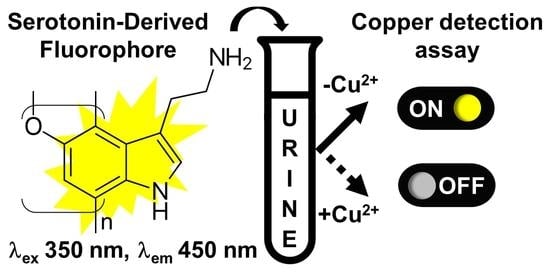
Serotonin-Derived Fluorophore: A Novel Fluorescent Biomaterial for Copper Detection in Urine
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Instrumentation
2.3. Assay Protocol
2.3.1. Synthesis of Serotonin-Derived Fluorophore
2.3.2. Quantum Yield Calculation
2.3.3. Copper Determination via the Fluorescence Quenching-Based Method
2.3.4. ICP-MS Measurements
3. Results and Discussion
3.1. Synthesis and Characterization of Serotonin-Derived Fluorophore (SEDF)
3.2. SEDF Fluorescence Quenching by Metal Ions
3.3. Copper Quantification via Quenching-Based Bioanalytical Assay
3.4. Copper Detection in Human Urine Samples
3.5. Assay Performance Compared to Other Fluorescence-Based Method for Copper Detection
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Maung, M.T.; Carlson, A.; Olea-Flores, M.; Elkhadragy, L.; Schachtschneider, K.M.; Navarro-Tito, N.; Padilla-Benavides, T. The molecular and cellular basis of copper dysregulation and its relationship with human pathologies. FASEB J. 2021, 35, 1–50. [Google Scholar] [CrossRef] [PubMed]
- Tsang, T.; Davis, C.I.; Brady, D.C. Copper biology. Curr. Biol. 2021, 31, R421–R427. [Google Scholar] [CrossRef] [PubMed]
- National Research Council (US). Committee on Copper in Drinking Water; National Academies Press (US): Washington, DC, USA, 2000. Available online: https://www.ncbi.nlm.nih.gov/books/NBK225407/ (accessed on 1 March 2023).
- Zuily, L.; Lahrach, N.; Fassler, R.; Genest, O.; Faller, P.; Sénèque, O.; Denis, Y.; Castanié-Cornet, M.P.; Genevaux, P.; Jakob, U.; et al. Copper Induces Protein Aggregation, a Toxic Process Compensated by Molecular Chaperones. MBio 2022, 13, e03251-21. [Google Scholar] [CrossRef] [PubMed]
- Donnelly, P.S.; Xiao, Z.; Wedd, A.G. Copper and Alzheimer’s disease. Curr. Opin. Chem. Biol. 2007, 11, 128–133. [Google Scholar] [CrossRef] [PubMed]
- Davies, K.M.; Mercer, J.F.B.; Chen, N.; Double, K.L. Copper dyshomoeostasis in Parkinson’s disease: Implications for pathogenesis and indications for novel therapeutics. Clin. Sci. 2016, 130, 565–574. [Google Scholar] [CrossRef]
- Ni, M.; You, Y.; Chen, J.; Zhang, L. Copper in depressive disorder: A systematic review and meta-analysis of observational studies. Psychiatry Res. 2018, 267, 506–515. [Google Scholar] [CrossRef]
- Arputharaj, E.; Singh, S.; Pasupuleti, R.R.; Dahms, H.U.; Huang, Y.L. Visible fluorescent sensing of Cu2+ ions in urine by reusable chitosan/L-histidine–stabilized silicon nanoparticles integrated thin layer chromatography sheet. Anal. Chim. Acta. 2022, 1231, 340418. [Google Scholar] [CrossRef]
- Ma, X.; Yang, Y.; Ma, R.; Zhang, Y.; Zou, X.; Zhu, S.; Ge, X.; Yuan, Y.; Zhang, W.; Zhu, G. Inorganic nanocrystal-dynamic porous polymer assemblies with effective energy transfer for sensitive diagnosis of urine copper. Chem. Sci. 2020, 11, 12187–12193. [Google Scholar] [CrossRef]
- Zhao, L.; Li, H.; Xu, Y.; Liu, H.; Zhou, T.; Huang, N.; Li, Y.; Ding, L. Selective detection of copper ion in complex real samples based on nitrogen-doped carbon quantum dots. Anal. Bioanal. Chem. 2018, 410, 4301–4309. [Google Scholar] [CrossRef]
- Cai, Y.; You, J.; You, Z.; Dong, F.; Du, S.; Zhang, L. Profuse color-evolution-based fluorescent test paper sensor for rapid and visual monitoring of endogenous Cu2+ in human urine. Biosens. Bioelectron. 2018, 99, 332–337. [Google Scholar] [CrossRef]
- Nsanzamahoro, S.; Wang, W.F.; Zhang, Y.; Shi, Y.P.; Yang, J.L. Synthesis of orange-emissive silicon nanoparticles as “off-on” fluorescence probe for sensitive and selective detection of L-methionine and copper. Talanta 2021, 231, 122369. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.J.; Liu, S.G.; Han, L.; Luo, H.Q.; Li, N.B. A ratiometric fluorescent and colorimetric dual-signal sensing platform based on N-doped carbon dots for selective and sensitive detection of copper(II) and pyrophosphate ion. Sens. Actuators B Chem. 2019, 283, 215–221. [Google Scholar] [CrossRef]
- Yang, Y.; Chen, P.; Liu, Y.; Cai, Z.; Wang, X.; Me, Y.; Ding, X.; Lin, L.; Jiang, H.; Zhang, Z.; et al. A colorimetric indicator-displacement assay based on stable Cu2+ selective carbon dots for fluorescence turn-on detection of pyrophosphate anions in urine. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2021, 251, 119479. [Google Scholar] [CrossRef] [PubMed]
- Mohammad-Zadeh, L.F.; Moses, L.; Gwaltney-Brant, S.M. Serotonin: A review. J. Vet. Pharmacol. Ther. 2008, 31, 187–199. [Google Scholar] [CrossRef]
- Jeon, K.; Andoy, N.M.O.; Schmitt, C.W.; Xue, Y.; Barner, L.; Sullan, R.M.A. Size-controlled synthesis of bioinspired polyserotonin nanoparticles with free radical scavenging activity. J. Mater. Chem. B 2021, 9, 634–637. [Google Scholar] [CrossRef]
- Nakatsuka, N.; Hasani-Sadrabadi, M.; Cheung, K.; Young, T.; Bahlakeh, G.; Moshaverinia, A.; Weiss, P.; Andrews, A. Polyserotonin Nanoparticles as Multifunctional Materials for Biomedical Applications. ACS Nano 2018, 12, 4761–4774. [Google Scholar] [CrossRef]
- Ishino, K.; Nishitani, S.; Man, Y.; Saito, A.; Sakata, T. Surface Characteristics and Formation of Polyserotonin Thin Films for Bioelectrical and Biocompatible Interfaces. ACS Appl. Mater. Interfaces 2022, 38, 8633–8642. [Google Scholar] [CrossRef] [PubMed]
- Kiratitanavit, W.; Bruno, F.F.; Xia, Z.; Yu, S.; Kumar, J.; Nagarajan, R. Biocatalytic synthesis of fluorescent conjugated polyserotonin. J. Renew. Mater. 2019, 7, 205–214. [Google Scholar] [CrossRef] [Green Version]
- Farahmand Nejad, M.A.; Ghasemi, F.; Hormozi-Nezhad, M.R. A wide-color-varying ratiometric nanoprobe for detection of norepinephrine in urine samples. Anal. Chim. Acta 2018, 1039, 124–131. [Google Scholar] [CrossRef]
- Koh, D.Y.; Kook, J.K.; Lee, S.W. Highly fluorescent oligodopamine (F-ODA) for accurate and sensitive detection of the neurotransmitter dopamine. Anal. Biochem. 2020, 591, 113571. [Google Scholar] [CrossRef]
- Moini, M.; To, U.; Schilsky, M.L. Recent advances in Wilson disease. Transl. Gastroenterol. Hepatol. 2021, 6, 21. [Google Scholar] [CrossRef]
- Brewer, G.J.; Gow, P.J.; Smallwood, R.A.; Angus, P.W.; Sewell, R.B.; Smith, A.L.; Wall, A.J. Diagnosis of Wilson’s disease: An experience over three decades. Gut 2002, 50, 136. [Google Scholar] [CrossRef] [PubMed]
- Walshe, J.M. The pattern of urinary copper excretion and its response to treatment in patients with Wilson’s disease. Qjm 2011, 104, 775–778. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gray, L.W.; Peng, F.; Molloy, S.A.; Pendyala, V.S.; Muchenditsi, A.; Muzik, O.; Lee, J.; Kaplan, J.H.; Lutsenko, S. Urinary Copper Elevation in a Mouse Model of Wilson’s Disease Is a Regulated Process to Specifically Decrease the Hepatic Copper Load. PLoS ONE 2012, 7, e38327. [Google Scholar] [CrossRef] [Green Version]
- Woimant, F.; Djebrani-Oussedik, N.; Poujois, A. New tools for Wilson’s disease diagnosis: Exchangeable copper fraction. Ann. Transl. Med. 2019, 7, S70. [Google Scholar] [CrossRef] [PubMed]
- Robson, A.F.; Lockett, P.; Tetlow, L.; Chaloner, C. Evaluation of 24-h urine containers for urine copper measurement by inductively coupled plasma mass spectrometry. Ann. Clin. Biochem. 2020, 57, 246–248. [Google Scholar] [CrossRef]
- Lakowicz, J.R. Principles of Fluorescence Spectroscopy, 3rd ed.; Springer: Boston, MA, USA, 2006; pp. 54–55. [Google Scholar] [CrossRef]
- Heuther, G.; Reimer, A.; Schmidt, F.; Schuff-Werner, P.; Brudny, M.M. Oxidation of the indole nucleus of 5-hydroxytryptamine and formation of dimers in the presence of peroxidase and H2O2. J. Neural Transm. Suppl. 1990, 32, 249–257. [Google Scholar] [CrossRef]
- Dai, Z.; Chauhan, S.; Green, T.K. Synthesis and enantioseparation of atropisomers of serotonin dimer. Tetrahedron Lett. 2015, 56, 4022–4024. [Google Scholar] [CrossRef]
- Wrona, M.Z.; Dryhurst, G. Electrochemical oxidation of 5-hydroxytryptamine in aqueous solution at physiological pH. Bioorg. Chem. 1990, 18, 291–317. [Google Scholar] [CrossRef]
- Wrona, M.Z.; Dryhurst, G. Oxidation Chemistry of 5-Hydroxytryptamine. 1. Mechanism and Products Formed at Micromolar Concentrations. J. Org. Chem. 1987, 52, 2817–2825. [Google Scholar] [CrossRef]
- Jones, C.E.; Underwood, C.K.; Coulson, E.J.; Taylor, P.J. Copper induced oxidation of serotonin: Analysis of products and toxicity. J. Neurochem. 2007, 102, 1035–1043. [Google Scholar] [CrossRef]
- Strambini, G.B.; Gabellieri, E. Quenching of indole luminescence by copper ions: A distance dependence study. J. Phys. Chem. 1991, 95, 4347–4352. [Google Scholar] [CrossRef]
- Bu, J.; Duan, H.; Wang, X. Fluorescence sensors for Cu2+ based on conjugated indole Schiff base. Res. Chem. Intermed. 2014, 40, 3119–3126. [Google Scholar] [CrossRef]
- Lin, J.H.; Yu, C.J.; Yang, Y.C.; Tseng, W.L. Formation of fluorescent polydopamine dots from hydroxyl radical-induced degradation of polydopamine nanoparticles. Phys. Chem. Chem. Phys. 2015, 17, 15124–15130. [Google Scholar] [CrossRef]
- An, T.; Lee, N.; Cho, H.J.; Kim, S.; Shin, D.S.; Lee, S.M. Ultra-selective detection of Fe2+ ion by redox mechanism based on fluorescent polymerized dopamine derivatives. RSC Adv. 2017, 7, 30582–30587. [Google Scholar] [CrossRef] [Green Version]
- García-Moreno, E.; Tomás, A.; Atrián-Blasco, E.; Gascón, S.; Romanos, E.; Rodriguez-Yoldi, J.M.; Cerrada, E.; Laguna, M. In vitro and in vivo evaluation of organometallic gold(I) derivatives as anticancer agents. Dalt. Trans. 2016, 45, 2462–2475. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Q.; Guo, W.; Jia, Y.; Xu, J. Comparison of chromium and iron distribution in serum and urine among healthy people and prediabetes and diabetes patients. Biomed. Res. Int. 2019, 2019, 3801639. [Google Scholar] [CrossRef] [Green Version]
- Serrat, F.B. Colorimetric method for determination of chlorine with 3,3′,5,5′-tetramethylbenzidine. Talanta 1994, 41, 2091–2094. [Google Scholar] [CrossRef]
- Palladino, P.; Torrini, F.; Scarano, S.; Minunni, M. 3,3′,5,5′-tetramethylbenzidine as multi-colorimetric indicator of chlorine in water in line with health guideline values. Anal. Bioanal. Chem. 2020, 412, 7861–7869. [Google Scholar] [CrossRef]
- Sarigul, N.; Korkmaz, F.; Kurultak, İ. A New Artificial Urine Protocol to Better Imitate Human Urine. Sci. Rep. 2019, 9, 1–11. [Google Scholar] [CrossRef] [Green Version]








| # | 1 [Cu2+] | F0/F | 2 [Cu2+] | 3 [Cu2+] | 4 Recovery (%) |
|---|---|---|---|---|---|
| 1 | 0.05 mM | 1.07 ± 0.06 | 0.04 mM ± 0.02 | 0.03 mM | 133% |
| 2 | 0.20 mM | 1.52 ± 0.11 | 0.22 mM ± 0.01 | 0.21 mM | 105% |
| 3 | 0.20 mM | 1.61 ± 0.03 | 0.25 mM ± 0.01 | 0.22 mM | 114% |
| 4 | 0.40 mM | 1.81 ± 0.04 | 0.43 mM ± 0.01 | 0.49 mM | 88% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lettieri, M.; Scarano, S.; Caponi, L.; Bertolini, A.; Saba, A.; Palladino, P.; Minunni, M. Serotonin-Derived Fluorophore: A Novel Fluorescent Biomaterial for Copper Detection in Urine. Sensors 2023, 23, 3030. https://doi.org/10.3390/s23063030
Lettieri M, Scarano S, Caponi L, Bertolini A, Saba A, Palladino P, Minunni M. Serotonin-Derived Fluorophore: A Novel Fluorescent Biomaterial for Copper Detection in Urine. Sensors. 2023; 23(6):3030. https://doi.org/10.3390/s23063030
Chicago/Turabian StyleLettieri, Mariagrazia, Simona Scarano, Laura Caponi, Andrea Bertolini, Alessandro Saba, Pasquale Palladino, and Maria Minunni. 2023. "Serotonin-Derived Fluorophore: A Novel Fluorescent Biomaterial for Copper Detection in Urine" Sensors 23, no. 6: 3030. https://doi.org/10.3390/s23063030
APA StyleLettieri, M., Scarano, S., Caponi, L., Bertolini, A., Saba, A., Palladino, P., & Minunni, M. (2023). Serotonin-Derived Fluorophore: A Novel Fluorescent Biomaterial for Copper Detection in Urine. Sensors, 23(6), 3030. https://doi.org/10.3390/s23063030