Determination of Vitamin C (Ascorbic Acid) Using High Performance Liquid Chromatography Coupled with Electrochemical Detection
Abstract
:1. Introduction
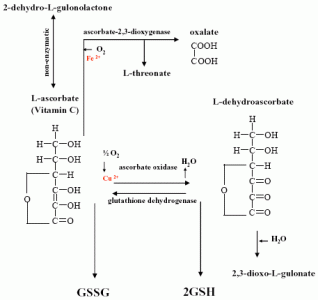
1.1 Biological function of vitamin C
1.2 Daily needs of vitamin C
1.3 Content of vitamin C in foods
1.4 Methods for ascorbic acid determination
2. Material and Methods
2.1 Chemicals, material and pH measurements
2.2 Flow injection analysis/High performance liquid chromatography with CoulArray or Coulochem electrochemical detector
CoulArray
Coulochem III
2.3 Preparation of real samples
2.4 Accuracy, precision and recovery
2.5 Descriptive statistics
3. Results and Discussion
3.1 Flow injection analysis coupled with CoulArray electrochemical detector to detect ascorbic acid
3.2 Flow injection analysis coupled with Coulochem III electrochemical detector to detect AA
3.3 High performance liquid chromatography coupled with CoulArray electrochemical detector to detect AA
3.4 Analysis of real samples
4. Conclusions
Acknowledgments
References
- Velisek, J.; Cejpek, K. Biosynthesis of food constituents: Vitamins. 2. Water-soluble vitamins: part 1 - a review. Czech. J. Food Sci. 2007, 25, 49–64. [Google Scholar]
- Linster, C.L.; Van Schaftingen, E. Vitamin C - Biosynthesis, recycling and degradation in mammals. Febs J. 2007, 274, 1–22. [Google Scholar]
- Davey, M.W.; Van Montagu, M.; Inze, D.; Sanmartin, M.; Kanellis, A.; Smirnoff, N.; Benzie, I.J.J.; Strain, J.J.; Favell, D.; Fletcher, J. Plant L-ascorbic acid: chemistry, function, metabolism, bioavailability and effects of processing. J. Sci. Food Agric. 2000, 80, 825–860. [Google Scholar]
- Noctor, G.; Foyer, C.H. Ascorbate and glutathione: Keeping active oxygen under control. Annu. Rev. Plant Physiol. Plant Molec. Biol. 1998, 49, 249–279. [Google Scholar]
- Kleszczewska, E. L-Ascorbic acid - clinical use, toxicity, properties, methods of determination and application in chemical analysis. Pharmazie 2000, 55, 640–644. [Google Scholar]
- Meister, A. Glutathione Ascorbic-Acid Antioxidant System in Animals. J. Biol. Chem. 1994, 269, 9397–9400. [Google Scholar]
- Englard, S.; Seifter, S. The Biochemical Functions of Ascorbic-Acid. Annu. Rev. Nutr. 1986, 6, 365–406. [Google Scholar]
- Levine, M.; Rumsey, S.C.; Daruwala, R.; Park, J.B.; Wang, Y.H. Criteria and recommendations for vitamin C intake. JAMA-J. Am. Med. Assoc. 1999, 281, 1415–1423. [Google Scholar]
- Gomez-Romero, M.; Arraez-Roman, D.; Segura-Carretero, A.; Fernandez-Gutierrez, A. Analytical determination of antioxidants in tomato: Typical components of the Mediterranean diet. J. Sep. Sci. 2007, 30, 452–461. [Google Scholar]
- Deicher, R.; Horl, W.H. Vitamin C in chronic kidney disease and hemodialysis patients. Kidney Blood Pressure Res. 2003, 26, 100–106. [Google Scholar]
- Ogiri, Y.; Sun, F.; Hayami, S.; Fujimura, A.; Yamamoto, K.; Yaita, M.; Kojo, S. Very low vitamin C activity of orally administered L-dehydroascorbic acid. J. Agric. Food Chem. 2002, 50, 227–229. [Google Scholar]
- Prasad, B.B.; Tiwari, K.; Singh, M.; Sharma, P.S.; Patel, A.K.; Srivastava, S. Molecularly imprinted polymer-based solid-phase microextraction fiber coupled with molecularly imprinted polymer-based sensor for ultratrace analysis of ascorbic acid. J. Chromatogr. A 2008, 1198, 59–66. [Google Scholar]
- Karlsen, A.; Blomhoff, R.; Gundersen, T.E. Stability of whole blood and plasma ascorbic acid. Eur. J. Clin. Nutr. 2007, 61, 1233–1236. [Google Scholar]
- Yao, X.; Wang, Y.T.; Chen, G. Simultaneous determination of am aminothiols, ascorbic acid and uric acid in biological samples by capillary electrophoresis with electrochemical detection. Biomed. Chromatogr. 2007, 21, 520–526. [Google Scholar]
- Koide, K.; Zhang, X.M.; Ohishi, K.; Usami, Y.; Hotta, Y.; Hiramitsu, T. Ascorbic acid concentration in rabbit vitreous measured by microdialysis with HPLC-electrochemical detection before and after vitreous surgery. Exp. Eye Res. 2006, 82, 868–873. [Google Scholar]
- Cofan, C.; Radovan, C. Simultaneous chronoamperometric sensing of ascorbic acid and acetaminophen at a boron-doped diamond electrode. Sensors 2008, 8, 3952–3969. [Google Scholar]
- Wang, Y.; Xu, H.; Zhang, J.M.; Li, G. Electrochemical sensors for clinic analysis. Sensors. 2008, 8, 2043–2081. [Google Scholar]
- Yogeswaran, U.; Chen, S.M. A review on the electrochemical sensors and biosensors composed of nanowires as sensing material. Sensors 2008, 8, 290–313. [Google Scholar]
- Chen, J.; Fang, Y.J. Flow injection technique for biochemical analysis with chemiluminescence detection in acidic media. Sensors 2007, 7, 448–458. [Google Scholar]
- Shaidarova, L.G.; Gedmina, A.V.; Chelnokova, I.A.; Budnikov, G.K. Electrocatalytic oxidation and flow-injection determination of ascorbic acid at a graphite electrode modified with a polyaniline film containing electrodeposited palladium. J. Anal. Chem. 2006, 61, 601–608. [Google Scholar]
- Matos, R.C.; Augelli, M.A.; Lago, C.L.; Angnes, L. Flow injection analysis-amperometric determination of ascorbic and uric acids in urine using arrays of gold microelectrodes modified by electrodeposition of palladium. Anal. Chim. Acta 2000, 404, 151–157. [Google Scholar]
- Janda, P.; Weber, J.; Dunsch, L.; Lever, A.B.P. Detection of ascorbic acid using a carbon fiber microelectrode coated with cobalt tetramethylpyridoporphyrazine. Anal. Chem. 1996, 68, 960–965. [Google Scholar]
- Behrens, W.A.; Madere, R. A Highly Sensitive High-Performance Liquid-Chromatography Method for the Estimation of Ascorbic and Dehydroascorbic Acid in Tissues, Biological-Fluids, and Foods. Anal. Biochem. 1987, 165, 102–107. [Google Scholar]
- Shakya, R.; Navarre, D.A. Rapid screening of ascorbic acid, glycoalkaloids, and phenolics in potato using high-performance liquid chromatography. J. Agric. Food Chem. 2006, 54, 5253–5260. [Google Scholar]
- Melendez-Martinez, A.J.; Vicario, I.M.; Heredia, F.J. Provitamin A carotenoids and ascorbic acid contents of the different types of orange juices marketed in Spain. Food Chem. 2007, 101, 177–184. [Google Scholar]
- Wang, J.; Chatrathi, M.P.; Tian, B.M.; Polsky, R. Microfabricated electrophoresis chips for simultaneous bioassays of glucose, uric acid, ascorbic acid, and acetaminophen. Anal. Chem. 2000, 72, 2514–2518. [Google Scholar]
- Davey, M.W.; Bauw, G.; VanMontagu, M. Simultaneous high-performance capillary electrophoresis analysis of the reduced and oxidised forms of ascorbate and glutathione. J. Chromatogr. B 1997, 697, 269–276. [Google Scholar]
- Klejdus, B.; Petrlova, J.; Potesil, D.; Adam, V.; Mikelova, R.; Vacek, J.; Kizek, R.; Kuban, V. Simultaneous determination of water- and fat-soluble vitamins in pharmaceutical preparations by high-performance liquid chromatography coupled with diode array detection. Anal. Chim. Acta. 2004, 520, 57–67. [Google Scholar]
- Wu, T.; Guan, Y.Q.; Ye, J.N. Determination of flavonoids and ascorbic acid in grapefruit peel and juice by capillary electrophoresis with electrochemical detection. Food Chem. 2007, 100, 1573–1579. [Google Scholar]
- Sun, X.M.; Niu, Y.; Bi, S.; Zhang, S.S. Determination of ascorbic acid in individual rat hepatocyte by capillary electrophoresis with electrochemical detection. J. Chromatogr. B 2008, 870, 46–50. [Google Scholar]
- Zuo, F.; Luo, C.H.; Zheng, Z.H.; Ding, X.B.; Peng, Y.X. Supramolecular assembly of betacyclodextrin-capped gold nanoparticles on ferrocene-functionalized ITO surface for enhanced voltammetric analysis of ascorbic acid. Electroanalysis 2008, 20, 894–899. [Google Scholar]
- Razmi, H.; Harasi, M. Voltammetric behavior and amperometric determination of ascorbic acid at cadmium pentacyanonitrosylferrate film modified GC electrode. Int. J. Electrochem. Sci. 2008, 3, 82–95. [Google Scholar]
- Abbaspour, A.; Khajehzadeh, A.; Noori, A. A simple and selective sensor for the determination of ascorbic acid in vitamin C tablets based on paptode. Anal. Sci. 2008, 24, 721–725. [Google Scholar]
- Liu, H.Q.; Tian, Y. Analytical application of pyramidal, rodlike, and spherical gold nanostructures: Simultaneous detection of ascorbic acid and uric acid. Electroanalysis 2008, 20, 1227–1233. [Google Scholar]
- Dai, H.; Wu, X.P.; Wang, Y.M.; Zhou, W.C.; Chen, G.N. An electrochemiluminescent biosensor for vitamin C based on inhibition of luminol electrochemiluminescence on graphite/poly(methyl methacrylate) composite electrode. Electrochim. Acta 2008, 53, 5113–5117. [Google Scholar]
- Nezamzadeh, A.; Amini, M.K.; Faghihian, H. Square-wave voltammetric determination of ascorbic acid based on its electrocatalytic oxidation at zeolite-modified carbon-paste electrodes. Int. J. Electrochem. Sci. 2007, 2, 583–594. [Google Scholar]
- Kizek, R.; Vacek, J.; Trnkova, L.; Jelen, F. Cyclic voltammetric study of the redox system of glutathione using the disulfide bond reductant tris(2-carboxyethyl)phosphine. Bioelectrochemistry 2004, 63, 19–24. [Google Scholar]
- Mikelova, R.; Baloun, J.; Petrlova, J.; Adam, V.; Havel, L.; Petrek, J.; Horna, A.; Kizek, R. Electrochemical determination of Ag-ions in environment waters and their action on plant embryos. Bioelectrochemistry 2007, 70, 508–518. [Google Scholar]
- Causon, R. Validation of chromatographic methods in biomedical analysis - Viewpoint and discussion. J. Chromatogr. B 1997, 689, 175–180. [Google Scholar]
- Bugianesi, R.; Serafini, M.; Simone, F.; Wu, D.Y.; Meydani, S.; Ferro-Luzzi, A.; Azzini, E.; Maiani, G. High-performance liquid chromatography with coulometric electrode array detector for the determination of quercetin levels in cells of the immune system. Anal. Biochem. 2000, 284, 296–300. [Google Scholar]
- Petrlova, J.; Mikelova, R.; Stejskal, K.; Kleckerova, A.; Zitka, O.; Petrek, J.; Havel, L.; Zehnalek, J.; Adam, V.; Trnkova, L.; Kizek, R. Simultaneous determination of eight biologically active thiol compounds using gradient elution-liquid chromatography with Coul-Array detection. J. Sep. Sci. 2006, 29, 1166–1173. [Google Scholar]
- Long, G.L.; Winefordner, J.D. Limit of Detection. Anal. Chem. 1983, 55, A712–A724. [Google Scholar]
- Masarik, M.; Stobiecka, A.; Kizek, R.; Jelen, F.; Pechan, Z.; Hoyer, W.; Jovin, T.M.; Subramaniam, V.; Palecek, E. Sensitive electrochemical detection of native and aggregated alphasynuclein protein involved in Parkinson's disease. Electroanalysis 2004, 16, 1172–1181. [Google Scholar]
- Kizek, R.; Trnkova, L.; Palecek, E. Determination of metallothionein at the femtomole level by constant current stripping chronopotentiometry. Anal. Chem. 2001, 73, 4801–4807. [Google Scholar]
- Hubalek, J.; Hradecky, J.; Adam, V.; Krystofova, O.; Huska, D.; Masarik, M.; Trnkova, L.; Horna, A.; Klosova, K.; Adamek, M.; Zehnalek, J.; Kizek, R. Spectrometric and voltammetric analysis of urease – Nickel nanoelectrode as an electrochemical sensor. Sensors 2007, 7, 1238–1255. [Google Scholar]
- Petrlova, J.; Krizkova, S.; Supalkova, V.; Masarik, M.; Adam, V.; Havel, L.; Kramer, K.J.; Kizek, R. The determination of avidin in genetically modified maize by voltammetric techniques. Plant Soil Environ. 2007, 53, 345–349. [Google Scholar]
- Petrlova, J.; Krizkova, S.; Zitka, O.; Hubalek, J.; Prusa, R.; Adam, V.; Wang, J.; Beklova, M.; Sures, B.; Kizek, R. Utilizing a chronopotentiometric sensor technique for metallothionein determination in fish tissues and their host parasites. Sens. Actuator B-Chem. 2007, 127, 112–119. [Google Scholar]
- Petrlova, J.; Masarik, M.; Potesil, D.; Adam, V.; Trnkova, L.; Kizek, R. Zeptomole detection of streptavidin using carbon paste electrode and square wave voltammetry. Electroanalysis 2007, 19, 1177–1182. [Google Scholar]
- Fabrik, I.; Krizkova, S.; Huska, D.; Adam, V.; Hubalek, J.; Trnkova, L.; Eckschlager, T.; Kukacka, J.; Prusa, R.; Kizek, R. Employment of electrochemical techniques for metallothionein determination in tumour cell lines and patients with a tumour disease. Electroanalysis 2008, 20, 1521–1532. [Google Scholar]
- Fabrik, I.; Ruferova, Z.; Hilscherova, K.; Adam, V.; Trnkova, L.; Kizek, R. A determination of metallothionein in larvae of freshwater midges (Chironomus riparius) using Brdicka reaction. Sensors 2008, 8, 4081–4094. [Google Scholar]
- Adam, V.; Baloun, J.; Fabrik, I.; Trnkova, L.; Kizek, R. An electrochemical detection of metallothioneins in nanolitres at zeptomole level. Sensors 2008, 8, 2293–2305. [Google Scholar]
- Adam, V.; Blastik, O.; Krizkova, S.; Lubal, P.; Kukacka, J.; Prusa, R.; Kizek, R. Application of the Brdicka reaction in determination of metallothionein in patients with tumours. Chem. Listy 2008, 102, 51–58. [Google Scholar]
- Adam, V.; Zitka, O.; Dolezal, P.; Zeman, L.; Horna, A.; Hubalek, J.; Sileny, J.; Krizkova, S.; Trnkova, L.; Kizek, R. Lactoferrin isolation using monolithic column coupled with spectrometric or micro-amperometric detector. Sensors. 2008, 8, 464–487. [Google Scholar]
- Zitka, O.; Stejskal, K.; Kleckerova, A.; Adam, V.; Beklova, M.; Horna, A.; Supalkova, V.; Havel, L.; Kizek, R. Utilizing electrochemical techniques for detection of biological samples. Chem. Listy 2007, 101, 225–231. [Google Scholar]
- Kand'ar, R.; Zakova, P.; Lotkova, H.; Kucera, O.; Cervinkova, Z. Determination of reduced and oxidized glutathione in biological samples using liquid chromatography with fluorimetric detection. J. Pharm. Biomed. Anal. 2007, 43, 1382–1387. [Google Scholar]
- Supalkova, V.; Huska, D.; Diopan, V.; Hanustiak, P.; Zitka, O.; Stejskal, K.; Baloun, J.; Pikula, J.; Havel, L.; Zehnalek, J.; Adam, V.; Trnkova, L.; Beklova, M.; Kizek, R. Electroanalysis of plant thiols. Sensors 2007, 7, 932–959. [Google Scholar]
- Supalkova, V.; Petrek, J.; Baloun, J.; Adam, V.; Bartusek, K.; Trnkova, L.; Beklova, M.; Diopan, V.; Havel, L.; Kizek, R. Multi-instrumental investigation of affecting of early somatic embryos of Spruce by cadmium(II) and lead(II) ions. Sensors 2007, 7, 743–759. [Google Scholar]
- Supalkova, V.; Beklova, M.; Baloun, J.; Singer, C.; Sures, B.; Adam, V.; Huska, D.; Pikula, J.; Rauscherova, L.; Havel, L.; Zehnalek, J.; Kizek, R. Affecting of aquatic vascular plant Lemna minor by cisplatin revealed by voltammetry. Bioelectrochemistry 2008, 72, 59–65. [Google Scholar]
- Krizkova, S.; Ryant, P.; Krystofova, O.; Adam, V.; Galiova, M.; Beklova, M.; Babula, P.; Kaiser, J.; Novotny, K.; Novotny, J.; Liska, M.; Malina, R.; Zehnalek, J.; Hubalek, J.; Havel, L.; Kizek, R. Multi-instrumental analysis of tissues of sunflower plants treated with silver(I) ions – Plants as bioindicators of environmental pollution. Sensors 2008, 8, 445–463. [Google Scholar]
- Ryant, P.; Dolezelova, E.; Fabrik, I.; Baloun, J.; Adam, V.; Babula, P.; Kizek, R. Electrochemical determination of low molecular mass thiols content in potatoes (Solanum tuberosum) cultivated in the presence of various sulphur forms and infected by late blight (Phytophora infestans). Sensors 2008, 8, 3165–3182. [Google Scholar]
- Babula, P.; Huska, D.; Hanustiak, P.; Baloun, J.; Krizkova, S.; Adam, V.; Hubalek, J.; Havel, L.; Zemlicka, M.; Horna, A.; Beklova, M.; Kizek, R. Flow injection analysis coupled with carbon electrodes as the tool for analysis of naphthoquinones with respect to their content and functions in biological samples. Sensors 2006, 6, 1466–1482. [Google Scholar]
- Supalkova, V.; Stavelikova, H.; Krizkova, S.; Adam, V.; Horna, A.; Havel, L.; Ryant, P.; Babula, P.; Kizek, R. Study of capsaicin content in various parts of pepper fruit by liquid chromatography with electrochemical detection. Acta Chim. Slov. 2007, 54, 55–59. [Google Scholar]
- Kolouchova-Hanzlikova, I.; Melzoch, K.; Filip, V.; Smidrkal, J. Rapid method for resveratrol determination by HPLC with electrochemical and UV detections in wines. Food Chem. 2004, 87, 151–158. [Google Scholar]
- Diopan, V.; Babula, P.; Shestivska, V.; Adam, V.; Zemlicka, M.; Dvorska, M.; Hubalek, J.; Trnkova, L.; Havel, L.; Kizek, R. Electrochemical and spectrometric study of antioxidant activity of pomiferin, isopomiferin, osajin and catalposide. J. Pharm. Biomed. Anal. 2008, 48, 127–133. [Google Scholar]
- Supalkova, V.; Petrek, J.; Havel, L.; Krizkova, S.; Petrlova, J.; Adam, V.; Potesil, D.; Babula, P.; Beklova, M.; Horna, A.; Kizek, R. Electrochemical sensors for detection of acetylsalicylic acid. Sensors 2006, 6, 1483–1497. [Google Scholar]
- Mikelova, R.; Hodek, P.; Hanustiak, P.; Adam, V.; Krizkova, S.; Havel, L.; Stiborova, M.; Horna, A.; Beklova, M.; Trnkova, L.; Kizek, R. Determination of isoflavones using liquid chromatography with electrochemical detection. Acta Chim. Slov. 2007, 54, 92–97. [Google Scholar]
- Billova, S.; Kizek, R.; Jelen, F.; Novotna, P. Square-wave voltammetric determination of cefoperazone in a bacterial culture, pharmaceutical drug, milk, and urine. Anal. Bioanal. Chem. 2003, 377, 362–369. [Google Scholar]
- Diculescu, V.C.; Vivan, M.; Brett, A.M.O. Voltammetric behavior of antileukemia drug glivec. Part III: In situ DNA oxidative damage by the Glivec electrochemical metabolite. Electroanalysis 2006, 18, 1963–1970. [Google Scholar]
- Beklova, M.; Krizkova, S.; Supalkova, V.; Mikelova, R.; Adam, V.; Pikula, J.; Kizek, R. Determination of bromadiolone in pheasants and foxes by differential pulse voltammetry. Int. J. Environ. Anal. Chem. 2007, 87, 459–469. [Google Scholar]
- Krizkova, S.; Beklova, M.; Pikula, J.; Adam, V.; Horna, A.; Kizek, R. Hazards of secondary bromadiolone intoxications evaluated using high-performance liquid chromatography with electrochemical detection. Sensors 2007, 7, 1271–1286. [Google Scholar]
- Potesil, D.; Petrlova, J.; Adam, V.; Vacek, J.; Klejdus, B.; Zehnalek, J.; Trnkova, L.; Havel, L.; Kizek, R. Simultaneous femtomole determination of cysteine, reduced and oxidized glutathione, and phytochelatin in maize (Zea mays L.) kernels using high-performance liquid chromatography with electrochemical detection. J. Chromatogr. A 2005, 1084, 134–144. [Google Scholar]
- Wang, J. Analytical Electrochemistry; Wiley-VCH: New York, 2000. [Google Scholar]






| Sample | Homogenate(μg mL1)a, b, c | Spiking AA(μg mL1)a, b | Homogenate+spikingAA (μg mL-1)a, b | Recovery(%) |
|---|---|---|---|---|
| Celaskon (tablets) | 9.8 ± 0.2 (2.0) | 5.0 ± 0.2 (4.0) | 15.6 ± 0.9 (5.8) | 105 |
| 15.9 ± 0.9 (5.7) | 24.3 ± 1.9 (7.8) | 95 | ||
| Citrus aurantium | 4.3 ± 0.1 (2.3) | 5.1 ± 0.1 (1.9) | 9.7 ± 0.3 (3.1) | 103 |
| 15.3 ± 1.0 (6.5) | 20.3 ± 1.6 (7.9) | 104 | ||
| Human serum | 7.1 ± 0.3 (4.2) | 4.8 ± 0.2 (4.2) | 12.1 ± 0.6 (5.0) | 102 |
| 15.1 ± 0.9 (6.0) | 21.7 ± 1.8 (8.3) | 98 | ||
© 2008 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Gazdik, Z.; Zitka, O.; Petrlova, J.; Adam, V.; Zehnalek, J.; Horna, A.; Reznicek, V.; Beklova, M.; Kizek, R. Determination of Vitamin C (Ascorbic Acid) Using High Performance Liquid Chromatography Coupled with Electrochemical Detection. Sensors 2008, 8, 7097-7112. https://doi.org/10.3390/s8117097
Gazdik Z, Zitka O, Petrlova J, Adam V, Zehnalek J, Horna A, Reznicek V, Beklova M, Kizek R. Determination of Vitamin C (Ascorbic Acid) Using High Performance Liquid Chromatography Coupled with Electrochemical Detection. Sensors. 2008; 8(11):7097-7112. https://doi.org/10.3390/s8117097
Chicago/Turabian StyleGazdik, Zbynek, Ondrej Zitka, Jitka Petrlova, Vojtech Adam, Josef Zehnalek, Ales Horna, Vojtech Reznicek, Miroslava Beklova, and Rene Kizek. 2008. "Determination of Vitamin C (Ascorbic Acid) Using High Performance Liquid Chromatography Coupled with Electrochemical Detection" Sensors 8, no. 11: 7097-7112. https://doi.org/10.3390/s8117097
APA StyleGazdik, Z., Zitka, O., Petrlova, J., Adam, V., Zehnalek, J., Horna, A., Reznicek, V., Beklova, M., & Kizek, R. (2008). Determination of Vitamin C (Ascorbic Acid) Using High Performance Liquid Chromatography Coupled with Electrochemical Detection. Sensors, 8(11), 7097-7112. https://doi.org/10.3390/s8117097