Non-Enzymatic Electrochemical Platform for Parathion Pesticide Sensing Based on Nanometer-Sized Nickel Oxide Modified Screen-Printed Electrodes. Food Chem. 2018, 255, 104–111. [Google Scholar] [CrossRef]
- Ensafi, A.A.; Noroozi, R.; Zandi–-Atashbar, N.; Rezaei, B. Cerium(IV) Oxide Decorated on Reduced Graphene Oxide, a Selective and Sensitive Electrochemical Sensor for Fenitrothion Determination. Sens. Actuators B Chem. 2017, 245, 980–987. [Google Scholar] [CrossRef]
- Sahoo, D.; Mandal, A.; Mitra, T.; Chakraborty, K.; Bardhan, M.; Dasgupta, A.K. Nanosensing of Pesticides by Zinc Oxide Quantum Dot: An Optical and Electrochemical Approach for the Detection of Pesticides in Water. J. Agric. Food Chem. 2018, 66, 414–423. [Google Scholar] [CrossRef]
- Karthik, R.; Kumar, J.V.; Chen, S.-M.; Kokulnathan, T.; Chen, T.-W.; Sakthinathan, S.; Chiu, T.-W.; Muthuraj, V. Development of Novel 3D Flower-like Praseodymium Molybdate Decorated Reduced Graphene Oxide: An Efficient and Selective Electrocatalyst for the Detection of Acetylcholinesterase Inhibitor Methyl Parathion. Sens. Actuators B Chem. 2018, 270, 353–361. [Google Scholar] [CrossRef]
- Yue, Y.; Jiang, L.; Li, Z.; Yuan, J.; Shi, H.; Feng, S. A Glassy Carbon Electrode Modified with a Monolayer of Zirconium(IV) Phosphonate for Sensing of Methyl-Parathion by Square Wave Voltammetry. Microchim. Acta 2019, 186, 433. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, R.C.; Sousa, C.P.; Freire, T.M.; Freire, R.M.; Denardin, J.C.; Fechine, P.B.A.; Becker, H.; Morais, S.; de Lima-Neto, P.; Correia, A.N. Chitosan-Magnetite Nanocomposite as a Sensing Platform to Bendiocarb Determination. Anal. Bioanal. Chem. 2018, 410, 7229–7238. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Q.; Xia, Y.; Wan, X.; Yang, S.; Cai, Z.; Ye, Y.; Li, G. Morphology-Dependent MnO2/Nitrogen-Doped Graphene Nanocomposites for Simultaneous Detection of Trace Dopamine and Uric Acid. Mater. Sci. Eng. C 2020, 109, 110615. [Google Scholar] [CrossRef]
- Cozzarini, L.; Bertolini, G.; Šuran-Brunelli, S.T.; Radivo, A.; Bracamonte, M.V.; Tavagnacco, C.; Goldoni, A. Metal Decorated Carbon Nanotubes for Electrocatalytic Water Splitting. Int. J. Hydrog. Energy 2017, 42, 18763–18773. [Google Scholar] [CrossRef]
- Pedrosa, V.A.; Miwa, D.; Machado, S.A.S.; Avaca, L.A. On the Utilization of Boron Doped Diamond Electrode as a Sensor for Parathion and as an Anode for Electrochemical Combustion of Parathion. Electroanalysis 2006, 18, 1590–1597. [Google Scholar] [CrossRef]
- Švorc, Ľ.; Rievaj, M.; Bustin, D. Green Electrochemical Sensor for Environmental Monitoring of Pesticides: Determination of Atrazine in River Waters Using a Boron-Doped Diamond Electrode. Sens. Actuators B Chem. 2013, 181, 294–300. [Google Scholar] [CrossRef]
- Costa, D.J.E.; Santos, J.C.S.; Sanches-Brandão, F.A.C.; Ribeiro, W.F.; Salazar-Banda, G.R.; Araujo, M.C.U. Boron-Doped Diamond Electrode Acting as a Voltammetric Sensor for the Detection of Methomyl Pesticide. J. Electroanal. Chem. 2017, 789, 100–107. [Google Scholar] [CrossRef]
- Majidi, M.R.; Asadpour-Zeynali, K.; Nazarpur, M. Determination of Fenitrothion in River Water and Commercial Formulations by Adsorptive Stripping Voltammetry with a Carbon Ceramic Electrode. J. AOAC Int. 2009, 92, 548–554. [Google Scholar] [CrossRef] [Green Version]
- Okumura, L.L.; Saczk, A.A.; de Oliveira, M.F.; de Fulgêncio, A.C.C.; Torrezani, L.; Gomes, P.E.N.; Peixoto, R.M. Electrochemical Feasibility Study of Methyl Parathion Determination on Graphite-Modified Basal Plane Pyrolytic Graphite Electrode. J. Braz. Chem. Soc. 2011, 22, 652–659. [Google Scholar] [CrossRef] [Green Version]
- Amare, M.; Abicho, S.; Admassie, S. Determination of Fenitrothion in Water Using a Voltammetric Sensor Based on a Polymer-Modified Glassy Carbon Electrode. J. AOAC Int. 2014, 97, 580–585. [Google Scholar] [CrossRef]
- Deroco, P.B.; Lourencao, B.C.; Fatibello-Filho, O. The Use of Modified Electrode with Carbon Black as Sensor to the Electrochemical Studies and Voltammetric Determination of Pesticide Mesotrione. Microchem. J. 2017, 133, 188–194. [Google Scholar] [CrossRef]
- Bolat, G.; Abaci, S.; Vural, T.; Bozdogan, B.; Denkbas, E.B. Sensitive Electrochemical Detection of Fenitrothion Pesticide Based on Self-Assembled Peptide-Nanotubes Modified Disposable Pencil Graphite Electrode. J. Electroanal. Chem. 2018, 809, 88–95. [Google Scholar] [CrossRef]
- Itkes, M.P.M.; de Oliveira, G.G.; Silva, T.A.; Fatibello-Filho, O.; Janegitz, B.C. Voltammetric Sensing of Fenitrothion in Natural Water and Orange Juice Samples Using a Single-Walled Carbon Nanohorns and Zein Modified Sensor. J. Electroanal. Chem. 2019, 840, 21–26. [Google Scholar] [CrossRef]
- Geto, A.; Noori, J.S.; Mortensen, J.; Svendsen, W.E.; Dimaki, M. Electrochemical Determination of Bentazone Using Simple Screen-Printed Carbon Electrodes. Environ. Int. 2019, 129, 400–407. [Google Scholar] [CrossRef]
- Zeng, Y.; Yu, D.; Yu, Y.; Zhou, T.; Shi, G. Differential Pulse Voltammetric Determination of Methyl Parathion Based on Multiwalled Carbon Nanotubes–Poly(Acrylamide) Nanocomposite Film Modified Electrode. J. Hazard. Mater. 2012, 217–218, 315–322. [Google Scholar] [CrossRef]
- Noyrod, P.; Chailapakul, O.; Wonsawat, W.; Chuanuwatanakul, S. The Simultaneous Determination of Isoproturon and Carbendazim Pesticides by Single Drop Analysis Using a Graphene-Based Electrochemical Sensor. J. Electroanal. Chem. 2014, 719, 54–59. [Google Scholar] [CrossRef]
- Dong, J.; Hou, J.; Jiang, J.; Ai, S. Innovative Approach for the Electrochemical Detection of Non-Electroactive Organophosphorus Pesticides Using Oxime as Electroactive Probe. Anal. Chim. Acta 2015, 885, 92–97. [Google Scholar] [CrossRef]
- Ma, H.; Wang, L.; Liu, Z.; Guo, Y. Ionic Liquid–Graphene Hybrid Nanosheets-Based Electrochemical Sensor for Sensitive Detection of Methyl Parathion. Int. J. Environ. Anal. Chem. 2016, 96, 161–172. [Google Scholar] [CrossRef]
- Wei, P.; Gan, T.; Wu, K. N-Methyl-2-Pyrrolidone Exfoliated Graphene as Highly Sensitive Analytical Platform for Carbendazim. Sens. Actuators B Chem. 2018, 274, 551–559. [Google Scholar] [CrossRef]
- Tan, X.; Liu, Y.; Zhang, T.; Luo, S.; Liu, X.; Tian, H.; Yang, Y.; Chen, C. Ultrasensitive Electrochemical Detection of Methyl Parathion Pesticide Based on Cationic Water-Soluble Pillar[5]Arene and Reduced Graphene Nanocomposite. RSC Adv. 2019, 9, 345–353. [Google Scholar] [CrossRef] [Green Version]
- Velusamy, V.; Palanisamy, S.; Chen, S.-W.; Balu, S.; Yang, T.C.K.; Banks, C.E. Novel Electrochemical Synthesis of Cellulose Microfiber Entrapped Reduced Graphene Oxide: A Sensitive Electrochemical Assay for Detection of Fenitrothion Organophosphorus Pesticide. Talanta 2019, 192, 471–477. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Masibi, K.K.; Fayemi, O.E.; Adekunle, A.S.; Al-Mohaimeed, A.M.; Fahim, A.M.; Mamba, B.B.; Ebenso, E.E. Electrochemical Detection of Endosulfan Using an AONP-PANI-SWCNT Modified Glassy Carbon Electrode. Materials 2021, 14, 723. [Google Scholar] [CrossRef] [PubMed]
- Sun, G.; Wang, P.; Ge, S.; Ge, L.; Yu, J.; Yan, M. Photoelectrochemical Sensor for Pentachlorophenol on Microfluidic Paper-Based Analytical Device Based on the Molecular Imprinting Technique. Biosens. Bioelectron. 2014, 56, 97–103. [Google Scholar] [CrossRef]
- Xie, C.; Gao, S.; Guo, Q.; Xu, K. Electrochemical Sensor for 2,4-Dichlorophenoxy Acetic Acid Using Molecularly Imprinted Polypyrrole Membrane as Recognition Element. Microchim. Acta 2010, 169, 145–152. [Google Scholar] [CrossRef]
- Shi, H.; Zhao, G.; Liu, M.; Zhu, Z. A Novel Photoelectrochemical Sensor Based on Molecularly Imprinted Polymer Modified TiO2 Nanotubes and Its Highly Selective Detection of 2,4-Dichlorophenoxyacetic Acid. Electrochem. Commun. 2011, 13, 1404–1407. [Google Scholar] [CrossRef]
- Li, H.; Wang, Z.; Wu, B.; Liu, X.; Xue, Z.; Lu, X. Rapid and Sensitive Detection of Methyl-Parathion Pesticide with an Electropolymerized, Molecularly Imprinted Polymer Capacitive Sensor. Electrochim. Acta 2012, 62, 319–326. [Google Scholar] [CrossRef]
- Bakas, I.; Hayat, A.; Piletsky, S.; Piletska, E.; Chehimi, M.M.; Noguer, T.; Rouillon, R. Electrochemical Impedimetric Sensor Based on Molecularly Imprinted Polymers/Sol–Gel Chemistry for Methidathion Organophosphorous Insecticide Recognition. Talanta 2014, 130, 294–298. [Google Scholar] [CrossRef]
- Motaharian, A.; Motaharian, F.; Abnous, K.; Hosseini, M.R.M.; Hassanzadeh-Khayyat, M. Molecularly Imprinted Polymer Nanoparticles-Based Electrochemical Sensor for Determination of Diazinon Pesticide in Well Water and Apple Fruit Samples. Anal. Bioanal. Chem. 2016, 408, 6769–6779. [Google Scholar] [CrossRef]
- Bow, Y.; Sutriyono, E.; Nasir, S.; Iskandar, I. Molecularly Imprinted Polymers (MIP) Based Electrochemical Sensor for Detection of Endosulfan Pesticide. Int. J. Adv. Sci. Eng. Inf. Technol. 2017, 7, 662–668. [Google Scholar] [CrossRef] [Green Version]
- Khadem, M.; Faridbod, F.; Norouzi, P.; Rahimi Foroushani, A.; Ganjali, M.R.; Shahtaheri, S.J.; Yarahmadi, R. Modification of Carbon Paste Electrode Based on Molecularly Imprinted Polymer for Electrochemical Determination of Diazinon in Biological and Environmental Samples. Electroanalysis 2017, 29, 708–715. [Google Scholar] [CrossRef]
- Shahtaheri, S.J.; Faridbod, F.; Khadem, M. Highly Selective Voltammetric Sensor Based on Molecularly Imprinted Polymer and Carbon Nanotubes to Determine the Dicloran Pesticide in Biological and Environmental Samples. Procedia Technol. 2017, 27, 96–97. [Google Scholar] [CrossRef]
- Ahmad, A.L.; Lah, N.F.C.; Low, S.C. Configuration of Molecular Imprinted Polymer for Electrochemical Atrazine Detection. J Polym. Res. 2018, 25, 243. [Google Scholar] [CrossRef]
- Khalifa, M.E.; Abdallah, A.B. Molecular Imprinted Polymer Based Sensor for Recognition and Determination of Profenofos Organophosphorous Insecticide. Biosens. Bioelectron. X 2019, 2, 100027. [Google Scholar] [CrossRef]
- Aghoutane, Y.; Diouf, A.; Österlund, L.; Bouchikhi, B.; El Bari, N. Development of a Molecularly Imprinted Polymer Electrochemical Sensor and Its Application for Sensitive Detection and Determination of Malathion in Olive Fruits and Oils. Bioelectrochemistry 2020, 132, 107404. [Google Scholar] [CrossRef]
- Durand, P.; Thomas, D. Use of Immobilized Enzyme Coupled with an Electrochemical Sensor for the Detection of Organophosphates and Carbamates Pesticides. J Environ. Pathol. Toxicol. Oncol. 1984, 5, 51–57. [Google Scholar]
- Palleschi, G.; Bernabei, M.; Cremisini, C.; Mascini, M. Determination of Organophosphorus Insecticides with a Choline Electrochemical Biosensor. Sens. Actuators B Chem. 1992, 7, 513–517. [Google Scholar] [CrossRef]
- Hassani, S.; Momtaz, S.; Vakhshiteh, F.; Maghsoudi, A.S.; Ganjali, M.R.; Norouzi, P.; Abdollahi, M. Biosensors and Their Applications in Detection of Organophosphorus Pesticides in the Environment. Arch. Toxicol. 2017, 91, 109–130. [Google Scholar] [CrossRef]
- Chauhan, N.; Pundir, C.S. An Amperometric Acetylcholinesterase Sensor Based on Fe3O4 Nanoparticle/Multi-Walled Carbon Nanotube-Modified ITO-Coated Glass Plate for the Detection of Pesticides. Electrochim. Acta 2012, 67, 79–86. [Google Scholar] [CrossRef]
- Kaur, B.; Srivastava, R.; Satpati, B. Nanocrystalline Titanosilicate-Acetylcholinesterase Electrochemical Biosensor for the Ultra-Trace Detection of Toxic Organophosphate Pesticides. ChemElectroChem 2015, 2, 1164–1173. [Google Scholar] [CrossRef]
- Chauhan, N.; Narang, J.; Jain, U. Amperometric Acetylcholinesterase Biosensor for Pesticides Monitoring Utilising Iron Oxide Nanoparticles and Poly(Indole-5-Carboxylic Acid). J. Exp. Nanosci. 2016, 11, 111–122. [Google Scholar] [CrossRef]
- Dong, P.; Jiang, B.; Zheng, J. A Novel Acetylcholinesterase Biosensor Based on Gold Nanoparticles Obtained by Electroless Plating on Three-Dimensional Graphene for Detecting Organophosphorus Pesticides in Water and Vegetable Samples. Anal. Methods 2019, 11, 2428–2434. [Google Scholar] [CrossRef]
- Gangadhara Reddy, K.; Madhavi, G.; Kumara Swamy, B.E. Mobilized Lipase Enzymatic Biosensor for the Determination of Chlorfenvinphos and Malathion in Contaminated Water Samples: A Voltammetric Study. J. Mol. Liq. 2014, 198, 181–186. [Google Scholar] [CrossRef]
- Alves, M. de F.; Corrêa, R.A.M. de S.; da Cruz, F.S.; Franco, D.L.; Ferreira, L.F. Electrochemical Enzymatic Fenitrothion Sensor Based on a Tyrosinase/Poly(2-Hydroxybenzamide)-Modified Graphite Electrode. Anal. Biochem. 2018, 553, 15–23. [Google Scholar] [CrossRef]
- Malvano, F.; Albanese, D.; Pilloton, R.; Di Matteo, M.; Crescitelli, A. A New Label-Free Impedimetric Affinity Sensor Based on Cholinesterases for Detection of Organophosphorous and Carbamic Pesticides in Food Samples: Impedimetric Versus Amperometric Detection. Food Bioprocess. Technol. 2017, 10, 1834–1843. [Google Scholar] [CrossRef]
- Mulchandani, A.; Mulchandani, P.; Chen, W.; Wang, J.; Chen, L. Amperometric Thick-Film Strip Electrodes for Monitoring Organophosphate Nerve Agents Based on Immobilized Organophosphorus Hydrolase. Anal. Chem. 1999, 71, 2246–2249. [Google Scholar] [CrossRef]
- Crew, A.; Lonsdale, D.; Byrd, N.; Pittson, R.; Hart, J.P. A Screen-Printed, Amperometric Biosensor Array Incorporated into a Novel Automated System for the Simultaneous Determination of Organophosphate Pesticides. Biosens. Bioelectron. 2011, 26, 2847–2851. [Google Scholar] [CrossRef] [PubMed]
- Laothanachareon, T.; Champreda, V.; Sritongkham, P.; Somasundrum, M.; Surareungchai, W. Cross-Linked Enzyme Crystals of Organophosphate Hydrolase for Electrochemical Detection of Organophosphorus Compounds. World J. Microbiol. Biotechnol. 2008, 24, 3049–3055. [Google Scholar] [CrossRef] [Green Version]
- Beleno Cabarcas, M.T.; Stoytcheva, M.; Zlatev, R.; Montero, G.; Velkova, Z.; Gochev, V. Chitosan Nanocomposite Modified OPH-Based Amperometric Sensor for Organophosphorus Pesticides Determination. CAC 2018, 14. [Google Scholar] [CrossRef]
- Deng, A.-P.; Yang, H. A Multichannel Electrochemical Detector Coupled with an ELISA Microtiter Plate for the Immunoassay of 2,4-Dichlorophenoxyacetic Acid. Sens. Actuators B Chem. 2007, 124, 202–208. [Google Scholar] [CrossRef]
- Bauer, C.G.; Eremenko, A.V.; Ehrentreich-Förster, E.; Bier, F.F.; Makower, A.; Halsall, H.B.; Heineman, W.R.; Scheller, F.W. Zeptomole-Detecting Biosensor for Alkaline Phosphatase in an Electrochemical Immunoassay for 2,4-Dichlorophenoxyacetic Acid. Anal. Chem. 1996, 68, 2453–2458. [Google Scholar] [CrossRef] [PubMed]
- Dzantiev, B.B.; Zherdev, A.V.; Yulaev, M.F.; Sitdikov, R.A.; Dmitrieva, N.M.; Moreva, I.Y. Electrochemical Immunosensors for Determination of the Pesticides 2,4-Dichlorophenoxyacetic and 2,4,5-Tricholorophenoxyacetic Acids. Biosens. Bioelectron. 1996, 11, 179–185. [Google Scholar] [CrossRef]
- Hleli, S.; Martelet, C.; Abdelghani, A.; Burais, N.; Jaffrezic-Renault, N. Atrazine Analysis Using an Impedimetric Immunosensor Based on Mixed Biotinylated Self-Assembled Monolayer. Sens. Actuators B Chem. 2006, 113, 711–717. [Google Scholar] [CrossRef]
- Bhardwaj, S.K.; Sharma, A.L.; Kim, K.-H.; Deep, A. Antibody Conjugated Glycine Doped Polyaniline Nanofilms as Efficient Biosensor for Atrazine. Mater. Res. Express 2017, 4, 125022. [Google Scholar] [CrossRef]
- Mehta, J.; Vinayak, P.; Tuteja, S.K.; Chhabra, V.A.; Bhardwaj, N.; Paul, A.K.; Kim, K.-H.; Deep, A. Graphene Modified Screen Printed Immunosensor for Highly Sensitive Detection of Parathion. Biosens. Bioelectron. 2016, 83, 339–346. [Google Scholar] [CrossRef]
- Mehta, J.; Bhardwaj, N.; Bhardwaj, S.K.; Tuteja, S.K.; Vinayak, P.; Paul, A.K.; Kim, K.-H.; Deep, A. Graphene Quantum Dot Modified Screen Printed Immunosensor for the Determination of Parathion. Anal. Biochem. 2017, 523, 1–9. [Google Scholar] [CrossRef]
- Supraja, P.; Tripathy, S.; Krishna Vanjari, S.R.; Singh, V.; Singh, S.G. Label Free, Electrochemical Detection of Atrazine Using Electrospun Mn2O3 Nanofibers: Towards Ultrasensitive Small Molecule Detection. Sens. Actuators B Chem. 2019, 285, 317–325. [Google Scholar] [CrossRef]
- Qian, J.; Li, J.; Fang, D.; Yu, Y.; Zhi, J. A Disposable Biofilm-Modified Amperometric Biosensor for the Sensitive Determination of Pesticide Biotoxicity in Water. RSC Adv. 2014, 4, 55473–55482. [Google Scholar] [CrossRef]
- Abu-Ali, H.; Nabok, A.; Smith, T.J.; Al-Shanawa, M. Development of a Novel Electrochemical Inhibition Sensor Array Based on Bacteria Immobilized on Modified Screen-Printed Gold Electrodes for Water Pollution Detection. Bionanoscience 2019, 9, 345–355. [Google Scholar] [CrossRef] [Green Version]
- Abu-Ali, H.; Nabok, A.; Smith, T.J. Electrochemical Inhibition Bacterial Sensor Array for Detection of Water Pollutants: Artificial Neural Network (ANN) Approach. Anal. Bioanal. Chem. 2019, 411, 7659–7668. [Google Scholar] [CrossRef] [Green Version]
- Fei, A.; Liu, Q.; Huan, J.; Qian, J.; Dong, X.; Qiu, B.; Mao, H.; Wang, K. Label-Free Impedimetric Aptasensor for Detection of Femtomole Level Acetamiprid Using Gold Nanoparticles Decorated Multiwalled Carbon Nanotube-Reduced Graphene Oxide Nanoribbon Composites. Biosens. Bioelectron. 2015, 70, 122–129. [Google Scholar] [CrossRef]
- Liu, M.; Khan, A.; Wang, Z.; Liu, Y.; Yang, G.; Deng, Y.; He, N. Aptasensors for Pesticide Detection. Biosens. Bioelectron. 2019, 130, 174–184. [Google Scholar] [CrossRef]
- Fu, J.; Dong, H.; Zhao, Q.; Cheng, S.; Guo, Y.; Sun, X. Fabrication of Refreshable Aptasensor Based on Hydrophobic Screen-Printed Carbon Electrode Interface. Sci. Total Environ. 2020, 712, 136410. [Google Scholar] [CrossRef]
- Sgobbi, L.F.; Machado, S.A.S. Functionalized Polyacrylamide as an Acetylcholinesterase-Inspired Biomimetic Device for Electrochemical Sensing of Organophosphorus Pesticides. Biosens. Bioelectron. 2018, 100, 290–297. [Google Scholar] [CrossRef]
- Karimian, N.; Fakhri, H.; Amidi, S.; Hajian, A.; Arduini, F.; Bagheri, H. A Novel Sensing Layer Based on Metal–Organic Framework UiO-66 Modified with TiO 2 –Graphene Oxide: Application to Rapid, Sensitive and Simultaneous Determination of Paraoxon and Chlorpyrifos. New J. Chem. 2019, 43, 2600–2609. [Google Scholar] [CrossRef]
- Aghaie, A.; Khanmohammadi, A.; Hajian, A.; Schmid, U.; Bagheri, H. Nonenzymatic Electrochemical Determination of Paraoxon Ethyl in Water and Fruits by Graphene-Based NiFe Bimetallic Phosphosulfide Nanocomposite as a Superior Sensing Layer. Food Anal. Methods 2019, 12, 1545–1555. [Google Scholar] [CrossRef]
- Cinti, S.; Neagu, D.; Carbone, M.; Cacciotti, I.; Moscone, D.; Arduini, F. Novel Carbon Black-Cobalt Phthalocyanine Nanocomposite as Sensing Platform to Detect Organophosphorus Pollutants at Screen-Printed Electrode. Electrochim. Acta 2016, 188, 574–581. [Google Scholar] [CrossRef]
- Yola, M.L. Electrochemical Activity Enhancement of Monodisperse Boron Nitride Quantum Dots on Graphene Oxide: Its Application for Simultaneous Detection of Organophosphate Pesticides in Real Samples. J. Mol. Liq. 2019, 277, 50–57. [Google Scholar] [CrossRef]
- Zheng, A.; Shen, C.; Tang, Q.; Gong, C.; Chow, C. Catalytic Chemosensing Assay for Selective Detection of Methyl Parathion Organophosphate Pesticide. Chem. Eur. J. 2019, 25, 9643–9649. [Google Scholar] [CrossRef]
- Xiang, H.; Cai, Q.; Li, Y.; Zhang, Z.; Cao, L.; Li, K.; Yang, H. Sensors Applied for the Detection of Pesticides and Heavy Metals in Freshwaters. J. Sens. 2020, 2020, 1–22. [Google Scholar] [CrossRef]
- Opekar, F.; Tůma, P. Dual-Channel Capillary Electrophoresis for Simultaneous Determination of Cations and Anions. J. Chromatogr. A 2016, 1446, 158–163. [Google Scholar] [CrossRef] [PubMed]
- Saurina, J.; López-Aviles, E.; Le Moal, A.; Hernández-Cassou, S. Determination of Calcium and Total Hardness in Natural Waters Using a Potentiometric Sensor Array. Anal. Chim. Acta 2002, 464, 89–98. [Google Scholar] [CrossRef]
- Cuartero, M.; Pankratova, N.; Cherubini, T.; Crespo, G.A.; Massa, F.; Confalonieri, F.; Bakker, E. In Situ Detection of Species Relevant to the Carbon Cycle in Seawater with Submersible Potentiometric Probes. Environ. Sci. Technol. Lett. 2017, 4, 410–415. [Google Scholar] [CrossRef]
- Stoodley, R.; Rodriguez Nuñez, J.R.; Bartz, T. Field and In-Lab Determination of Ca2+ in Seawater. J. Chem. Educ. 2014, 91, 1954–1957. [Google Scholar] [CrossRef]
- Li, M.; Li, Y.-T.; Li, D.-W.; Long, Y.-T. Recent Developments and Applications of Screen-Printed Electrodes in Environmental Assays—A Review. Anal. Chim. Acta 2012, 734, 31–44. [Google Scholar] [CrossRef]
- Yin, T.; Yu, H.; Ding, J.; Qin, W. An Integrated Screen-Printed Potentiometric Strip for Determination of Ca2+ in Seawater. J. Electrochem. Soc. 2019, 166, B589–B593. [Google Scholar] [CrossRef]
- Schwarz, J.; Trommer, K.; Gerlach, F.; Mertig, M. All-Solid-State Screen-Printed Sensors for Potentiometric Calcium(II) Determinations in Environmental Samples. Am. J. Anal. Chem. 2018, 9, 113–123. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Liu, Y.; Yan, R.; Gao, Y.; Wang, P. Bimetallic AuCu Nanoparticles Coupled with Multi-Walled Carbon Nanotubes as Ion-to-Electron Transducers in Solid-Contact Potentiometric Sensors. Electrochim. Acta 2020, 331, 135370. [Google Scholar] [CrossRef]
- Yin, T.; Li, J.; Qin, W. An All-Solid-State Polymeric Membrane Ca2+ -Selective Electrode Based on Hydrophobic Alkyl-Chain-Functionalized Graphene Oxide. Electroanalysis 2017, 29, 821–827. [Google Scholar] [CrossRef]
- Akhter, F.; Nag, A.; Alahi, M.E.E.; Liu, H.; Mukhopadhyay, S.C. Electrochemical Detection of Calcium and Magnesium in Water Bodies. Sens. Actuators A Phys. 2020, 305, 111949. [Google Scholar] [CrossRef]
- Levin, M.B.; Khripoun, G.A.; Korneev, S.M.; Mikhelson, K.N. Water Hardness Electrodes with Ionophores Containing Oxy- and Ester-Groups. Russ. J. Electrochem. 2018, 54, 391–399. [Google Scholar] [CrossRef]
- Wang, N.; Kanhere, E.; Tao, K.; Wu, J.; Miao, J.; Triantafyllou, M.S. Water Hardness Determination Using Disposable MEMS-Based Electrochemical Sensor. In Proceedings of the 2018 IEEE 8th International Nanoelectronics Conferences (INEC), Kuala Lumpur, Malaysia, 3–5 January 2018; pp. 43–44. [Google Scholar]
- He Zhang; Rongyan Chuai; Xin Li; Bing Zhang Design, Preparation and Performance Study of On-Chip Flow-Through Amperometric Sensors with an Integrated Ag/AgCl Reference Electrode. Micromachines 2018, 9, 114. [CrossRef] [Green Version]
- Haldorai, Y.; Kim, J.Y.; Vilian, A.T.E.; Heo, N.S.; Huh, Y.S.; Han, Y.-K. An Enzyme-Free Electrochemical Sensor Based on Reduced Graphene Oxide/Co3O4 Nanospindle Composite for Sensitive Detection of Nitrite. Sens. Actuators B Chem. 2016, 227, 92–99. [Google Scholar] [CrossRef]
- Tian, F.; Li, H.; Li, M.; Li, C.; Lei, Y.; Yang, B. Synthesis of One-Dimensional Poly(3,4-Ethylenedioxythiophene)-Graphene Composites for the Simultaneous Detection of Hydroquinone, Catechol, Resorcinol, and Nitrite. Synth. Met. 2017, 226, 148–156. [Google Scholar] [CrossRef]
- Wang, P.; Wang, M.; Zhou, F.; Yang, G.; Qu, L.; Miao, X. Development of a Paper-Based, Inexpensive, and Disposable Electrochemical Sensing Platform for Nitrite Detection. Electrochem. Commun. 2017, 81, 74–78. [Google Scholar] [CrossRef]
- Chen, W.; Niu, X.; Li, X.; Li, X.; Li, G.; He, B.; Li, Q.; Sun, W. Investigation on Direct Electrochemical and Electrocatalytic Behavior of Hemoglobin on Palladium-Graphene Modified Electrode. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 80, 135–140. [Google Scholar] [CrossRef]
- Bagheri, H.; Hajian, A.; Rezaei, M.; Shirzadmehr, A. Composite of Cu Metal Nanoparticles-Multiwall Carbon Nanotubes-Reduced Graphene Oxide as a Novel and High Performance Platform of the Electrochemical Sensor for Simultaneous Determination of Nitrite and Nitrate. J. Hazard. Mater. 2017, 324, 762–772. [Google Scholar] [CrossRef]
- Sivakumar, M. An Electrochemical Selective Detection of Nitrite Sensor for Polyaniline Doped Graphene Oxide Modified Electrode. Int. J. Electrochem. Sci. 2017, 4835–4846. [Google Scholar] [CrossRef]
- Rabti, A.; Ben Aoun, S.; Raouafi, N. A Sensitive Nitrite Sensor Using an Electrode Consisting of Reduced Graphene Oxide Functionalized with Ferrocene. Microchim. Acta 2016, 183, 3111–3117. [Google Scholar] [CrossRef]
- Zhang, Y.; Nie, J.; Wei, H.; Xu, H.; Wang, Q.; Cong, Y.; Tao, J.; Zhang, Y.; Chu, L.; Zhou, Y.; et al. Electrochemical Detection of Nitrite Ions Using Ag/Cu/MWNT Nanoclusters Electrodeposited on a Glassy Carbon Electrode. Sens. Actuators B Chem. 2018, 258, 1107–1116. [Google Scholar] [CrossRef]
- Huang, S.-S.; Liu, L.; Mei, L.-P.; Zhou, J.-Y.; Guo, F.-Y.; Wang, A.-J.; Feng, J.-J. Electrochemical Sensor for Nitrite Using a Glassy Carbon Electrode Modified with Gold-Copper Nanochain Networks. Microchim. Acta 2016, 183, 791–797. [Google Scholar] [CrossRef]
- Mo, R.; Wang, X.; Yuan, Q.; Yan, X.; Su, T.; Feng, Y.; Lv, L.; Zhou, C.; Hong, P.; Sun, S.; et al. Electrochemical Determination of Nitrite by Au Nanoparticle/Graphene-Chitosan Modified Electrode. Sensors 2018, 18, 1986. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kang, S.; Zhang, H.; Wang, G.; Zhang, Y.; Zhao, H.; Zhou, H.; Cai, W. Highly Sensitive Detection of Nitrite by Using Gold Nanoparticle-Decorated α-Fe 2 O 3 Nanorod Arrays as Self-Supporting Photo-Electrodes. Inorg. Chem. Front. 2019, 6, 1432–1441. [Google Scholar] [CrossRef]
- Zhe, T.; Sun, X.; Wang, Q.; Liu, Y.; Li, R.; Li, F.; Wang, L. A Screen Printed Carbon Electrode Modified with a Lamellar Nanocomposite Containing Dendritic Silver Nanostructures, Reduced Graphene Oxide, and β-Cyclodextrin for Voltammetric Sensing of Nitrite. Microchim. Acta 2019, 186, 319. [Google Scholar] [CrossRef]
- Rostami, M.; Abdi, G.; Kazemi, S.H.; Alizadeh, A. Nanocomposite of Magnetic Nanoparticles/Graphene Oxide Decorated with Acetic Acid Moieties on Glassy Carbon Electrode: A Facile Method to Detect Nitrite Concentration. J. Electroanal. Chem. 2019, 847, 113239. [Google Scholar] [CrossRef]
- Nasraoui, S.; Al-Hamry, A.; Teixeira, P.R.; Ameur, S.; Paterno, L.G.; Ben Ali, M.; Kanoun, O. Electrochemical Sensor for Nitrite Detection in Water Samples Using Flexible Laser-Induced Graphene Electrodes Functionalized by CNT Decorated by Au Nanoparticles. J. Electroanal. Chem. 2021, 880, 114893. [Google Scholar] [CrossRef]
- Zhao, Z.; Zhang, J.; Wang, W.; Sun, Y.; Li, P.; Hu, J.; Chen, L.; Gong, W. Synthesis and Electrochemical Properties of Co3O4-RGO/CNTs Composites towards Highly Sensitive Nitrite Detection. Appl. Surf. Sci. 2019, 485, 274–282. [Google Scholar] [CrossRef]
- Li, C.; Chen, D.; Wang, Y.; Lai, X.; Peng, J.; Wang, X.; Zhang, K.; Cao, Y. Simultaneous Electrochemical Detection of Nitrite and Hydrogen Peroxide Based on 3D Au-RGO/FTO Obtained Through a One-Step Synthesis. Sensors 2019, 19, 1304. [Google Scholar] [CrossRef] [Green Version]
- Sheng, Q.; Liu, D.; Zheng, J. A Nonenzymatic Electrochemical Nitrite Sensor Based on Pt Nanoparticles Loaded Ni(OH) 2/Multi-Walled Carbon Nanotubes Nanocomposites. J. Electroanal. Chem. 2017, 796, 9–16. [Google Scholar] [CrossRef]
- Hui, N.; Chai, F.; Lin, P.; Song, Z.; Sun, X.; Li, Y.; Niu, S.; Luo, X. Electrodeposited Conducting Polyaniline Nanowire Arrays Aligned on Carbon Nanotubes Network for High Performance Supercapacitors and Sensors. Electrochim. Acta 2016, 199, 234–241. [Google Scholar] [CrossRef]
- Wu, W.; Li, Y.; Jin, J.; Wu, H.; Wang, S.; Ding, Y.; Ou, J. Sensing Nitrite with a Glassy Carbon Electrode Modified with a Three-Dimensional Network Consisting of Ni7S6 and Multi-Walled Carbon Nanotubes. Microchim. Acta 2016, 183, 3159–3166. [Google Scholar] [CrossRef]
- Rao, D.; Sheng, Q.; Zheng, J. Self-Assembly Preparation of Gold Nanoparticle Decorated 1-Pyrenemethylamine Functionalized Graphene Oxide–Carbon Nanotube Composites for Highly Sensitive Detection of Nitrite. Anal. Methods 2016, 8, 4926–4933. [Google Scholar] [CrossRef]
- Bhat, S.A.; Pandit, S.A.; Rather, M.A.; Rather, G.M.; Rashid, N.; Ingole, P.P.; Bhat, M.A. Self-Assembled AuNPs on Sulphur-Doped Graphene: A Dual and Highly Efficient Electrochemical Sensor for Nitrite (NO 2 −) and Nitric Oxide (NO). New J. Chem. 2017, 41, 8347–8358. [Google Scholar] [CrossRef]
- Aksu, Z.; Alanyalıoğlu, M. Fabrication of Free-Standing Reduced Graphene Oxide Composite Papers Doped with Different Dyes and Comparison of Their Electrochemical Performance for Electrocatalytical Oxidation of Nitrite. Electrochim. Acta 2017, 258, 1376–1386. [Google Scholar] [CrossRef]
- Wang, L.; Kim, J.; Cui, T. Self-Assembled Graphene and Copper Nanoparticles Composite Sensor for Nitrate Determination. Microsyst. Technol. 2018, 24, 3623–3630. [Google Scholar] [CrossRef]
- Can, F.; Korkut Ozoner, S.; Ergenekon, P.; Erhan, E. Amperometric Nitrate Biosensor Based on Carbon Nanotube/Polypyrrole/Nitrate Reductase Biofilm Electrode. Mater. Sci. Eng. C 2012, 32, 18–23. [Google Scholar] [CrossRef]
- Madasamy, T.; Pandiaraj, M.; Balamurugan, M.; Bhargava, K.; Sethy, N.K.; Karunakaran, C. Copper, Zinc Superoxide Dismutase and Nitrate Reductase Coimmobilized Bienzymatic Biosensor for the Simultaneous Determination of Nitrite and Nitrate. Biosens. Bioelectron. 2014, 52, 209–215. [Google Scholar] [CrossRef]
- Manea, F.; Remes, A.; Radovan, C.; Pode, R.; Picken, S.; Schoonman, J. Simultaneous Electrochemical Determination of Nitrate and Nitrite in Aqueous Solution Using Ag-Doped Zeolite-Expanded Graphite-Epoxy Electrode. Talanta 2010, 83, 66–71. [Google Scholar] [CrossRef]
- Hu, J.; Sun, J.; Bian, C.; Tong, J.; Shanhong, X. 3D Dendritic Nanostructure of Silver-Array: Preparation, Growth Mechanism and Application in Nitrate Sensor. Electroanalysis 2013, 25, 546–556. [Google Scholar] [CrossRef]
- Li, Y.; Sun, J.; Bian, C.; Tong, J.; Xia, S. Electrodeposition of Copper Nano-Clusters at a Platinum Microelectrode for Trace Nitrate Determination. Procedia Eng. 2010, 5, 339–342. [Google Scholar] [CrossRef] [Green Version]
- da Silva, I.S.; de Araujo, W.R.; Paixão, T.R.L.C.; Angnes, L. Direct Nitrate Sensing in Water Using an Array of Copper-Microelectrodes from Flat Flexible Cables. Sens. Actuators B Chem. 2013, 188, 94–98. [Google Scholar] [CrossRef]
- Stortini, A.M.; Moretto, L.M.; Mardegan, A.; Ongaro, M.; Ugo, P. Arrays of Copper Nanowire Electrodes: Preparation, Characterization and Application as Nitrate Sensor. Sens. Actuators B Chem. 2015, 207, 186–192. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Han, H.; Pan, D.; Zhang, P. Fabrication of a Micro-Needle Sensor Based on Copper Microspheres and Polyaniline Film for Nitrate Determination in Coastal River Waters. J. Electrochem. Soc. 2019, 166, B1038–B1043. [Google Scholar] [CrossRef]
- Lotfi Zadeh Zhad, H.R.; Lai, R.Y. Comparison of Nanostructured Silver-Modified Silver and Carbon Ultramicroelectrodes for Electrochemical Detection of Nitrate. Anal. Chim. Acta 2015, 892, 153–159. [Google Scholar] [CrossRef]
- Yang, H.; Cui, H.; Tang, W.; Li, Z.; Han, N.; Xing, F. A Critical Review on Research Progress of Graphene/Cement Based Composites. Compos. Part A Appl. Sci. Manuf. 2017, 102, 273–296. [Google Scholar] [CrossRef]
- Xiao, Q.; Feng, M.; Liu, Y.; Lu, S.; He, Y.; Huang, S. The Graphene/Polypyrrole/Chitosan-Modified Glassy Carbon Electrode for Electrochemical Nitrite Detection. Ionics 2018, 24, 845–859. [Google Scholar] [CrossRef]
- Yao, Y.; Ping, J. Recent Advances in Graphene-Based Freestanding Paper-like Materials for Sensing Applications. TrAC Trends Anal. Chem. 2018, 105, 75–88. [Google Scholar] [CrossRef]
- Thirumalraj, B.; Palanisamy, S.; Chen, S.-M.; Zhao, D.-H. Amperometric Detection of Nitrite in Water Samples by Use of Electrodes Consisting of Palladium-Nanoparticle-Functionalized Multi-Walled Carbon Nanotubes. J. Colloid Interface Sci. 2016, 478, 413–420. [Google Scholar] [CrossRef]
- Fajerwerg, K.; Ynam, V.; Chaudret, B.; Garçon, V.; Thouron, D.; Comtat, M. An Original Nitrate Sensor Based on Silver Nanoparticles Electrodeposited on a Gold Electrode. Electrochem. Commun. 2010, 12, 1439–1441. [Google Scholar] [CrossRef]
- Ghanbari, K. Silver Nanoparticles Dispersed in Polypyrrole Matrixes Coated on Glassy Carbon Electrode as a Nitrate Sensor. Anal. Bioanal. Electrochem. 2013, 5, 46–58. [Google Scholar]
- Xi, R.; Zhang, S.-H.; Zhang, L.; Wang, C.; Wang, L.-J.; Yan, J.-H.; Pan, G.-B. Electrodeposition of Pd-Pt Nanocomposites on Porous GaN for Electrochemical Nitrite Sensing. Sensors 2019, 19, 606. [Google Scholar] [CrossRef] [Green Version]
- Bonyani, M.; Mirzaei, A.; Leonardi, S.G.; Neri, G. Silver Nanoparticles/Polymethacrylic Acid (AgNPs/PMA) Hybrid Nanocomposites-Modified Electrodes for the Electrochemical Detection of Nitrate Ions. Measurement 2016, 84, 83–90. [Google Scholar] [CrossRef]
- Runsewe, D.; Betancourt, T.; Irvin, J.A. Biomedical Application of Electroactive Polymers in Electrochemical Sensors: A Review. Materials 2019, 12, 2629. [Google Scholar] [CrossRef] [Green Version]
- Roldán-Carmona, C.; Malinkiewicz, O.; Soriano, A.; Mínguez Espallargas, G.; Garcia, A.; Reinecke, P.; Kroyer, T.; Dar, M.I.; Nazeeruddin, M.K.; Bolink, H.J. Flexible High Efficiency Perovskite Solar Cells. Energy Environ. Sci. 2014, 7, 994. [Google Scholar] [CrossRef]
- Ge, Y.; Jamal, R.; Zhang, R.; Zhang, W.; Yu, Z.; Yan, Y.; Liu, Y.; Abdiryim, T. Electrochemical Synthesis of Multilayered PEDOT/PEDOT-SH/Au Nanocomposites for Electrochemical Sensing of Nitrite. Microchim. Acta 2020, 187, 248. [Google Scholar] [CrossRef]
- Wang, G.; Morrin, A.; Li, M.; Liu, N.; Luo, X. Nanomaterial-Doped Conducting Polymers for Electrochemical Sensors and Biosensors. J. Mater. Chem. B 2018, 6, 4173–4190. [Google Scholar] [CrossRef]
- Arvas, M.B.; Gorduk, O.; Gencten, M.; Sahin, Y. Preparation of a Novel Electrochemical Sensor for Phosphate Detection Based on a Molybdenum Blue Modified Poly(Vinyl Chloride) Coated Pencil Graphite Electrode. Anal. Methods 2019, 11, 3874–3881. [Google Scholar] [CrossRef]
- Topcu, C.; Caglar, B.; Onder, A.; Coldur, F.; Caglar, S.; Guner, E.K.; Cubuk, O.; Tabak, A. Structural Characterization of Chitosan-Smectite Nanocomposite and Its Application in the Development of a Novel Potentiometric Monohydrogen Phosphate-Selective Sensor. Mater. Res. Bull. 2018, 98, 288–299. [Google Scholar] [CrossRef]
- Haung, Y.; Ye, Y.; Zhao, G.; Wu, X.; Kan, Y.; Mur, L.; Han, J.; Qin, H. An All-Solid-State Phosphate Electrode with H3PO4 Doped Polyaniline as the Sensitive Layer. Int. J. Electrochem. Sci. 2017, 12, 4677–4691. [Google Scholar] [CrossRef]
- Ding, X.; Behbahani, M.; Gruden, C.; Seo, Y. Characterization and Evaluation of Phosphate Microsensors to Monitor Internal Phosphorus Loading in Lake Erie Sediments. J. Environ. Manag. 2015, 160, 193–200. [Google Scholar] [CrossRef] [Green Version]
- Talarico, D.; Arduini, F.; Amine, A.; Moscone, D.; Palleschi, G. Screen-Printed Electrode Modified with Carbon Black Nanoparticles for Phosphate Detection by Measuring the Electroactive Phosphomolybdate Complex. Talanta 2015, 141, 267–272. [Google Scholar] [CrossRef] [PubMed]
- Bhat, K.S.; Nakate, U.T.; Yoo, J.-Y.; Wang, Y.; Mahmoudi, T.; Hahn, Y.-B. Nozzle-Jet-Printed Silver/Graphene Composite-Based Field-Effect Transistor Sensor for Phosphate Ion Detection. ACS Omega 2019, 4, 8373–8380. [Google Scholar] [CrossRef] [PubMed]
- Cinti, S.; Talarico, D.; Palleschi, G.; Moscone, D.; Arduini, F. Novel Reagentless Paper-Based Screen-Printed Electrochemical Sensor to Detect Phosphate. Anal. Chim. Acta 2016, 919, 78–84. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Wang, L.; Bourouina, T.; Cui, T. Ion Sensitive Field Effect Transistor Based on Graphene and Ionophore Hybrid Membrane for Phosphate Detection. Microsyst. Technol. 2019, 25, 3357–3364. [Google Scholar] [CrossRef]
- Barhoumi, L.; Baraket, A.; Nooredeen, N.M.; Ali, M.B.; Abbas, M.N.; Bausells, J.; Errachid, A. Silicon Nitride Capacitive Chemical Sensor for Phosphate Ion Detection Based on Copper Phthalocyanine - Acrylate-Polymer. Electroanalysis 2017, 29, 1586–1595. [Google Scholar] [CrossRef] [Green Version]
- Zina, F.; Nooredeen, N.M.; Azzouzi, S.; Ali, M.B.; Abbas, M.N.; Errachid, A. Novel Sensitive Impedimetric Microsensor for Phosphate Detection Based on a Novel Copper Phthalocyanine Derivative. Anal. Lett. 2018, 51, 371–386. [Google Scholar] [CrossRef]
- Li, L.; Shang, G.; Qin, W. Potentiometric Sensing of Aqueous Phosphate by Competition Assays Using Ion-Exchanger Doped-Polymeric Membrane Electrodes as Transducers. Analyst 2016, 141, 4573–4577. [Google Scholar] [CrossRef]
- Cui, J.; Ogabiela, E.E.; Hui, J.; Wang, Y.; Zhang, Y.; Tong, L.; Zhang, J.; Adeloju, S.B.; Zhang, X.; Wu, Y. Electrochemical Biosensor Based on Pt/Au Alloy Nanowire Arrays for Phosphate Detection. J. Electrochem. Soc. 2015, 162, B62–B67. [Google Scholar] [CrossRef]
- Kabir, M.F.; Rahman, M.T.; Gurung, A.; Qiao, Q. Electrochemical Phosphate Sensors Using Silver Nanowires Treated Screen Printed Electrodes. IEEE Sens. J. 2018, 18, 3480–3485. [Google Scholar] [CrossRef]
- Kazem, K.; Payam, H.; Hamid, A.S. Cobalt-Graphene Nanocomposite Electrode for Phosphate Sensing. Anal. Bioanal. Electrochem. 2017, 9, 521–534. [Google Scholar]
- Kolliopoulos, A.V.; Kampouris, D.K.; Banks, C.E. Rapid and Portable Electrochemical Quantification of Phosphorus. Anal. Chem. 2015, 87, 4269–4274. [Google Scholar] [CrossRef] [Green Version]
- Ahmad, R.; Ahn, M.-S.; Hahn, Y.-B. ZnO Nanorods Array Based Field-Effect Transistor Biosensor for Phosphate Detection. J. Colloid Interface Sci. 2017, 498, 292–297. [Google Scholar] [CrossRef]
- Ogabiela, E.; Adeloju, S.B.; Cui, J.; Wu, Y.; Chen, W. A Novel Ultrasensitive Phosphate Amperometric Nanobiosensor Based on the Integration of Pyruvate Oxidase with Highly Ordered Gold Nanowires Array. Biosens. Bioelectron. 2015, 71, 278–285. [Google Scholar] [CrossRef]
- Power, A.C.; Gorey, B.; Chandra, S.; Chapman, J. Carbon Nanomaterials and Their Application to Electrochemical Sensors: A Review. Nanotechnol. Rev. 2018, 7, 19–41. [Google Scholar] [CrossRef]
- Talbi, M.; Al-Hamry, A.; Ali, M.B.; Kanoun, O. Carbon Screen Printed Electrodes Functionalized with Cu(II)Pc for Phosphate Detection. In Proceedings of the 2020 17th International Multi-Conference on Systems, Signals Devices (SSD), Monastir, Tunisia, 20–23 July 2020; pp. 869–872. [Google Scholar]
- Hariganesh, S.; Vadivel, S.; Maruthamani, D.; Rangabhashiyam, S. Disinfection by-products in drinking water: Detection and treatment methods. In Disinfection By-products in Drinking Water; Butterworth-Heinemann: Oxford, UK, 2020; pp. 279–304. ISBN 978-0-08-102977-0. [Google Scholar]
- Valentini, F.; Biagiotti, V.; Lete, C.; Palleschi, G.; Wang, J. The Electrochemical Detection of Ammonia in Drinking Water Based on Multi-Walled Carbon Nanotube/Copper Nanoparticle Composite Paste Electrodes. Sens. Actuators B Chem. 2007, 128, 326–333. [Google Scholar] [CrossRef]
- Sun, W.; Hou, F.; Gong, S.; Han, L.; Wang, W.; Shi, F.; Xi, J.; Wang, X.; Li, G. Direct Electrochemistry and Electrocatalysis of Hemoglobin on Three-Dimensional Graphene Modified Carbon Ionic Liquid Electrode. Sens. Actuators B Chem. 2015, 219, 331–337. [Google Scholar] [CrossRef]
- Dai, H.; Xu, H.; Wu, X.; Lin, Y.; Wei, M.; Chen, G. Electrochemical Behavior of Thionine at Titanate Nanotubes-Based Modified Electrode: A Sensing Platform for the Detection of Trichloroacetic Acid. Talanta 2010, 81, 1461–1466. [Google Scholar] [CrossRef]
- Peverly, A.A.; Peters, D.G. Electrochemical Determination of Trihalomethanes in Water by Means of Stripping Analysis. Anal. Chem. 2012, 84, 6110–6115. [Google Scholar] [CrossRef]
- Cetó, X.; Saint, C.; Chow, C.W.K.; Voelcker, N.H.; Prieto-Simón, B. Electrochemical Fingerprints of Brominated Trihaloacetic Acids (HAA3) Mixtures in Water. Sens. Actuators B Chem. 2017, 247, 70–77. [Google Scholar] [CrossRef]
- Qian, D.; Li, W.; Chen, F.; Huang, Y.; Bao, N.; Gu, H.; Yu, C. Voltammetric Sensor for Trichloroacetic Acid Using a Glassy Carbon Electrode Modified with Au@Ag Nanorods and Hemoglobin. Microchim. Acta 2017, 184, 1977–1985. [Google Scholar] [CrossRef]
- Al-Zahrani, E.; Soomro, M.T.; Bashami, R.M.; Rehman, A.U.; Danish, E.; Ismail, I.M.I.; Aslam, M.; Hameed, A. Fabrication and Performance of Magnetite (Fe3O4) Modified Carbon Paste Electrode for the Electrochemical Detection of Chlorite Ions in Aqueous Medium. J. Environ. Chem. Eng. 2016, 4, 4330–4341. [Google Scholar] [CrossRef]
- Wang, Y.-H.; Yu, C.-M.; Gu, H.-Y.; Tu, Y.-F. The Hemoglobin-Modified Electrode with Chitosan/Fe3O4 Nanocomposite for the Detection of Trichloroacetic Acid. J. Solid State Electrochem. 2016, 20, 1337–1344. [Google Scholar] [CrossRef]
- Zhao, W.; Li, X.; Wen, Z.; Niu, X.; Shen, Q.; Sun, Z.; Dong, R.; Sun, W. Application of Ionic Liquid-Graphene-NiO Hollowsphere Composite Modified Electrode for Electrochemical Investigation on Hemoglobin and Electrocatalysis to Trichloroacetic Acid. Int. J. Electrochem. Sci. 2017, 12, 2025–2034. [Google Scholar] [CrossRef]
- Kong, L.; Du, Z.; Xie, Z.; Chen, R.; Jia, S.; Dong, R.; Sun, Z.; Sun, W. Electrochemistry of Hemoglobin-Ionic Liquid-Graphene-SnO2 Nanosheet Composite Modified Electrode and Electrocatalysis. Int. J. Electrochem. Sci. 2017, 12, 2297–2305. [Google Scholar] [CrossRef]
- Yan, H.; Chen, X.; Shi, Z.; Feng, Y.; Li, J.; Lin, Q.; Wang, X.; Sun, W. Direct Electrochemistry of Myoglobin on TiO2 and Alginate Composite Modified Carbon Ionic Liquid Electrode via the Electrodeposition Method. J. Solid State Electrochem. 2016, 20, 1783–1792. [Google Scholar] [CrossRef]
- Shi, F.; Zheng, W.; Wang, W.; Hou, F.; Lei, B.; Sun, Z.; Sun, W. Application of Graphene–Copper Sulfide Nanocomposite Modified Electrode for Electrochemistry and Electrocatalysis of Hemoglobin. Biosens. Bioelectron. 2015, 64, 131–137. [Google Scholar] [CrossRef]
- Najafi, M.; Darabi, S.; Tadjarodi, A.; Imani, M. Determination of Trichloroacetic Acid (TCAA) Using CdO Nanoparticles Modified Carbon Paste Electrode. Electroanalysis 2013, 25, 487–492. [Google Scholar] [CrossRef]
- Zeng, Z.; Fang, X.; Miao, W.; Liu, Y.; Maiyalagan, T.; Mao, S. Electrochemically Sensing of Trichloroacetic Acid with Iron(II) Phthalocyanine and Zn-Based Metal Organic Framework Nanocomposites. ACS Sens. 2019, 4, 1934–1941. [Google Scholar] [CrossRef]
- Cetó, X.; Saint, C.P.; Chow, C.W.K.; Voelcker, N.H.; Prieto-Simón, B. Electrochemical Detection of N-nitrosodimethylamine Using a Molecular Imprinted Polymer. Sens. Actuators B Chem. 2016, 237, 613–620. [Google Scholar] [CrossRef]
- Chen, W.; Weng, W.; Niu, X.; Li, X.; Men, Y.; Sun, W.; Li, G.; Dong, L. Boron-Doped Graphene Quantum Dots Modified Electrode for Electrochemistry and Electrocatalysis of Hemoglobin. J. Electroanal. Chem. 2018, 823, 137–145. [Google Scholar] [CrossRef]
- Zhu, L.; Li, X.; Deng, Y.; Zou, R.; Shao, B.; Yan, L.; Ruan, C.; Sun, W. Construction and Electrochemical Behavior of Hemoglobin Sensor Based on ZnO Doped Carbon Nanofiber Modified Electrode. J. Iran. Chem. Soc. 2020. [Google Scholar] [CrossRef]
- Xie, H.; Luo, G.; Niu, Y.; Weng, W.; Zhao, Y.; Ling, Z.; Ruan, C.; Li, G.; Sun, W. Synthesis and Utilization of Co3O4 Doped Carbon Nanofiber for Fabrication of Hemoglobin-Based Electrochemical Sensor. Mater. Sci. Eng. C 2020, 107, 110209. [Google Scholar] [CrossRef] [PubMed]
- Bashami, R.M.; Soomro, M.T.; Khan, A.N.; Aazam, E.S.; Ismail, I.M.I.; El-Shahawi, M.S. A Highly Conductive Thin Film Composite Based on Silver Nanoparticles and Malic Acid for Selective Electrochemical Sensing of Trichloroacetic Acid. Anal. Chim. Acta 2018, 1036, 33–48. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.-H.; Mugherli, L.; Rivron, C.; Tran-Thi, T.-H. Innovative Colorimetric Sensors for the Selective Detection of Monochloramine in Air and in Water. Sens. Actuators B Chem. 2015, 208, 622–627. [Google Scholar] [CrossRef]
- Serrano, M.; Silva, M.; Gallego, M. Determination of 14 Haloketones in Treated Water Using Solid–Phase Microextraction and Gas Chromatography–Mass Spectrometry. J. Chromatogr. A 2015, 1407, 208–215. [Google Scholar] [CrossRef]
- Nasraoui, S.; Al-Hamry, A.; Ameur, S.; Ben Ali, M.; Kanoun, O. Graphene Induced Using 405 Nm Laser as Electrode Material for the Electrochemical Sensing Application. In Proceedings of the 2019 5th International Conference on Nanotechnology for Instrumentation and Measurement (NanofIM), Sfax, Tunisia, 30 October 2019; pp. 1–5. [Google Scholar]
- Tirawattanakoson, R.; Rattanarat, P.; Ngamrojanavanich, N.; Rodthongkum, N.; Chailapakul, O. Free Radical Scavenger Screening of Total Antioxidant Capacity in Herb and Beverage Using Graphene/PEDOT: PSS-Modified Electrochemical Sensor. J. Electroanal. Chem. 2016, 767, 68–75. [Google Scholar] [CrossRef]
- Gao, F.; Zheng, D.; Tanaka, H.; Zhan, F.; Yuan, X.; Gao, F.; Wang, Q. An Electrochemical Sensor for Gallic Acid Based on Fe2O3/Electro-Reduced Graphene Oxide Composite: Estimation for the Antioxidant Capacity Index of Wines. Mater. Sci. Eng. C 2015, 57, 279–287. [Google Scholar] [CrossRef]
- Souza, L.P.; Calegari, F.; Zarbin, A.J.G.; Marcolino-Júnior, L.H.; Bergamini, M.F. Voltammetric Determination of the Antioxidant Capacity in Wine Samples Using a Carbon Nanotube Modified Electrode. J. Agric. Food Chem. 2011, 59, 7620–7625. [Google Scholar] [CrossRef]
- Ziyatdinova, G.; Salikhova, I.; Budnikov, H. Chronoamperometric Estimation of Cognac and Brandy Antioxidant Capacity Using MWNT Modified Glassy Carbon Electrode. Talanta 2014, 125, 378–384. [Google Scholar] [CrossRef]
- Talarico, D.; Arduini, F.; Constantino, A.; Del Carlo, M.; Compagnone, D.; Moscone, D.; Palleschi, G. Carbon Black as Successful Screen-Printed Electrode Modifier for Phenolic Compound Detection. Electrochem. Commun. 2015, 60, 78–82. [Google Scholar] [CrossRef]
- Della Pelle, F.; Di Battista, R.; Vázquez, L.; Palomares, F.J.; Del Carlo, M.; Sergi, M.; Compagnone, D.; Escarpa, A. Press-Transferred Carbon Black Nanoparticles for Class-Selective Antioxidant Electrochemical Detection. Appl. Mater. Today 2017, 9, 29–36. [Google Scholar] [CrossRef] [Green Version]
- Raymundo-Pereira, P.A.; Campos, A.M.; Prado, T.M.; Furini, L.N.; Boas, N.V.; Calegaro, M.L.; Machado, S.A.S. Synergy between Printex Nano-Carbons and Silver Nanoparticles for Sensitive Estimation of Antioxidant Activity. Anal. Chim. Acta 2016, 926, 88–98. [Google Scholar] [CrossRef] [Green Version]
- Hui, K.H.; Ambrosi, A.; Pumera, M.; Bonanni, A. Improving the Analytical Performance of Graphene Oxide towards the Assessment of Polyphenols. Chem. Eur. J. 2016, 22, 3830–3834. [Google Scholar] [CrossRef]
- Hammani, H.; Boumya, W.; Laghrib, F.; Farahi, A.; Lahrich, S.; Aboulkas, A.; El Mhammedi, M.A. Electro-Catalytic Effect of Al 2 O 3 Supported onto Activated Carbon in Oxidizing Phenol at Graphite Electrode. Mater. Today Chem. 2017, 3, 27–36. [Google Scholar] [CrossRef]
- Quynh, B.T.P.; Byun, J.Y.; Kim, S.H. Non-Enzymatic Amperometric Detection of Phenol and Catechol Using Nanoporous Gold. Sens. Actuators B Chem. 2015, 221, 191–200. [Google Scholar] [CrossRef]
- Spătaru, T.; Spătaru, N. Voltammetric Detection of Phenol at Platinum–Polytyramine Composite Electrodes in Acidic Media. J. Hazard. Mater. 2010, 180, 777–780. [Google Scholar] [CrossRef]
- Nurul Karim, M.; Lee, H.J. Amperometric Phenol Biosensor Based on Covalent Immobilization of Tyrosinase on Au Nanoparticle Modified Screen Printed Carbon Electrodes. Talanta 2013, 116, 991–996. [Google Scholar] [CrossRef]
- Gu, B.X.; Xu, C.X.; Zhu, G.P.; Liu, S.Q.; Chen, L.Y.; Li, X.S. Tyrosinase Immobilization on ZnO Nanorods for Phenol Detection. J. Phys. Chem. B 2009, 113, 377–381. [Google Scholar] [CrossRef]
- Yi, H. Adsorption Stripping Voltammetry of Phenol at Nafion-Modified Glassy Carbon Electrode in the Presence of Surfactants. Talanta 2001, 55, 1205–1210. [Google Scholar] [CrossRef]
- Pan, G.; Zhao, G.; Wei, M.; Wang, Y.; Zhao, B. Design of Nanogold Electrochemical Immunosensor for Detection of Four Phenolic Estrogens. Chem. Phys. Lett. 2019, 732, 136657. [Google Scholar] [CrossRef]
- Nameghi, M.A.; Danesh, N.M.; Ramezani, M.; Alibolandi, M.; Abnous, K.; Taghdisi, S.M. An Ultrasensitive Electrochemical Sensor for 17β-Estradiol Using Split Aptamers. Anal. Chim. Acta 2019, 1065, 107–112. [Google Scholar] [CrossRef] [PubMed]
- Florea, A.; Cristea, C.; Vocanson, F.; Săndulescu, R.; Jaffrezic-Renault, N. Electrochemical Sensor for the Detection of Estradiol Based on Electropolymerized Molecularly Imprinted Polythioaniline Film with Signal Amplification Using Gold Nanoparticles. Electrochem. Commun. 2015, 59, 36–39. [Google Scholar] [CrossRef]
- Wang, Y.; Luo, J.; Liu, J.; Li, X.; Kong, Z.; Jin, H.; Cai, X. Electrochemical Integrated Paper-Based Immunosensor Modified with Multi-Walled Carbon Nanotubes Nanocomposites for Point-of-Care Testing of 17β-Estradiol. Biosens. Bioelectron. 2018, 107, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Silva, C.C.G.; Silva, L.M.; e Silva, B.C.; Garrido, S.S.; Boldrin, M.V.; De Souza, D. Cathodic Stripping Voltammetric Determination of β-Cyfluthrin, a Pyrethroid Insecticide, Using Polished Silver Solid Amalgam Electrode. J. Solid State Electrochem. 2020, 24, 1819–1826. [Google Scholar] [CrossRef]
- Punde, N.S.; Rajpurohit, A.S.; Srivastava, A.K. Sensitive Electrochemical Platform Based on Nano-Cylindrical Strontium Titanate/N-Doped Graphene Hybrid Composite for Simultaneous Detection of Diphenhydramine and Bromhexine. Electrochim. Acta 2019, 319, 727–739. [Google Scholar] [CrossRef]
- Li, J.; Kuang, D.; Feng, Y.; Zhang, F.; Liu, M. Voltammetric Determination of Bisphenol A in Food Package by a Glassy Carbon Electrode Modified with Carboxylated Multi-Walled Carbon Nanotubes. Microchim. Acta 2011, 172, 379–386. [Google Scholar] [CrossRef]
- Huang, Y.; Li, X.; Zheng, S. A Novel and Label-Free Immunosensor for Bisphenol A Using Rutin as the Redox Probe. Talanta 2016, 160, 241–246. [Google Scholar] [CrossRef]
- Liu, Y.; Yao, L.; He, L.; Liu, N.; Piao, Y. Electrochemical Enzyme Biosensor Bearing Biochar Nanoparticle as Signal Enhancer for Bisphenol A Detection in Water. Sensors 2019, 19, 1619. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.; Ren, T.; Ma, M.; Wang, Z.; Zhan, G.; Li, C. Voltammetric Sensing of Bisphenol A Based on a Single-Walled Carbon Nanotubes/Poly{3-Butyl-1-[3-(N-Pyrrolyl)Propyl] Imidazolium Ionic Liquid} Composite Film Modified Electrode. Electrochim. Acta 2013, 111, 49–56. [Google Scholar] [CrossRef]
- Kunene, K.; Sabela, M.; Kanchi, S.; Bisetty, K. High Performance Electrochemical Biosensor for Bisphenol A using Screen Printed Electrodes Modified with Multiwalled Carbon Nanotubes Functionalized with Silver-Doped Zinc Oxide. Waste Biomass Valori. 2020, 11, 1085–1096. [Google Scholar] [CrossRef]
- Li, R.; Wang, Y.; Deng, Y.; Liu, G.; Hou, X.; Huang, Y.; Li, C. Enhanced Biosensing of Bisphenol A Using a Nanointerface Based on Tyrosinase/Reduced Graphene Oxides Functionalized with Ionic Liquid. Electroanalysis 2016, 28, 96–102. [Google Scholar] [CrossRef]
- Tan, F.; Cong, L.; Li, X.; Zhao, Q.; Zhao, H.; Quan, X.; Chen, J. An Electrochemical Sensor Based on Molecularly Imprinted Polypyrrole/Graphene Quantum Dots Composite for Detection of Bisphenol A in Water Samples. Sens. Actuators B Chem. 2016, 233, 599–606. [Google Scholar] [CrossRef]
- Najafi, M.; Khalilzadeh, M.A.; Karimi-Maleh, H. A New Strategy for Determination of Bisphenol A in the Presence of Sudan I Using a ZnO/CNTs/Ionic Liquid Paste Electrode in Food Samples. Food Chem. 2014, 158, 125–131. [Google Scholar] [CrossRef]
- Deng, P.; Xu, Z.; Kuang, Y. Electrochemical Determination of Bisphenol A in Plastic Bottled Drinking Water and Canned Beverages Using a Molecularly Imprinted Chitosan–Graphene Composite Film Modified Electrode. Food Chem. 2014, 157, 490–497. [Google Scholar] [CrossRef]
- Zhou, L.; Wang, J.; Li, D.; Li, Y. An Electrochemical Aptasensor Based on Gold Nanoparticles Dotted Graphene Modified Glassy Carbon Electrode for Label-Free Detection of Bisphenol A in Milk Samples. Food Chem. 2014, 162, 34–40. [Google Scholar] [CrossRef]
- Supraja, P.; Tripathy, S.; Krishna Vanjari, S.R.; Singh, V.; Singh, S.G. Electrospun Tin (IV) Oxide Nanofiber Based Electrochemical Sensor for Ultra-Sensitive and Selective Detection of Atrazine in Water at Trace Levels. Biosens. Bioelectron. 2019, 141, 111441. [Google Scholar] [CrossRef]
- Rather, J.A.; De Wael, K. Fullerene-C60 Sensor for Ultra-High Sensitive Detection of Bisphenol-A and Its Treatment by Green Technology. Sens. Actuators B Chem. 2013, 176, 110–117. [Google Scholar] [CrossRef]
- Chawla, S.; Rawal, R.; Kumar, D.; Pundir, C.S. Amperometric Determination of Total Phenolic Content in Wine by Laccase Immobilized onto Silver Nanoparticles/Zinc Oxide Nanoparticles Modified Gold Electrode. Anal. Biochem. 2012, 430, 16–23. [Google Scholar] [CrossRef]
- Pino, F.; Mayorga-Martinez, C.C.; Merkoçi, A. High-Performance Sensor Based on Copper Oxide Nanoparticles for Dual Detection of Phenolic Compounds and a Pesticide. Electrochem. Commun. 2016, 71, 33–37. [Google Scholar] [CrossRef]
- Li, H.-Y.; Wang, X.-L.; Wang, Z.-X.; Jiang, W. Sensitive Determination of Bisphenol A Based on Ag Nanoparticles/Polyguanine Modified Electrode. Russ. J. Electrochem. 2017, 53, 132–139. [Google Scholar] [CrossRef]
- Chen, W.-Y.; Mei, L.-P.; Feng, J.-J.; Yuan, T.; Wang, A.-J.; Yu, H. Electrochemical Determination of Bisphenol A with a Glassy Carbon Electrode Modified with Gold Nanodendrites. Microchim. Acta 2015, 182, 703–709. [Google Scholar] [CrossRef]
- Tajik, S.; Beitollahi, H.; Nejad, F.G.; Shoaie, I.S.; Khalilzadeh, M.A.; Asl, M.S.; Van Le, Q.; Zhang, K.; Jang, H.W.; Shokouhimehr, M. Recent Developments in Conducting Polymers: Applications for Electrochemistry. RSC Adv. 2020, 10, 37834–37856. [Google Scholar] [CrossRef]
- Zhang, J.; Ellis, H.; Yang, L.; Johansson, E.M.J.; Boschloo, G.; Vlachopoulos, N.; Hagfeldt, A.; Bergquist, J.; Shevchenko, D. Matrix-Assisted Laser Desorption/Ionization Mass Spectrometric Analysis of Poly(3,4-Ethylenedioxythiophene) in Solid-State Dye-Sensitized Solar Cells: Comparison of In Situ Photoelectrochemical Polymerization in Aqueous Micellar and Organic Media. Anal. Chem. 2015, 87, 3942–3948. [Google Scholar] [CrossRef] [PubMed]
- Mazzotta, E.; Malitesta, C.; Margapoti, E. Direct Electrochemical Detection of Bisphenol A at PEDOT-Modified Glassy Carbon Electrodes. Anal. Bioanal. Chem. 2013, 405, 3587–3592. [Google Scholar] [CrossRef]
- Poorahong, S.; Thammakhet, C.; Thavarungkul, P.; Limbut, W.; Numnuam, A.; Kanatharana, P. Amperometric Sensor for Detection of Bisphenol A Using a Pencil Graphite Electrode Modified with Polyaniline Nanorods and Multiwalled Carbon Nanotubes. Microchim. Acta 2012, 176, 91–99. [Google Scholar] [CrossRef]
- Suresh, S.; Srivastava, V.C.; Mishra, I.M. Adsorption of Catechol, Resorcinol, Hydroquinone, and Their Derivatives: A Review. Int. J. Energy Environ. Eng. 2012, 3, 32. [Google Scholar] [CrossRef]
- Bieniek, G. Simultaneous Determination of Phenol, Cresol, Xylenol Isomers and Napthols in Urine by Capillary Gas Chromatography. J. Chromatogr. B Biomed. Sci. Appl. 1996, 682, 167–172. [Google Scholar] [CrossRef]
- Li, J.; Xu, Z.; Liu, M.; Deng, P.; Tang, S.; Jiang, J.; Feng, H.; Qian, D.; He, L. Ag/N-Doped Reduced Graphene Oxide Incorporated with Molecularly Imprinted Polymer: An Advanced Electrochemical Sensing Platform for Salbutamol Determination. Biosens. Bioelectron. 2017, 90, 210–216. [Google Scholar] [CrossRef]
- Yang, Y.J.; Weikun, L. Simultaneous Determination of Catechol, Hydroquinone, and Resorcinol on CTAB Functionalized Graphene Oxide/Multiwalled Carbon Nanotube Modified Electrode. Fuller. Nanotub. Carbon Nanostructures 2015, 23, 410–417. [Google Scholar] [CrossRef]
- Wang, J.; Yang, J.; Xu, P.; Liu, H.; Zhang, L.; Zhang, S.; Tian, L. Gold Nanoparticles Decorated Biochar Modified Electrode for the High-Performance Simultaneous Determination of Hydroquinone and Catechol. Sens. Actuators B Chem. 2020, 306, 127590. [Google Scholar] [CrossRef]
- Yang, S.; Yang, M.; Liu, Q.; Wang, X.; Fa, H.; Wang, Y.; Hou, C. An Ultrasensitive Electrochemical Sensor Based on Multiwalled Carbon Nanotube@Reduced Graphene Oxide Nanoribbon Composite for Simultaneous Determination of Hydroquinone, Catechol and Resorcinol. J. Electrochem. Soc. 2019, 166, B547–B553. [Google Scholar] [CrossRef]
- Liu, L.; Ma, Z.; Zhu, X.; Zeng, R.; Tie, S.; Nan, J. Electrochemical Behavior and Simultaneous Determination of Catechol, Resorcinol, and Hydroquinone Using Thermally Reduced Carbon Nano-Fragment Modified Glassy Carbon Electrode. Anal. Methods 2016, 8, 605–613. [Google Scholar] [CrossRef]
- Chen, T.W.; Yu, X.N.; Li, S.J. Simultaneous Determination of Dihydroxybenzene Isomers Using Glass Carbon Electrode Modified with 3D CNT-Graphene Decorated with Au Nanoparticles. Int. J. Electrochem. Sci. 2019, 14, 7037–7046. [Google Scholar] [CrossRef]
- Li, J.; Xia, J.; Zhang, F.; Wang, Z.; Liu, Q. An Electrochemical Sensor Based on Copper-Based Metal-Organic Frameworks-Graphene Composites for Determination of Dihydroxybenzene Isomers in Water. Talanta 2018, 181, 80–86. [Google Scholar] [CrossRef]
- Deng, M.; Lin, S.; Bo, X.; Guo, L. Simultaneous and Sensitive Electrochemical Detection of Dihydroxybenzene Isomers with UiO-66 Metal-Organic Framework/Mesoporous Carbon. Talanta 2017, 174, 527–538. [Google Scholar] [CrossRef]
- Zhang, H.; Huang, Y.; Hu, S.; Huang, Q.; Wei, C.; Zhang, W.; Yang, W.; Dong, P.; Hao, A. Self-Assembly of Graphitic Carbon Nitride Nanosheets–Carbon Nanotube Composite for Electrochemical Simultaneous Determination of Catechol and Hydroquinone. Electrochim. Acta 2015, 176, 28–35. [Google Scholar] [CrossRef]
- Fotouhi, L.; Dorraji, P.S.; Keshmiri, Y.S.S.; Hamtak, M. Electrochemical Sensor Based on Nanocomposite of Multi-Walled Carbon Nanotubes/TiO2 Nanoparticles in Chitosan Matrix for Simultaneous and Separate Determination of Dihydroxybenzene Isomers. J. Electrochem. Soc. 2018, 165, B202–B211. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, S.; Li, S.; Qu, J. Simultaneous Determination of Hydroquinone and Catechol Using a Glassy Carbon Electrode Modified with Au@Pd Loaded on Reduced Graphene Oxide. Anal. Methods 2018, 10, 1331–1338. [Google Scholar] [CrossRef]
- Singh, P. Composites Based on Conducting Polymers and Carbon Nanotubes for Supercapacitors. In Conducting Polymer Hybrids; Kumar, V., Kalia, S., Swart, H.C., Eds.; Springer Series on Polymer and Composite Materials; Springer International Publishing: Cham, Switzerland, 2017; pp. 305–336. ISBN 978-3-319-46456-5. [Google Scholar]
- Jiang, H.; Zhang, D.; He, Z.; Lian, Q.; Xue, Z.; Zhou, X.; Lu, X. A Novel Sensitive Electrochemical Sensor for the Simultaneous Determination of Hydroquinone and Catechol Using Tryptophan-Functionalized Graphene. Anal. Lett. 2015, 48, 1426–1436. [Google Scholar] [CrossRef]
- Song, D.; Xia, J.; Zhang, F.; Bi, S.; Xiang, W.; Wang, Z.; Xia, L.; Xia, Y.; Li, Y.; Xia, L. Multiwall Carbon Nanotubes-Poly(Diallyldimethylammonium Chloride)-Graphene Hybrid Composite Film for Simultaneous Determination of Catechol and Hydroquinone. Sens. Actuators B Chem. 2015, 206, 111–118. [Google Scholar] [CrossRef]
- Cuartero, M. Electrochemical Sensors for In-Situ Measurement of Ions in Seawater. Sens. Actuators B Chem. 2021, 334, 129635. [Google Scholar] [CrossRef]








| Analyte | EU (mg/L) | WHO (mg/L) | USA-EPA [mg/L] |
|---|---|---|---|
| Nitrate | 50 | 50 | 10 |
| Nitrite | 0.50 | 3 | 1 |
| Phosphate | - | 5 | - |
| Ammonium | 0.2 | 1.5 at Alkaline pH | - |
| Chlorate | 0.250 | 0.7 | - |
| 1,2-dichloroethane | 0.003 | 0.03 | 0.04 |
| Epichlorohydrin | 0.00010 | 0.0004 | 0.3 |
| Trihalomethanes (total) | 0.1 | 0.1 | 0.08 |
| Haloacetic acids (HAA5) | 0.06 | - | 0.06 |
| Halogenated acetonitriles | - | 0.02 | - |
| Trichlroacetaldehyde | - | 0.1 | - |
| 2,4,6-Trichlorophenols | - | 0.2 | 0.3 |
| Bisphenol A | 0.0025 | 2.5 | |
| Pesticides | 0.0001 | 0.00003–0.2 * | |
| Total pesticides | 0.0005 | ||
| Calcium | - | 10–500 | - |
| Magnesium | - | 52.1 | - |
| Detection Method | Materials | Analyte | LOD (µM) | Dynamic Range (µM) | Comments | Reference |
|---|---|---|---|---|---|---|
| SWV * | BDD | Parathion | 0.043 | - | Low interference with organic pollutants compared to HMDE * | [90] |
| SWV | BDD | Atrazine | 0.01 | 0.05–40 | Good selectivity and repeatability | [91] |
| SWV DPV * | BDD | Methomyl | 19 1.2 | 66–420 5.0–410.0 | Good recovery with real samples | [92] |
| adsorptive stripping SWV | Sol-gel carbon ceramic electrode | Fenitrothion | 0.0016 | 5000–1,000,000.1–50 | Demonstrated on-site monitoring | [93] |
| SWV | Graphite-modified basal plane pyrolytic graphite electrode | Methyl parathion | 3 | 79.0–263.3 | Applied for drinking water | [94] |
| adsorptive stripping SWV | Poly(4-amino-3-hydroxynaphthalene Sulfonic acid) modified GCE | Fenitrothion | 0.7 × 10−3 | 0.001–6.6 | Good recovery in spiked water samples | [95] |
| SWV | Sarbon black modified GCE | Mesotrione | 0.026 | 0.040–7.2 | Applied for real water samples and juice | [96] |
| cyclic voltammetry and SWV | Peptide nanotubes on modified pencil graphite electrode | Fenitrothion | 0.0196 | 0.114–1.712 | Good recovery in spiked water samples | [97] |
| DP adsorptive cathodic stripping voltammetric | Single-walled carbon nanohorns and zein modified GCE | Fenitrothion | 0.012 | 0.99–12 | Good repeatability and reproducibility applied for real water samples and juice | [98] |
| SWV | Screen-printed carbon electrode | Bentazone | 0.034 | - | Analysis time 10 s, reusable at least 15 times, sensitivity of 0.0987 × 106 μA/M | [99] |
| adsorptive stripping voltammetry | Nano poly(3-methyl thiophene)/multiwalled carbon nanotubes | Isoproturon, Voltage cypermethrin Deltamethrin fenvalerate Dicofol | 26–100 | 1.43–4.47 1.45–4.47 0.26–4.47 0.34–4.47 1.09–4.47 0.967–4.47 | Good recovery in spiked water samples | [100] |
| DPV | Multiwalled carbon nanotubes-poly(acrylamide) nanocomposite | Methyl parathion | 0.002 | 0.005–10 | Demonstrated for environmental water samples | [101] |
| SWV | Graphene-based electrochemical sensor | Isoproturon: | 20 | 20–1000 | Demonstrated for water, soil and vegetable samples | [102] |
| DPV | Graphene quantum dots with oxime as electroactive probe | Fenthion | 6.8 × 10−6 | 1.0 × 10−5–5.0 × 10−2 | Performance demonstrated for water and soil samples | [103] |
| DPV | Ionic liquid–graphene nanosheets | Methyl parathion | 1.9 × 10−5 | 0.09–0.04 | Satisfactory stability and reproducibility demonstrated for spiked water samples | [104] |
| DPV | N-methyl-2-pyrrolidone exfoliated graphene | Carbendazim | 0.78 M | 0.005–1.57 × 10−6 M | Demonstrated for ground water, soil, and cucumber samples | [105] |
| DPV | Pillar[5]arene/reduced graphene nanocomposite | Methyl parathion | 3 × 10−10 M | 0.001–150 × 10−6 M | Demonstrated for soil and waste water samples | [106] |
| DPV | Cellulose microfiber entrapped reduced graphene oxide | Fenitrothion | 0.008 | Linear range up to 1134 | Demonstrated for different water samples | [107] |
| Detection Method | Materials | Analyte | LOD (µM) | Dynamic Range (µM) | Comments | Reference |
|---|---|---|---|---|---|---|
| CV | Molecularly imprinted polypyrrole membrane | 2,4-Dichloro-phenoxy acetic acid | 0.83 | 1.0–10 | Successful determination in real samples | [109] |
| photoelectrochemical technique | Polypyrrole-based MIP composite with TiO2 * | 2,4-Dichloro-phenoxyacetic acid | 0.01 | - | Demonstrated for spiked water samples | [110] |
| capacitive sensor | Polyquercetin-polyresorcinol-Gold nanoparticles by MIP technique | Methyl parathion | 3.4 × 10−4 | 0.07–1 | Good recovery and low interference in water samples | [111] |
| impedimetric | MIP/sol–gel, different monomers | Methidathion | Proof-of-concept experiments | [112] | ||
| cyclic voltammetry and SWV | MIP suspension polymerization, modification of carbon paste electrode | Diazinon | 7.9 × 10−5 | 2.5 × 10−3–0.10.1–2.0 | Good recovery in water and apple samples | [113] |
| potentiometric | Methacrylic acid (functional monomer), ethylene glycol dimethyl acrylate (cross-linker) | Endosulfan | 20 | 20 to 12 × 10−3 | Nernstian response, good stability | [114] |
| DPV | Methacrylic acid, ethylene glycol dimethacrylate and carbon nanotubes | Diazinon | 1.3 × 10−4 | 5 × 10−4–1 | No sample preparation for human urine, tap, and river water samples | [115] |
| SWV | MIP with carbon nanotubes | Dicloran | 4.8 × 10−4 | 1 × 10−3–1 | No interference | [116] |
| cyclic voltammetry | MIP from methacrylic acid, ethylene glycol dimethacrylate | Atrazine | 716.26 | Proof-of-concept, effects of ethanol solution analyzed | [117] | |
| cyclic voltammetry | MIP/graphene oxide modified glassy carbon electrode | Profenofos | 5 × 10−3 | 0.05–3500 | Stable; enhanced selectivity vs. other structurally similar pesticides | [118] |
| DPV and EIS* | Acrylamide based MIP on gold electrode | Malathion | 1.79 × 10−7 | 1 × 10−7–0.017 | Good recovery in olive oil and fruit samples | [119] |
| Detection Technique | Electrode/ Materials | Analyte | LOD (µM) | Detection Range (µM) | Comments | Reference |
|---|---|---|---|---|---|---|
| CV, EIS | Screen-printed SPEs/carbon ink | Ca2+ | 1.0 | 10–10 × 104 | Detection in seawater | [160] |
| Potentiometric, EIS | Solid-contact ISE (SC-ISEs) | Ca2+ | 0.6 | 1–10 × 104 | Mineral water and tap water | [162] |
| Potentiometric | Glassy Carbon Electrode/Ca2+-ISM | Ca2+ | 0.16 | 0.3–1000 | Diluted artificial seawater | [163] |
| EIS | MWCNTs/PDMS | Ca2+ and Mg2+ | - | 29.41–5882.35 | In water bodies | [164] |
| EIS Potentiometric | Ionophores ISEs | Ca2+ Mg2+ | 100 100 | In artificial fish-breeding water | [165] | |
| SWASV * | MEMS-Based sensor on top of a silicon wafer | Ca2+ and Mg2+ | 29.41 | 294.1–1470.59 | - | [166] |
| Amperometric | On-chip amperometric sensor with ion exchange membrane | Mg2+ | 5 | - | - | [167] |
| Detection Principle | Electrode/Materials | Analytes | LOD (µM) | Dynamic Range (µM) | Comments | Refrerence |
|---|---|---|---|---|---|---|
| Chronoamperometry | Co3O4/RGO*/GCE+ | Nitrite | 0.14 | 1–380 | Tap water. Recovery: 99.3–101.5%. | [168] |
| DPV | PEDOT-Gr*/Ta | Nitrite | 7 | 20–2000 M | PBS. (RSD) 50 continuous CV cycles 4.5%. | [169] |
| DPV | GNPs/graphene/MCE | Nitrite | 0.1 | 0.3–720 | Lake water, river water, industrial sewage, and milk. Recovery: 96.0–103%. | [170] |
| CV * | Nafion/Hb *-Pd-GR */CILE | Nitrite | 0.2 | 40–500 | Tap water and Medical facial peel. Recovery: 96.17–101.24% | [171] |
| SWV | Cu/MWCNT */rGO/GCE | Nitrate Nitrite | 0.02 0.03 | 0.1–75 | Tap and mineral waters, sausages, salami, and cheese samples. Recovery: 98.3–102.5% | [172] |
| Amperometry | PANI@GO/GCE | Nitrite | 0.5 | 0.002–44 | Phosphate buffer | [173] |
| Amperometry | Ferrocene/rGO/SPCE * | Nitrite | 0.35 | 2.5–1450 | Spiked mineral water. Recovery: 95% and 101% | [174] |
| Amperometry | Ag/Cu/MWNT/GCE | Nitrite | 0.2 | 1–1000 | Lake water, Drinking water and Seawater. Recovery: 92–105% | [175] |
| DPV | Au Cu NCNs/GCE | Nitrite | 0.2 | 10–4000 | After storing in a refrigerator at 4 °C for 35 days, the peak current responses were still retained 98.60% of the initial values | [176] |
| Amperometry | GO-CS-AuNPs/GCE | Nitrite | 0.3 | 0.9–18.9 | Phosphate buffer | [177] |
| Amperometry | AuNPs-Fe2O3/FTO | Nitrite | 0.07 | 1–1000 | Tap and rain water samples | [178] |
| LSV * | 3D lamellar nanocomposite/AgNS */rGO/β-cyclodextrin/SPCE | Nitrite | 0.24 | 1–2000 | Nitrite in spiked pickles.(RSD) = 2.35% (n = 5) | [179] |
| DPV | Fe3O4/GO/COOH/GCE | Nitrite | 0.37 | 1–85 and 90–600 | Phosphate buffer | [180] |
| SWV | LIG/f-MWCNT-AuNPs | Nitrite | 0.9 | 10–140 | Tap water | [181] |
| Amperometry | Co3O4-rGO/CNTs/GCE | Nitrite | 0.016 | 8000–56,000 | Recoveries: 95.7–102.2% 83.3% of initial sensitivity after one month storage | [182] |
| CV * | 3D Au-rGO/FTO | Nitrite | - | 20.99–5740 | Phosphate buffer | [183] |
| Amperometry | Pt/Ni(OH)2/MWCNTs/GCE | Nitrite | 0.13 | 0.4–5670 | Milk Recoveries 96–104% | [184] |
| Amperometry | PANI/CNTs/GCE | Nitrite | 1.6 | - | PBS RSD 3.4% (n = 9) | [185] |
| Amperometry | Ni7S6/MWCNTs/GCE | Nitrite | 0.3 | 1.0–4002 | Lake later, Tape and water Pickle water | [186] |
| DPV | GO–MWCNT–PMA *–Au/GCE | Nitrite | 0.67 | 2–10,000 | Water RSD (n = 5) 4% | [187] |
| DPV | AuNPs-S-Gr/GCE | Nitrite | 0.003 | 12.5–680.92 | Water RSD (n = 3) 0.87% | [188] |
| Amperometry | rGO/Acr paper | Nitrite | 0.12 | 0.40–3600 | Milk and water | [189] |
| DPV | Self-assembled graphene CuNP/AuE | Nitrate | 7.98 | 10–90 | Lake water | [190] |
| Amperometry | CNT/PPy * film electrode with Nitrate reductase | Nitrate | 170 | 440–1450 | Nitrate in water | [191] |
| CV | Cu, Zn (SOD1 *) and nitrate reductase (NaR) coimmobilized on CNT–PPy modified Pt * electrode (NaR–SOD1–CNT–PPy–Pt) | Nitrate Nitrite | 0.2 0.05 | 0.5–10,000 0.0001–1000 | Human plasma, whole blood and saliva samples | [192] |
| CV | Ag-doped zeolite-expanded graphite-epoxy electrode | Nitrate | 100 | 1000–10,000 | Spiked tap water | [193] |
| SWV | Ag dendritic nanostructure on Au microelectrode array | Nitrate Nitrite | 2 | 2–1000 | River and lake water | [194] |
| CV | Cu* nanoclusters, electrodeposited on Pt microelectrode | Nitrate | - | 12.5–300 | Fresh water | [195] |
| SWV | Cu microelectrode array | Nitrate | 1.8 | 10–1070 | Mineral water | [196] |
| LSV | Cu nanowire array | Nitrate | 1.7–3 | 10–400 | Mineral water | [197] |
| DPV | Cu microspheres decorated on polyaniline on microneedle | Nitrate | 8 | 20–6000 | Pre-treated river water | [198] |
| CV | Ag branchlike on Ag or carbon ultramicroelectrodes | Nitrate | 3.2–5.1 | 4–1000 | Synthetic aquifer | [199] |
| Method | Electrode/Materials | Analyte | Linear Range (µM) | LOD (µM) | Comments | Ref |
|---|---|---|---|---|---|---|
| Voltammetry (DPV) | molybdenum blue modified PVC*/pencil graphite electrode | K2HPO4 | - | 0.021 | Measurement in soil sample | [212] |
| Potentiometry | Chitosan-clay/PVC | K2HPO4 | 1–104 | 0.6 | - | [213] |
| Chronoamperometry | Doped PANI*/gold electrode | KH2PO4 | 1–100 | 1 | Response time < 1 s, electrode lifetime > 40 days in solution | [214] |
| Potentiometry | Graphene nanocomposite/Co microelectrode | KH2PO4 | 0.1–1 | 0.01 | Effective measurement in lake water and sediment samples for soluble phosphorus (SPR) | [215] |
| Chronoamperometry | Carbon black NPs-SPE | KH2PO4 | 10−8 | 0.1 | Drinking, river, aquarium, and waste water samples with satisfactory recovery values, absence of silicate interference, stable sensor (>3 months in a dry condition at RT), for online in-situ analysis. | [216] |
| I−V measurement | Silver/Graphene Composite/FET* | PO43– | 5–6000 | 0.2 | Long-term stability, excellent reproducibility, and good selectivity, low-cost and applicable in real water samples | [217] |
| Amperometry | Paper CB-SPE* | K2HPO4 | 10–300 | 4 | High reproducibility, long storage stability, reagentless, RSD < 6% | [218] |
| I−V measurement | Graphene/ionophore hybrid membrane ISFET* | PO43– | - | 2800 | Good performance and selectivity, response time 10 s | [219] |
| Potentiometry | CuPc* Acrylate-Polymer/Silicon | K2HPO4 | 0.001–10 | 0.001 | High specificity | [220] |
| Impedimetry | CuPc/Au electrode | Na2HPO4 | 10−4–1000 | 0.00838 | - | [221] |
| Potentiometry | Zn2+/BPMP- Cu2+/BPMP* | K2HPO4 | 3–50 | 1 0.5 | Good selectivity and stability | [222] |
| Amperometry | Platinum/Au nanowires Arrays | Thiamine pyrophosphate (TPP) | 248–1456 | 45 | Good selectivity, storage in citrate buffer at 4 °C in refrigerator and measurement every three days showed good stability | [223] |
| Voltammetry | Silver Nanowires/SPE | Na2HPO4 | 5–1000 | 3 | Good repeatability and recovery | [224] |
| Potentiometry | Cobalt NPs-RGO*/GCE* | KH2PO4 | 1–10,000 | - | Measurement in tap and well water samples | [225] |
| Voltammetry | Graphite SPE | KH2PO4 | 0.003–0.115 | 0.02 | Dissolved phosphorus sensing in canal water samples | [226] |
| Potentiometry | ZnO NRs* FET | PO43– | 0.1–7000 | 0.05 | - | [227] |
| Amperometry | PyOx*/Au nanowires | KH2PO4 | 12.5–1000 | 0.1 | Good selectivity, stability > two weeks of repeated use in water samples (recovery of 96.67 ± 4.9%) | [228] |
| Method | Electrode Modification | Analyte | LOD (µM) | Linearity (µM) | Ref |
|---|---|---|---|---|---|
| CV | CILE */CTS */Hb */GR-CuS * composite | TCA * | 200 | 3000–64 × 103 | [243] |
| CV | CILE/CTS/Hb/3d GR * | TCA | 130 | 400–26 × 103 | [233] |
| CV | CPE */CdO | TCA | 2.3 | 3–230 | [244] |
| SWV | GCE */Iron pthalocyanine/ZIF-8 * | TCA | 1.89 × 10−3 | [245] | |
| EIS | GCE/MIP * | NDMA * | 0.011 | 0.13–3.1 | [246] |
| CV | CILE/Nafion/Hb/borondoped graphene qunatumdots | TCA, NaNO2, H2O2 | 53 | 100–300 × 103 | [247] |
| CV | CILE/Nafion/Hb/ZnO-CNF * | TCA and NaNo2 | 1.33 × 103 | 4 × 103–150 × 103 | [248] |
| CV | CILE/Nafion/Hb/Co3O4-CNF | TCA, KBrO3 and NaNo2 | 1.33 × 103 | 40 × 103–260 × 103 | [249] |
| SWV | GCE/AgNp-Malic acid | TCA | 30 × 10−3 | 01-2 & 4–100 | [250] |
| Detection Method | Eltrode/Materials | Analyte | LOD (µM) | Dynamic Range (µM) | Comments | Reference |
|---|---|---|---|---|---|---|
| Amperometry | GCE-MWCNTs | Gallic acid | 0.19 | 0.66–52.8 | Cognac and brandie, sample dilution not required | [257] |
| SWV | SPE-CB * | Catechol, gallic acid, caffeic acid, and tyrosol | 0.1, 1, 0.8, and 2 | 1–50 1–50 10–100 10–100 | Foods and beverages | [258] |
| DPV | Press-produced CB transducer | Tyrosol Hydroxytrosol | 20 6 | 10–75 10–75 | Olive oil | [259] |
| DPV | GCE/nano-carbons-AgNPs | Gallic acid | 0.063 | 0.5–8.5 | Wine, sample dilution not required | [260] |
| DPV | GCE-GR/boron doped | Gallic acid | - | - | Tea infusion | [261] |
| DPV | GCE-GR reduced-Fe2O3/Chitosan | Gallic acid | 0.51 | 1–0.01 | Red/white, wine, sample dilution not required | [255] |
| Chronoamperometry | SPE-GR/PEDOT/PSS * | Trolox | 0.59 | 5–30 | Herbs and herbal beverages | [254] |
| DPV | Al2O3/AC-CPE * | Phenol | 0.151 | 10–1000 | Natural waters and olive oil | [262] |
| Amperometry | Nanoporous gold/Si wafer | Catechol | 0.5 | 20–200 | PBS | [263] |
| CV | Platinum–polytyramine composite/graphite substrate | Phenol | - | 3 × 10−2–10 × 10−3 | Industrial waste waters | [264] |
| SWV | Tyr *-AuNPs */SPE | Phenol | 1.47 | 1.47–441 | Sensitivity of 15.7 mA ppm−1 Regional water samples | [265] |
| Amperometry | Tyr-ZnO nanorods/Au | Phenol | 0.6 | 0.6–2020–50 | Sensitivity of 103.08 µA/mM | [266] |
| Stripping voltammetry | Nafion-Modified GCE | Phenol | 0.001 | 0.008–10 | Water samples | [267] |
| DPV | HEX-AET *-gold nanoparticles-Glassy Carbon Elecrode | Phenolic estrogens DES DE >BPA > HEX | 0.0054 0.0033 0.0043 0.0054 | - | Satisfactory linear range and selectivity | [268] |
| DPV | DNA aptamers/AuSPE * | 17 β-estradiol | 5 × 10−7 in tap water/7 × 10−7 in milk | 1.5 × 10−6–10−4/10−4–0.07 | Excellent selectivity | [269] |
| LSV | MIP */AuNPs/Au | 17 β-estradiol | 1.09 × 10−9 | 3.6 × 10−9–3.6 × 10−3 | Broad linear range, high sensitivity, selectivity, and reproducibility, simple to fabricate, easy to operate. | [270] |
| DPV | MWCNTs/THI/AuNPs/SPWE | 17 β-estradiol | 0.0002 | 1.79 × 10−7–0.0018 | Cost effective, wireless connection with smart phone | [271] |
| SWCASV | Polished silver solid amalgam electrode | Pyrethroid insecticide (beta-cyfluthrin (βCF)) | - | 1.2 × 10−6–3.0 × 10−5 | Low detection limit with a high level of precision and accuracy | [272] |
| AdSDPV | SrTiO3/N-GNS * | Pharmaceutical compound: Diphenhydramine | 0.0021 | 0.038–100.0 ×10−6 | Good recoveries in synthetic pharmaceutical samples and human body fluids, good candidate for real application | [273] |
| Voltammetry | c-MWCNTs/GCE | BPA | 5.0 × 10−3 | (10–104) × 10−3 | Recovery: 98.4–102.8%. | [274] |
| LSV | BSA/Anti-BPA/AuNP/MWCNT/GCE | BPA | 8.7 × 10−3 | 0.01–1 | Food fresh-keeping film. Recovery: 97.3–103% | [275] |
| Amperometry | BCNP/Tyr/Nafion/GCE | BPA | 3.18 × 10−3 | 0.02–10 | Water samples. Recovery: 96.67–108%. | [276] |
| DPV | SWCNTs/Poly-IL/GCE | BPA | 10−3 | 5.0 × 10−3–30 | Leaching from plastic drinking bottle. | [277] |
| DPV | Lac/Ag–ZnO/MWCNTs/CSPE | BPA | 6 × 10−3 | 0.5–2.99 | High level of precision and accuracy. BPA in plastic bottles. | [278] |
| Amperometry | Tyr-DAPPT–rGO/GCE | BPA | 3.5 × 10−3 | 1.0 × 10−3–38 | Commercial plastic drinking bottles. | [279] |
| DPV | MIPPy/GQDs/GCE | BPA | 0.04 | 0.1–50 | Tap and sea water samples, with recoveries of 94.5% and 93.7% | [280] |
| SWV | ZnO/CNT/IL/CPE | BPA | 9 × 10−3 | 0.002–700 | Food samples. | [281] |
| Derivative Voltammetry | MIP/CS/Gr/ABPE | BPA | 6 × 10−3 | 8.0–2.0 | Plastic bottled drinking water and canned beverages. | [282] |
| DPV | AuNP/Gr/GCE | BPA | 5 × 10−3 | 0.01–10 | Milk samples with recoveries of 105%. | [283] |
| Detection Method | Materials | LOD (µM) HQ, CC and RC | Dynamic Range (µM) | Comments | References |
|---|---|---|---|---|---|
| CV | CTAB-GO/MWCNTs/GCE | 0.03 0.01 0.2 | 0.1–200 0.1–400 1–100 | Tap water | [297] |
| DPV | WBC*/Au-850–15/GCE | 0.002 0.004 - | 0.008–7.0 0.01–7.0 - | Tap | [298] |
| DPV | MWCNTs@RGONR*/GCE | 3.89 1.73 5.77 | 15–921 15–1101 15–1301 | Tap, River | [299] |
| DPV | CN-F*/GCE | 0.5 0.8 0.4 | 10–120 10–120 10–120 | River | [300] |
| DPV | 3D* CNT-Gr/AuNPs/GCE | 0.8 0.95 0.1 | 0.0–80 0.0–80 0.0–80 | Tap, River | [301] |
| DPV | Cu-MOF*-Gr/GCE | 0.59 0.33 - | 1.0–100 1.0–100 - | Tap water | [302] |
| DPV | UiO-66/MPC*-3/GCE | 0.056 0.072 3.51 | 0.5–100 0.4–100 30–400 | Tap-Lake | [303] |
| DPV | CNNS*-CNT/GCE | 0.13 0.09 - | 1–200 1–250 - | Tap | [304] |
| DPV | Chit*/MWCNTs/Ti2/GCE | 0.06 0.07 0.52 | 0.4–276 0.4–159 3.0–657 | River, Tap | [305] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kanoun, O.; Lazarević-Pašti, T.; Pašti, I.; Nasraoui, S.; Talbi, M.; Brahem, A.; Adiraju, A.; Sheremet, E.; Rodriguez, R.D.; Ben Ali, M.; et al. A Review of Nanocomposite-Modified Electrochemical Sensors for Water Quality Monitoring. Sensors 2021, 21, 4131. https://doi.org/10.3390/s21124131
Kanoun O, Lazarević-Pašti T, Pašti I, Nasraoui S, Talbi M, Brahem A, Adiraju A, Sheremet E, Rodriguez RD, Ben Ali M, et al. A Review of Nanocomposite-Modified Electrochemical Sensors for Water Quality Monitoring. Sensors. 2021; 21(12):4131. https://doi.org/10.3390/s21124131
Chicago/Turabian StyleKanoun, Olfa, Tamara Lazarević-Pašti, Igor Pašti, Salem Nasraoui, Malak Talbi, Amina Brahem, Anurag Adiraju, Evgeniya Sheremet, Raul D. Rodriguez, Mounir Ben Ali, and et al. 2021. "A Review of Nanocomposite-Modified Electrochemical Sensors for Water Quality Monitoring" Sensors 21, no. 12: 4131. https://doi.org/10.3390/s21124131
APA StyleKanoun, O., Lazarević-Pašti, T., Pašti, I., Nasraoui, S., Talbi, M., Brahem, A., Adiraju, A., Sheremet, E., Rodriguez, R. D., Ben Ali, M., & Al-Hamry, A. (2021). A Review of Nanocomposite-Modified Electrochemical Sensors for Water Quality Monitoring. Sensors, 21(12), 4131. https://doi.org/10.3390/s21124131