Understanding of Coupled Terrestrial Carbon, Nitrogen and Water Dynamics—An Overview
Abstract
:1. Introduction
2. Scientific Background and State of Knowledge
2.1. Overview of Terrestrial Ecosystems and Climate
2.2. Terrestrial C Cycling
2.3. Coupling of the C and Water Cycles
2.4. Coupling of the C and N Cycles
3. Monitoring of C and Water Cycling in Terrestrial Ecosystems
3.1. Global Flux Tower Network (FLUXNET)
3.2. CO2 Concentration Measurements, Data Assimilation and CarbonTracker
3.3. Stable C Isotope Measurements
3.4. Satellite Monitoring
3.5. Other Airborne Measurements
4. Modeling of C and Water Dynamics in Terrestrial Ecosystems
4.1. Land Surface and Ecosystem Modeling
4.2. Spatially-distributed Hydrological Processes Modeling
4.3. Modeling Dynamics of Stable C Isotopic Exchange between Ecosystem and the Atmosphere
4.4. Modeling Coupled C, N and Water Dynamics—An Ecohydrological Approach
4.5. Applications of Remotely-sensed Data in Ecohydrological Modeling
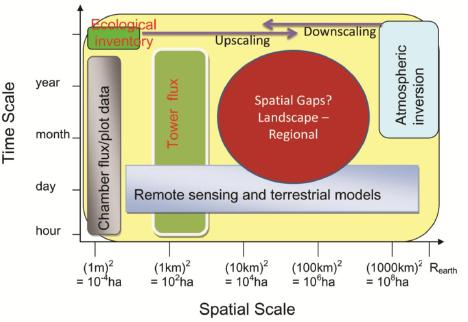
5. Research gaps in C and Water Flux Estimates and Scaling Approaches
6. Future Research Directions
6.1. Development of a Spatially Explicit Ecohydrological Modeling Framework
6.1.1. Reviewing of the Existing EASS Model [112]
6.1.2. Reviewing of the TerrainLab Model [157]
6.1.3. Development of an Ecohydrological Model by Coupling of EASS with TerrainLab
6.1.4. Model Calibration and Validation for EASS-TerrainLab
6.1.5. Model Sensitivity Analysis and Runs under Different Scenarios
6.2. Landscape and Regional C and Water Fluxes Estimation: An Upscaling Framework
7. Summary
Acknowledgments
References and Notes
- Treut, L.; Somerville, H.R.; Cubasch, U.; Ding, Y.; Mauritzen, C.; Mokssit, A.; Peterson, T.; Prather, M. Historical Overview of Climate Change. In Climate Change 2007: The Physical Science Basis. Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change; Solomon, S., Qin, D., Manning, M., Chen, Z., Marquis, M., Averyt, K.B., Tignor, M., Miller, H.L., Eds.; Cambridge University Press: Cambridge, UK, 2007; pp. 104–105. [Google Scholar]
- Betts, R.A.; Cox, P.M.; Woodward, F.I. Simulated responses of potential vegetation to doubled-CO2 climate change and feedbacks on near-surface temperature. Global Ecol. Biogeogr. 2000, 9, 171–180. [Google Scholar]
- Cox, P.M.; Betts, R.A.; Jones, C.D.; Spall, S.A.; Totterdell, I.J. Acceleration of global warming due to carbon-cycle feedbacks in a coupled climate model. Nature 2000, 408, 184. [Google Scholar]
- Pielke, R.A., Sr. Influence of the spatial distribution of vegetation and soils on the prediction of cumulus convective rainfall. Rev. Geophys. 2001, 39, 151–177. [Google Scholar]
- Friedlingstein, P.; Dufresne, J.L.; Cox, P.M.; Rayner, P. How positive is the feedback between climate change and the carbon cycle? Tellus B 2003, 55, 692–700. [Google Scholar]
- Seneviratne, S.I.; Luthi, D.; Litschi, M.; Schar, C. Land-atmosphere coupling and climate change in Europe. Nature 2006, 443, 205–209. [Google Scholar]
- Betts, A.K.; Desjardins, R.L.; Worth, D. Impact of agriculture, forest and cloud feedback on the surface energy budget in BOREAS. Agric. For. Meteor. 2007, 142, 156–169. [Google Scholar]
- Betts, R.A.; Boucher, O.; Collins, M.; Cox, P.M.; Falloon, P.D.; Gedney, N.; Hemming, D.L.; Huntingford, C.; Jones, C.D.; Sexton, D.M.H.; Webb, M.J. Projected increase in continental runoff due to plant responses to increasing carbon dioxide. Nature 2007, 448, 1037–1045. [Google Scholar]
- Baldocchi, D.D. Breathing of the terrestrial biosphere: lessons learned from a global network of carbon dioxide flux measurement systems. Aust. J. Bot. 2008, 56, 1–26. [Google Scholar]
- Running, S.W.; Nemani, R.R.; Heinsch, F.A.; Zhao, M.; Reeves, M.; Jolly, M. A continuous satellite-derived measure of global terrestrial primary productivity: Future science and applications. Bioscience 2004, 56, 547–560. [Google Scholar]
- Kalma, J.D.; McVicar, T.R.; McCabe, M.F. Estimating Land Surface Evaporation: A Review of Methods Using Remotely Sensed Surface Temperature Data. Surv. Geophys. 2008, 29, 421–469. [Google Scholar]
- Verstraeten, W.W.; Veroustraete, F.; Feyen, J. Assessment of evapotranspiration and soil moisture content across different scales of observation. Sensors 2008, 8, 70–117. [Google Scholar]
- Marquis, M.; Tans, P. Carbon crucible. Science 2008, 320, 460–470. [Google Scholar]
- Canadell, J.G.; Quere, C.; Raupacha, M.R.; Fielde, C.B.; Buitenhuisc, E.T.; Ciais, P.; Conwayg, T.J.; Gillettc, N.P.; Houghtonh, R.A.; Marlandi, G. Contributions to accelerating atmospheric CO2 growth from economic activity, carbon intensity, and efficiency of natural sinks. Proc. Natl. Acad. Sci. 2007, 104, 18866–18870. [Google Scholar]
- Prentice, I.C.; Farquhar, G.D.; Fasham, M.J.R. The Carbon Cycle and Atmospheric Carbon Dioxide; Houghton, J.T., Ed.; Cambridge University Press: Cambridge, UK, 2001; pp. 183–238. [Google Scholar]
- Falk, M.; Wharton, S.; Schroeder, M. Flux partitioning in an old-growth forest: seasonal and interannual dynamics. Tree Physiol. 2008, 28, 509–520. [Google Scholar]
- Levin, S.A. Complex adaptive systems: exploring the known, unknown and unknowable. Bull. Amer. Meteor. Soc. 2002, 40, 1–19. [Google Scholar]
- Ma, S.; Baldocchi, D.D.; Xu, L. Interannual variability in carbon dioxide exchage of an oak/grass sacanna and open grassland in California. Agric. For. Meteor. 2007, 147, 157–171. [Google Scholar]
- Black, T.A.; Chen, W.J.; Barr, A.G. Increased carbon sequestration by a boreal deciduous forest in years with a warm spring. Geophys. Res. Lett. 2000, 27, 1271–1274. [Google Scholar]
- Baldocchi, D.D.; Falge, E.; Gu, L. Fluxnet: a new tool to study the temporal and spatial variability of ecosystem-scale carbon dioxide, water vapor, and energy flux densities. Bull Amer. Meteorl. Soc. 2001, 82, 2415–2434. [Google Scholar]
- Baldocchi, D.D.; Wilson, K.B. Modeling CO2 fluxes and water vapor exchange of a temperate broadleaved forest across hourly to decadal time scales. Ecol. Mod. 2001, 142, 155–184. [Google Scholar]
- Barr, A.G.; Black, T.A.; Hogg, E.H. Inter-annual variability in the leaf area index of a boreal aspen-hazelnut forest in relation to net ecosystem production. Agric. For. Meteor. 2004, 126, 237–255. [Google Scholar]
- Law, B.E.; Falge, E.; Gu, L. Environmental controls over carbon dioxide and water vapor exchange of terrestrial vegetation. Agric. For. Meteor. 2002, 113, 97–120. [Google Scholar]
- Ball, J.T.; Woodrow, I.E.; Berry, J.A. A Model Predicting Stomatal Conductnance Amd Its Contribution to the Control of Photosynthesis under Different Environmental Conditions; Martinus Nijhoff: Dordrecht, Netherland, 1987; pp. 221–224. [Google Scholar]
- Levis, S.; Foley, J.A.; Pollard, D. Potential high-latitude vegetation feedbacks on CO2-induced climate change. Geophys. Res. Lett 1999, 26, 747–750. [Google Scholar]
- Rodriguez-Iturbe, I. Ecohydrology: A hydrologic perspective of climate-soil-vegetation dynamics. Water Resour. Res. 2000, 36, 3–9. [Google Scholar]
- Joos, F.; Prentice, I.C.; Sitch, S.; Meyer, R.; Hooss, G.; Plattner, G.K.; Gerber, S.; Hasselmann, K. Global warming feedbacks on terrestrial carbon uptake under the intergovernmental panel on climate change (IPCC) emission scenarios. Global Biogeochem. Cycles 2001, 15, 891–907. [Google Scholar]
- Arain, M.A.; Yuan, F.M.; Black, T.A. Soil-plant nitrogen cycling modulated carbon exchanges in a Western Temperate Conifer Forest in Canada. Agric. For. Meteor. 2006, 140, 171–192. [Google Scholar]
- Blanken, P.D.; Black, T.A. The canopy conductance of a boreal aspen forest, Prince Albert national park, Canada. Hydro. Proces. 2004, 18, 1561–1578. [Google Scholar]
- Snyder, P.K.; Delire, C.L.; Foley, J.A. Evaluating the influence of different vegetation biomes on the global climate. Clim. Dyn. 2004, 23, 279–302. [Google Scholar]
- Jarvis, P.G. Interpretation of variations in leaf water potential and stomatal conductance found in canopies in field. Philos. Trans. R Soc. Lond B Biol. Sci. 1976, 273, 593–610. [Google Scholar]
- Harris, P.P.; Huntingford, C.; Cox, P.M.; Gash, J.H.C.; Malhi, Y. Effect of soil moisture on canopy conductance of Amazonian rainforest. Agric. For. Meteor. 2004, 122, 215–227. [Google Scholar]
- Rodriguez-Iturbe, I.; Porporato, A.; Laio, F.; Ridolfi, L. Plants in water-controlled ecosystems: active role in hydrologic processes and response to water stress - i. scope and general outline. Adv. Water Resour. 2001, 24, 695–705. [Google Scholar]
- Parton, W.J.; Ojima, D.S.; Cole, C.V.; Schimel, D.S. A general model for soil organic matter dynamics: sensitivity to litter chemistry, texture and management. Soil Sci. Soc. Am. J. 1993, 39, 147–167. [Google Scholar]
- D'odorico, P.; Porporato, A.; Laio, F.; Ridolfi, L.; Rodriguez-Iturbe, I. Probabilistic modeling of nitrogen and carbon dynamics in water-limited ecosystems. Ecol. Model. 2004, 179, 205–219. [Google Scholar]
- Hedges, J.I. Global biogeochemical cycles: progress and problems. Mar. Chem. 1992, 39, 67–93. [Google Scholar]
- Wang, X.-C.; Chen, R.F.; Gardner, G.B. Sources and transport of dissolved and particulate organic carbon in the Mississippi River estuary and adjacent coastal waters of the northern Gulf of Mexico. Marine Chem. 2004, 89, 241–256. [Google Scholar]
- Meybeck, M. Carbon, nitrogen, and phosphorous transport by world rivers. Am. J. Sci. 1982, 282, 401–450. [Google Scholar]
- Vitousek, P.M.; Howarth, R.W. Nitrogen limitation on land and in the sea: how can it occur? Biogeochemistry 1991, 13, 87–115. [Google Scholar]
- Allison, S.D.; Czimczik, C.I.; Treseder, K.K. Microbial activity and soil respiration under nitrogen addition in Alaskan boreal forest. Glob. Change Biol. 2008, 14, 1–13. [Google Scholar]
- Vitousek, P.M.; Aber, J.D.; Howarth, R.W. Human alteration of the global nitrogen cycle: sources and consequences. Ecol. Appl. 1997, 7, 737–750. [Google Scholar]
- Galloway, J.N.; Cowling, E.B. Reactive nitrogen and the world: 200 years of change. Ambio. 2002, 31, 64–71. [Google Scholar]
- Brumme, R.; Khanna, P. Ecological and site historical aspects of N dynamics and current N status in temperate forests. Glob. Change Biol. 2008, 14, 125–141. [Google Scholar]
- Galloway, J.N.; Cowling, E.B. Reactive nitrogen and the world: 200 years of change. J. Human Environ. 2002, 31, 64–71. [Google Scholar]
- Aber, J.D.; McDowell, W.; Nadelhoffer, K. Nitrogen saturation in temperate forest ecosystems. Bioscience 1998, 48, 921–934. [Google Scholar]
- Aber, J.D.; Nadelhoffer, K.J.; Steudler, P.; Melillo, J.M. Nitrogen saturation in northern forest ecosystems. BioScience 1989, 39, 378–386. [Google Scholar]
- Bauer, G.A.; Bazzaz, F.A.; Minocha, R.; Long, S.; Magill, A.; Aber, J.; Berntson, G.M. Effects of chronic N additions on tissue chemistry, photosynthetic capacity, and carbon sequestration potential of a red pine (Pinus resinosa Ait.) stand in the NE United States. Forest Ecol. Manag. 2004, 196, 173–186. [Google Scholar]
- Agren, G.I.; Bosatta, E. Nitrogen saturation of terrestrial ecosystems. Environ. Pollut. 1988, 54, 185–197. [Google Scholar]
- Nihlgard, B. The ammonium hypothesis—an additional explanation to the forest dieback. Europe. Ambio. 1985, 14, 2–8. [Google Scholar]
- Townsend, A.R.; Braswell, B.H.; Holland, E.A.; Penner, J.E. Spatial and temporal patterns in terrestrial carbon storage due to deposition of fossil fuel nitrogen. Ecol. Appl. 1996, 6, 806–814. [Google Scholar]
- Pregitzer, K.S.; Burton, J.A.; Zak, D.R. Simulated chronic nitrogen deposition increases carbon storage in Northern Temperate forests. Glob. Change Biol. 2008, 14, 142–153. [Google Scholar]
- Olsson, P.; Linder, S.; Giesler, R. Fertilization of boreal forest reduces both autotrophic and heterotrophic soil respiration. Glob. Change Biol. 2005, 11, 1745–1753. [Google Scholar]
- Leggett, Z.H.; Kelting, D.L. Fertilization effects on carbon pools in loblolly pine plantations on two upland sites. Soil Sci. Soc. Amer. J. 2006, 70, 279–286. [Google Scholar]
- Nadelhoffer, K.J.; Emmett, B.A.; Gundersen, P. Nitrogen deposition makes a minor contribution to carbon sequestration in temperate forests. Nature 1999, 398, 145–148. [Google Scholar]
- Korner, C. Biosphere responses to CO2 enrichment. Ecol. Appl. 2000, 10, 1590–1619. [Google Scholar]
- De Vries, W.; Reinds, G.J.; Gundersen, P.; Sterba, H. The impact of nitrogen deposition on carbon sequestration in European forests and forest soils. Glob. Change Biol. 2006, 12, 1151–1173. [Google Scholar]
- Schulze, E.D. Air pollution and forest decline in a spruce (Picea abies) forest. Science 1989, 244, 776–783. [Google Scholar]
- Gundersen, P.; Emmett, B.A.; Kjønaas, O.J.; Koopmans, C.J.; Tietema, A. Impact of nitrogen deposition on nitrogen cycling in forests: a synthesis of NITREX data. Forest Ecol. Manag. 1998, 101, 37–55. [Google Scholar]
- Majdi, H.; Andersson, P. Fine root production and turnover in a Norway spruce stand in northern Sweden: effects of nitrogen and water manipulation. Ecosystems 2005, 8, 191–199. [Google Scholar]
- Nadelhoffer, K.J. The potential effects of nitrogen deposition on fine-root production in forest ecosystems. New Phytol. 2000, 147, 131–139. [Google Scholar]
- Bowden, R.D.; Rullo, G.; Sevens, G.R. Soil fluxes of carbon dioxide, nitrous oxide, and methane at a productive temperate deciduous forest. J. Environ. Quality 2000, 29, 268–276. [Google Scholar]
- Bowden, R.D.; Davidson, E.; Savage, K. Chronic nitrogen additions reduce total soil respiration and microbial respiration in temperate forest soils at the Harvard forest. For. Ecol. Manage 2004, 196, 43–56. [Google Scholar]
- Burton, A.J.; Pregitzer, K.; Crawford, J.N. Simulated chronic NO3- deposition reduces soil respiration in northern hardwood forests. Glob. Change Biol. 2004, 10, 1080–1091. [Google Scholar]
- Cleveland, C.C.; Townsend, A.R. Nutrient additions to a tropical rain forest drive substantial soil carbon dioxide losses to the atmosphere. Proc. Natl. Acad. Sci. 2006, 103, 10316–10321. [Google Scholar]
- Phillips, R.P.; Fahey, T.J. Fertilization effects on fineroot biomass, rhizosphere microbes and respiratory fluxes in hardwood forest soils. New Physiologist 2007, 176, 655–664. [Google Scholar]
- Mo, J.M.; Brown, S.; Xue, J.H. Response of litter decomposition to simulated N deposition in disturbed, rehabilitated and mature forests in subtropical China. Plant Soil 2006, 282, 135–151. [Google Scholar]
- Mo, J.M.; Zhang, W.; Zhu, W. Nitrogen addition reduces soil respiration in a mature tropical forest in southern China. Glob. Change Biol. 2008, 14, 403–412. [Google Scholar]
- Hyvonen, R.; Persson, T.; Andersson, S.; Olsson, B.; Agren, G.I.; Linder, S. Impact of long-term nitrogen addition on carbon stocks in trees and soils in northern Europe. Biogeochemistry 2008, 89, 121–137. [Google Scholar]
- Law, B.E.; Waring, R.H.; Anthoni, P.M. Measurements of gross and net ecosystem, productivity and water vapour exchange of a Pinus ponderosa ecosystem, and evaluation of two generalized models. Glob. Change Biol. 2000, 6, 155–168. [Google Scholar]
- Barford, C.C.; Wofsy, S.C.; Goulden, M.L. Factors controlling long- and short-term sequestration of atmospheric CO2 in a mid-latitude forest. Science 2001, 294, 1688–1691. [Google Scholar]
- Amiro, B.D.; Barr, A.G.; Black, T.A. Carbon, energy and water fluxes at mature disturbed forest sites, Saskatchewan, Canada. Agric. For. Meteor. 2006, 136, 237–251. [Google Scholar]
- Stoy, P.C.; Katul, G.G.; Siqueira, M.B.S.; Juang, J.-Y.; Novick, K.A.; Mccarthy, H.R.; Oishi, A.C.; Uebelherr, C.M.; Kim, H.S.; Oren, R. Separating the effects of climate and vegetation on evapotranspiration along a successional chronosequence in the southeastern US. Glob. Change Biol. 2006, 12, 2115–2135. [Google Scholar]
- Chen, B.; Chen, J.M.; Mo, G.; Yuen, C-W.; Margolis, H.; Higuchi, K.; Chan, D. Modeling and scaling coupled energy, water, and carbon fluxes based on remote sensing: an application to Canada's landmass. J. Hydrometeor. 2007, 8, 123–143. [Google Scholar]
- Urbanski, S.; Barford, C.; Wofsy, S. Factors controlling CO2 exchange on timescales from hourly to decadal at Harvard Forest. J. Geophys. Res. 2007, 112, G02020. [Google Scholar] [CrossRef]
- Gloor, M.; Bakwin, P.; Hurst, D.; Lock, L.; Draxler, R.; Tans, P. What is the concentration footprint of a tall tower? J. Geophys. Res. 2001, 106, 831–840. [Google Scholar]
- Lin, J.C.; Gerbig, C.; Wofsy, S.C.; Andrews, A.E.; Daube, B.C.; Grainger, C.A.; Stephens, B.B.; Bakwin, P.S.; Hollinger, D.Y. Measuring fluxes of trace gases at regional scales by Lagrangian observations: application to the CO2 Budget and Rectification Airborne (COBRA) study. J. Geophys. Res. 2004, 109, D15304. [Google Scholar] [CrossRef]
- Chen, B.; Chen, J.M.; Mo, G.; Black, T.A.; Worthy, D.E.J. Comparison of regional carbon flux estimates from CO2 concentration measurements and remote sensing based footprint integration. Global Biogeochem. Cycles 2008, 22, GB2012. [Google Scholar] [CrossRef]
- Peters, W.; Jacobson, A.; Sweeney, C.; Andrews, A.; Conway, T.; Masarie, K. An atmospheric perspective on North American carbon dioxide exchange: CarbonTracker. Proc. Natl. Acad. Sci. 2007, 104, 18925–18930. [Google Scholar]
- Bakwin, P.S.; Davis, K.J.; Yi, C.; Wofsy, S.C.; Munger, J.W.; Haszpra, L.; Barcza, Z. Regional carbon dioxide fluxes from mixing ratio data. Tellus B 2004, 56, 301–311. [Google Scholar]
- Bakwin, P.S.; Tans, P.P.; Zhao, C.; Ussler, W.; Quesnell, E. Measurements of carbon dioxide on a very tall tower. Tellus B 1995, 37, 535–549. [Google Scholar]
- Bakwin, P.S.; Tans, P.P.; Hurst, D.F.; Zhao, C. Measurements of carbon dioxide on very tall towers: results of the NOAA/CMDL program. Tellus B 1998, 50, 401–415. [Google Scholar]
- Wofsy, S.C.; Harriss, R.C. The North American Carbon Program (NACP) Report for the US Interagency Carbon Cycle Science Program; US Interagency Carbon Cycle Science Program: Washington, DC, USA, 2002. [Google Scholar]
- Betts, A.K.; Helliker, B.; Berry, J. Coupling between CO2, water vapor, temperature and radon and their fluxes in an idealized equilibrium boundary layer over land. J. Geophys. Res. 2004, 109, D18103. [Google Scholar] [CrossRef]
- Helliker, B.R.; Berry, J.A.; Betts, A.K.; Bakwin, P.S.; Davis, K.J.; Denning, A.S.; Ehleringer, J.R.; Miller, J.B.; Butler, M.P.; Ricciuto, D.M. Estimates of net CO2 flux by application of equilibrium boundary layer concepts to CO2 and water vapor measurements from a tall tower. J. Geophys. Res. 2004, 109, D20106. [Google Scholar] [CrossRef]
- Dolman, A.J.; Cerbig, C.; Noilhan, J.; Sarrat, C.; Miglietta, F. Detecting regional variability in sources and sinks of carbon dioxide: a synthesis. Biogeosciences 2009, 6, 1015–1026. [Google Scholar]
- Matross, D.; Andrews, A.; Pathmathevan, M. Estimating regional carbon exchange in New England and Quebec by combining atmospheric, ground-based and satellite data. Tellus B 2006, 58, 344–358. [Google Scholar]
- Tans, P.P.; Fung, I.Y.; Takahashi, T. Observational constraints on the global atmospheric CO2 budget. Science 1990, 247, 1431–1438. [Google Scholar]
- Gurney, K.R.; Law, R.M.; Denning, A.S.; Rayner, P.J.; Baker, D.; Bousquet, P.; Bruhwiler, L.; Chen, Y.-H.; Ciais, P.; Fan, S.M.; Fung, I.Y.; Gloor, M.; Heimann, M.; Higuchi, K.; John, J.; Maki, T.; Maksyutov, S.; Masarie, K.; Peylin, P.; Prather, M.; Bernard, C.P.; Randerson, J.; Sarmiento, J.; Taguchi, S.; Takahashi, T.; Yuen, C.-W. Towards robust regional estimates of CO2 sources and sinks using atmospheric transport models. Nature 2002, 415, 626–630. [Google Scholar]
- Stephens, B.B.; Gurney, K.R.; Tans, P.P.; Sweeney, C.; Peters, W.; Bruhwiler, L.; Ciais, P.; Ramonet, M.; Bousquet, P.; Nakazawa, T. Weak northern and strong tropical land carbon uptake from vertical profiles of atmospheric CO2. Science 2007, 316, 1732–1735. [Google Scholar]
- Yang, Z.; Washenfelder, R.A.; Keppel-Aleks, G.; Krakauer, N.Y.; Randerson, J.T.; Tans, P.P.; Sweeney, C.; Wennberg, P.O. New constraints on Northern Hemisphere growing season net flux. Geophys. Res. Lett. 2007, 34, L12807. [Google Scholar] [CrossRef]
- Rodenbeck, C.; Houweling, S.; GLoor, M.; Heimann, M. CO2 flux history 1982-2001 inferred from atmospheric data using a global inversion of atmospheric transport. Atmos. Chem. Phys. 2003, 3, 1919–1964. [Google Scholar]
- Tans, P.P. On calculating the transfer of carbon-13 in reservoir models of the carbon cycle. Tellus B 1980, 32, 464–469. [Google Scholar]
- Tans, P.P.; Berry, J.A.; Keeling, R.F. Oceanic 13C/12C observation: A new window on CO2 uptake by the oceans. Global Biogeochem. Cycles 1993, 7, 353–368. [Google Scholar]
- Suits, N.S.; Denning, A.S.; Berry, J.A.; Still, C.J.; Kaduk, J.; Miller, J.B.; Baker, I.T. Simulation of carbon isotope discrimination of the terrestrial biosphere. Global Biogeochem. Cycles 2005, 19, GB1017. [Google Scholar] [CrossRef]
- Farquhar, G.D.; Richards, R.A. Isotopic composition of plant carbon correlates with water-use efficiency of wheat genotypes. Aust. J. Plant. Physiol. 1984, 11, 539–552. [Google Scholar]
- Farquhar, G.D.; Hubick, K.T.; Condon, A.G.; Richards, R.A. Carbon Isotope Discrimination and Plant Water Use Efficiency. In StableIsotopes in Ecological Research; Rundel, P.W., Ed.; Academic: San Diego, CA. USA, 1988; pp. 21–40. [Google Scholar]
- Condon, A.G.; Richards, R.A.; Farquhar, G.D. Relationship between carbon isotope discrimination, water use efficiency and transpiration efficiency for dryland wheat. Aust. J. Agric. Res. 1993, 44, 1693–1711. [Google Scholar]
- Hall, A.E.; Ismail, A.M.; Menendez, C.M. Implications for Plant Breeding of Genotypic and Drought Induced Differences in Water Use Efficiency, Carbon Isotope Discrimination and Gas Exchange. In Stable Isotopes and Plant Carbon-Water Relations; Ehleringer, J.R., Hall, A.E., Farquhar, G.D., Eds.; Academic: San Diego, CA. USA, 1993; pp. 19–28. [Google Scholar]
- Ekblad, A.; Hogberg, P. Natural abundance of 13C in CO2 respired from forest soils reveals speed of link between tree photosynthesis and root respiration. Oecologia 2001, 127, 305–308. [Google Scholar]
- Bowling, D.R.; McDowell, N.G.; Bond, B.J.; Law, B.E.; Ehleringer, J.R. 13C content of ecosystem respiration is linked to precipitation and vapor pressure deficit. Oecologia 2002, 131, 113–124. [Google Scholar]
- Berry, S.C.; Varney, G.T.; Flanagan, L.B. Leaf δ13C in Pinus resinosa trees and understory plants: Variation associated with light and CO2 gradients. Oecologia 1997, 109, 499–506. [Google Scholar]
- Buchmann, N.; Guehl, J.M.; Barigah, T.S.; Ehleringer, J.R. Interseasonal comparison of CO2 concentrations, isotopic composition, and carbon dynamics in an Amazonian rainforest (French Guiana). Oecolgia 1997, 110, 120–131. [Google Scholar]
- Buchmann, N.; Kao, W.Y.; Ehleringer, J.R. Influence of stand structure on carbon-13 of vegetation, soils, and canopy air within deciduous and evergreen forests in Utah, United States. Oecologia 1997, 110, 109–119. [Google Scholar]
- Le Roux, X.; Bariac, T.; Sinoquet, H.; Genty, B.; Piel, C.; Mariotti, A.; Girardin, C.; Richard, P. Spatial distribution of leaf water use efficiency and carbon isotope discrimination within an isolated tree crown. Plant Cell Environ. 2001, 24, 1021–1032. [Google Scholar]
- Keeling, C.D. A mechanism for cyclic enrichment of carbon-12 by terrestrial plants. Geochim. Cosmochim. Acta 1961, 24, 299–313. [Google Scholar]
- Schleser, G.H.; Jayasekera, R. δ13C variations of leaves in forests as an indication of reassimilated CO2 from the soil. Oecologia 1985, 65, 536–542. [Google Scholar]
- Lloyd, J.; Kruijt, B.; Hollinger, D.Y. Vegetation effects on the isotopic composition of atmospheric CO2 at local and regional scales: theoretical aspects and a comparison between rain forest in Amazonia and a boreal forest in Siberia. Austr. J. Plant Phys. 1996, 23, 371–399. [Google Scholar]
- Sternberg, L.S.O. A model to estimate carbon dioxide recycling in forests using 13C/12C ratios and concentrations of ambient carbon dioxide. Agric. Aor. Meteor. 1989, 48, 163–173. [Google Scholar]
- Sternberg, L.S.O.; Moreira, M.Z.; Martinelli, L.A.; Victoria, R.L.; Barbosa, E.M.; Bonates, L.C.M.; Nepstad, D.C. Carbon dioxide recycling in two Amazonian tropical forests. Agric. For. Meteor. 1997, 88, 259–268. [Google Scholar]
- Yakir, D.; Wang, X.F. Fluxes of CO2 and water between terrestrial vegetation and the atmosphere estimated from isotope measurements. Nature 1996, 380, 515–517. [Google Scholar]
- Bowling, D.R.; Tans, P.P.; Monson, R.K. Partitioning net ecosystem carbon exchange with isotopic fluxes of CO2. Glob. Change Biol. 2001, 7, 127–145. [Google Scholar]
- Ogee, J.; Brunet, Y.; Loustau, D.; Berbigier, P.; Delzon, S. MuSICA, a CO2, water and energy multi-layer, multi-leaf pine forest model: Evaluation from hourly to yearly time scales and sensitivity analysis. Glob. Change Biol. 2003, 9, 697–717. [Google Scholar]
- Lai, C.T.; Schauer, A.J.; Owensby, C.; Ham, J.M.; Ehleringer, J.R. Isotopic air sampling in a tallgrass prairie to partition net ecosystem CO2 exchange. J. Geophys. Res. 2003, 108, 4566. [Google Scholar]
- Knohl, A.; Buchmann, N. Partitioning the net CO2 flux of a deciduous forest into respiration and assimilation using stable carbon isotopes. Global Biogeochem. Cycles 2005, 19, GB4008. [Google Scholar] [CrossRef]
- Bowling, D.R.; Sargent, S.D.; Tanner, B.D.; Ehleringer, J.R. Tunable diode laser absorption spectroscopy for stable isotope studies of ecosystem-atmosphere CO2 exchange. Agri. For. Meteor. 2003, 118, 1–19. [Google Scholar]
- Lai, C.T.; Ehleringer, J.R.; Tans, P.P.; Wofsy, S.C.; Urbanski, S.P.; Hollinger, D.Y. Estimating photosynthetic 13C discrimination in terrestrial CO2 exchange from canopy to regional scales. Global Biogeochem. Cycles 2004, 18, GB1041. [Google Scholar] [CrossRef]
- Griffis, T.J.; Baker, J.M.; Zhang, J. Seasonal dynamics and partitioning of isotopic CO2 exchange in a C3/C4 managed ecosystem. Agric. For. Meteor. 2005, 132, 1–19. [Google Scholar]
- Gillies, R.R.; Cui, J.; Carlson, T.N.; Kustas, W.P.; Humes, K.S. Verification of a method for obtaining surface soil water content and energy fluxes from remote measurements of NDVI and surface radiant temperature. Inter. J. Remot. Sens. 1997, 18, 3145–3166. [Google Scholar]
- Kite, G.W.; Droogers, P. Comparing evapotranspiration estimates from satellites, hydrological models and field data. J. Hydrol. 2000, 229, 3–18. [Google Scholar]
- Su, Z. Remote sensing of land use and vegetation for mesoscale hydrological studies. Inter. J. Remot. Sens. 2000, 21, 213–233. [Google Scholar]
- Su, Z. The surface energy balance system (SEBS) for estimation of the turbulent heat fluxes. Hydro. Earth Sci. 2002, 6, 85–99. [Google Scholar]
- Dirmeyer, P.A. Vegetation stress as a feedback mechanism in mid-latitude drought. J. Climate 1994, 7, 1463–1483. [Google Scholar]
- Pielke, R.S., Sr.; Avissar, R.; Raupach, M. Interactions between the atmosphere and terrestrial ecosystems: influence on weather and climate. Glob. Change Biol. 1998, 4, 461–475. [Google Scholar]
- Betts, A.K.; Ball, J.H. Albedo over the boreal forest. J. Geophys. Res. 1997, 102, 901–909. [Google Scholar]
- Hamazaki, T.; Kaneko, Y.; Kuze, A. Carbon Dioxide Monitoring from the Gosat Satellite, Geo-Imagery Bridging Continents. In Proceedings of Commission VII, ISPRS Congress; Istanbul, Turkey, July 2004. [Google Scholar]
- Crisp, D. Orbiting Carbon Observatory (OCO) mission. Adv. Space. Res. 2004, 34, 700–709. [Google Scholar]
- Coops, N.; Wulder, M.; Culvenor, D.; St-Onge, B. Comparison of forest attributes extracted from fine spatial resolution multispectral and lidar data. Can. J. Remote Sens. 2004, 30, 855–866. [Google Scholar]
- Coops, N.C.; Hilker, T.; Wulder, M.A.; St-Onge, B.; Newnham, G.; Siggins, A.; Trofymow, J.A. Estimating canopy structure of Douglas-fir forest stands from discrete-return lidar. Trees 2007, 21, 295–310. [Google Scholar]
- Hilker, T.; Coops, N.C.; Hall, F.G.; Black, T.A.; Chen, B.; Krishnan, P.; Wulder, M.A.; Sellers, P.J.; Middleton, E.M.; Huemmrich, K.F. A modeling approach for upscaling gross ecosystem production to the landscape scale using remote sensing data. J. Geophys Res. 2008, 113, G03006. [Google Scholar] [CrossRef]
- Chen, B.; Black, A.; Coops, N.C.; Hilker, T.; Trofymow, T.; Nesic, Z.; Morgenstern, K. Assessing tower flux footprint climatology and scaling between remotely sensed and eddy covariance measurements. Boundary-Layer Meteor. 2009, 130, 137–167. [Google Scholar]
- Rannik, U.; Kolari, P.; Vesala, T.; Hari, P. Uncertainties in measurement and modelling of net ecosystem exchange of a forest. Agric. For. Meteor. 2006, 138, 244–257. [Google Scholar]
- Cox, P.M.; Betts, R.A.; Bunton, C.B.; Essery, R.L.H.; Rowntree, P.R.; Smith, J. The impact of new land surface physics on the GCM simulation of climate and climate sensitivity. Climate Dyn. 1999, 15, 183–203. [Google Scholar]
- Gedney, N.; Cox, P.M.; Betts, R.A.; Boucher, O.; Huntingford, C.; Stott, P.A. Detection of a direct carbon dioxide effect in continental river runoff records. Nature 2006, 439, 835–838. [Google Scholar]
- Dickinson, R.E.; Berry, J.A.; Bonan, G.B. Nitrogen controls on climate model evapotranspiration. J. Climat. 2002, 15, 278–295. [Google Scholar]
- Matthews, E.; Hansen, J. Land Surface Modeling: A Mini-Workshop. Available online: http://www.giss.nasa.gov/meetings/landsurface1998/section3.html/ (accessed on October 20, 2009).
- Seth, A.; Giorgi, F.; Dickinson, R.E. Simulating fluxes from heterogeneous land surfaces: Explicit subgrid method employing the biosphere-atmosphere transfer scheme (BATS). J. Geophys. Res. 1994, 99, 651–667. [Google Scholar]
- Manabe, S. Climate and the ocean circulation I. The atmospheric circulation and the hydrology of the Earth's surface. Mon. Wea. Rev. 1969, 97, 739–774. [Google Scholar]
- Deardorff, J.W. Efficient prediction of ground surface temperature and moisture, with inclusion of a layer of vegetation. J. Geophys. Res. 1978, 83, 1889–1903. [Google Scholar]
- Sellers, P.J. Modeling the exchange of energy, water, and carbon between continentals and the atmosphere. Science 1997, 275, 502–509. [Google Scholar]
- Sellers, P.J.; Mintz, Y.; Sud, Y.C.; Dalcher, A. A Simple Biosphere Model (SiB) for Use Within General-Circulation Models. J. Atmos. Sci. 1986, 43, 505–531. [Google Scholar]
- Verseghy, D.L. CLASS: A Canadian land surface scheme for gcms. Int. J. Climatol. 1999, 11, 111–133. [Google Scholar]
- Verseghy, D.L.; McFarlane, N.A.; Lazare, M. CLASS: A Canadian land surface scheme for GCMs: II. Vegetation model and coupled runs. Int. J. Climatol. 1993, 13, 347–370. [Google Scholar]
- Chen, B.; Chen, J.M.; Ju, W. Remote sensing based ecosystem-atmosphere simulation Scheme (EASS)—model formulation and test with multiple-year data. Ecol. Model. 2007, 209, 277–300. [Google Scholar]
- Henderson-Sellers, A.; Yang, Z.-L.; Dickinson, R.E. The project for intercomparison of land-surface parameterization schemes. Bull. Amer. Meteorol. Soc. 1993, 74, 1335. [Google Scholar]
- Wang, S.; Grant, R.F.; Verseghy, D.L.; Black, T.A. Modeling carbon-coupled energy and water dynamics of boreal aspen forest in a general circulation model land surface scheme. Int. J. Climatol. 2002, 22, 1249–1265. [Google Scholar]
- Kickert, R.N.; Tonella, G.; Simonov, A.; Krupa, S. Predictive modeling of effects under global change. Environ. Pollut. 1999, 100, 87–132. [Google Scholar]
- Kley, D.; Kleinmann, M.; Sandermann, H.; Krupa, S. Photochemical oxidants: state of the science. Environ. Pollut. 1999, 100, 19–42. [Google Scholar]
- Schwalm, C.R.; Ek, A.R. Climate change and site: relevant mechanisms and modeling techniques. For. Ecol. Manage. 2001, 150, 241–258. [Google Scholar]
- Bonan, G.B. Comparison of two land surface process models using prescribed forcings. J. Geophys. Res. 1994, 99, 803–818. [Google Scholar]
- Sellers, P.J.; Randall, D.A.; Collatz, G.J. A revised land surface parameterization (SiB2) for atmospheric GCMs. Part I: model formulation. J. Climat. 1996, 9, 676–705. [Google Scholar]
- Williams, M.S.; Law, B.E.; Anthoni, P.M.; Unsworth, W.H. Use of a simulation model and ecosystems flux data to examine carbon-water interactions in ponderosa pine. Tree Physiol. 2001, 21, 287–298. [Google Scholar]
- Foley, J.A.; Prentice, I.C.; Ramankutty, N.; Levis, S.; Pollard, D.; Sitch, S.; Haxeltine, A. An integrated biosphere model of land surface processes, terrestrial carbon balance, and vegetation dynamics. Global Biogeochem. Cycles 1996, 10, 603–628. [Google Scholar]
- Bonan, G.B. Land-atmospheric interactions for climate system models: Coupling biophysical, biogeochemical and ecosystem dynamical processes. Remote Sens. Environ. 1995, 51, 57–73. [Google Scholar]
- Wang, S.; Grant, R.F.; Verseghy, D.L.; Black, T.A. Modeling carbon dynamics of boreal forest ecosystems using the Canadian land surface scheme. Clim. Chang. 2002, 55, 451–477. [Google Scholar]
- Holdridge, L. Determination of world plant formations from simple climatic data. Science 1947, 105, 367–368. [Google Scholar]
- Prentice, K.; Fung, I.Y. The sensitivity of terrestrial carbon storage to climate change. Nature 1990, 346, 48–51. [Google Scholar]
- Prentice, I.C.; Cramer, W.; Harrison, S.P.; Leemans, R.; Monserud, R.A.; Solomon, A.M. A global biome model based on plant physiology and dominance, soil properties, and climate. J. Biogeog. 1992, 19, 117–134. [Google Scholar]
- Zhang, Y.; Chen, W.; Cihlar, J. A process-based model for quantifying the impact of climate change on permafrost thermal regimes. J. Geophys. Res. 2003, 108, 4695. [Google Scholar]
- Viterbo, P.; Beljaars, A.C.M. An improved land surface parameterization scheme in the ECMWF model and its validation. J. Climat. 1995, 8, 2716–2748. [Google Scholar]
- Christopher, S.R.; Ek, A.R. A process-based model of forest ecosystems driven by meteorology. Ecol. Model. 2004, 179, 317–348. [Google Scholar]
- de Rosnay, P.; Drusch, M.; Boone, A.; Balsamo, G.; Decharme, B.; Harris, P.; Kerr, Y.; Pellarin, T.; Polcher, J.; Wigneron, J.-P. AMMA Land Surface Model Intercomparison Experiment coupled to the Community Microwave Emission Model: ALMIP-MEM. J. Geophys. Res. 2009, 114, D05108. [Google Scholar] [CrossRef]
- Liang, X.; Lettenmaier, D.P.; Wood, E.F.; Burges, S.J. A simple hydrologically based model of land surface water and energy fluxes for GSMs. J. Geophys. Res. 1994, 99, 14415–14428. [Google Scholar]
- Liang, X.; Guo, J.; Leung, L.R. Assessment of the effects of spatial resolutions on daily water flux simulations. J. Hydrol. 2004, 298, 287–310. [Google Scholar]
- Beldring, S.; Engeland, K.; Roald, L.A.; Sælthun, N.R.; Voksø, A. Estimation of parameters in a distributed precipitation runoff model for Norway. Hydro. Earth System Sci. 2003, 7, 304–316. [Google Scholar]
- Brath, A.; Montanari, A.; Toth, E. Analysis of the effects of different scenarios of historical data availability on the calibration of a spatially-distributed hydrological model. J. Hydro. 2004, 291, 232–253. [Google Scholar]
- Christensen, N.; Lettenmaier, D.P. A multimodel ensemble approach to assessment of climate change impacts on the hydrology and water resources of the Colorado River basin. Hydro. Earth System Sci. 2007, 11, 1417–1434. [Google Scholar]
- Reed, S.; Koren, V.; Smith, M.; Zhang, Z.; Moreda, F.; Seo, D.J. Overall distributed model intercomparison project results. J. Hydro. 2004, 298, 27–60. [Google Scholar]
- Ciais, P.; Tans, P.P.; Trolier, M; White, J.W.C.; Francey, R.J. A large Northern Hemisphere terrestrial CO2 sink indicated by the 13C/12C ratio of atmospheric CO2. Science 1995, 269, 1098–1102. [Google Scholar]
- Ponton, S.; Flanagan, L.B.; Alstard, K.; Johnson, B.G.; Morgenstern, K.; Kljun, N.; Black, T.A.; Barr, A.G. Comparison of ecosystem water-use efficiency among Douglas-fir forest, aspen forest and grassland using eddy covariance and carbon isotope techniques. Glob. Change Biol. 2006, 12, 294–310. [Google Scholar]
- Lai, C.-T.; Schauer, A.J.; Owensby, C. Regional CO2 fluxes inferred from mixing ratio measurements: estimates from flask air samples in central Kansas, USA. Tellus B 2006, 58, 523–536. [Google Scholar]
- Lai, C.T.; Ehleringer, J.; Schauer, A.; Tanss, P.P.; Hollinger, D.; Paw, K.T.U.; Munger, J.; Wofsy, S. Canopy-scale δ13C of photosynthetic and respiratory CO2 fluxes: observations in forest biomes across the United States. Glob. Change Biol. 2005, 11, 633–643. [Google Scholar]
- Baldocchi, D.D.; Bowling, D.R. Modeling the discrimination of 13C above and within a temperate broad-leaved forest canopy on hourly to seasonal time scales. Plant Cell Environ. 2003, 26, 231–244. [Google Scholar]
- Farquhar, G.D.; Ehleringer, J.R.; Hubick, K.T. Carbon isotope discrimination and photosynthesis. Annu. Rev. Plant Phys. Plant Mol. Biol. 1989, 40, 503–537. [Google Scholar]
- Farquhar, G.D.; O'Leary, M.H.; Berry, J.A. On the relationship between carbon isotope discrimination and the intercellular carbon dioxide concentration in leaves. Aust. J. Plant Physi. 1982, 9, 121–137. [Google Scholar]
- Pataki, D.E.; Ehleringer, J.R.; Flanagan, L.B.; Yakir, D.; Bowling, D.R.; Still, C.J.; Buchmann, N.; Kaplan, J.O.; Berry, J.A. The application and interpretation of Keeling plots in terrestrial carbon cycle research. Global Biogeochem. Cycles 2003, 17, 1022. [Google Scholar]
- Bowling, D.R.; Baldocchi, D.D.; Monson, R.K. Dynamics of isotope exchange of carbon dioxide in a Tennessee deciduous forest. Global Biogeochem. Cycles 1999, 13, 903–921. [Google Scholar]
- Baldocchi, D.D.; Harley, P.C. Scaling carbon dioxide and water vapour exchange from leaf to canopy in a deciduous forest: model testing and application. Plant Cell Environ 1995, 18, 1157–1173. [Google Scholar]
- Leuning, R. Estimation of scalar source/sink distributions in plant canopies using Lagrangian dispersion analysis: Corrections for atmospheric stability and comparison with a multilayer canopy model. Boundary-Layer Meteor 2000, 96, 293–314. [Google Scholar]
- Lloyd, J.; Francey, R.J.; Mollicone, D.; Raupach, M.R.; Sogachev, A.; Arneth, A.; Byers, J.N.; Kelliher, F.M.; Rebmann, C.; Valentini, R.; Wong, S.C.; Bauer, G.; Schulze, E.D. Vertical profiles, boundary layer budgets, and regional flux estimates for CO2 and its 13C/12C ratio and for water vapor above a forest/bog mosaic in central Siberia. Global Biogeochem. Cycles 2001, 15, 267–284. [Google Scholar]
- Chen, B.; Chen, J.M.; Huang, L.; Tans, P.P. Simulating dynamics of δ13C of CO2 in the planetary boundary layer over a boreal forest region: Covariation between surface fluxes and atmospheric mixing. Tellus B 2006, 58, 537–549. [Google Scholar]
- Chen, B.; Chen, J.M.; Tans, P.P.; Huang, L. Modeling dynamics of stable carbon isotopic exchange between a boreal ecosystem and the Atmosphere. Glob. Change Biol. 2006, 12, 1842–1867. [Google Scholar]
- Chen, B.; Chen, J.M. Diurnal, seasonal and inter-annual variability of carbon isotope discrimination at the canopy level in response to environmental factors in a boreal forest ecosystem. Plant Cell Environ. 2007, 30, 1223–1239. [Google Scholar]
- Grant, R.F.; Flanagan, L.B. Modeling stomatal and nonstomatal effects of water deficits on CO2 fixation in a semiarid grassland. J. Geophys. Res. 2007, 112, G03011. [Google Scholar] [CrossRef]
- Grant, R.F.; Black, T.A.; den Hartog, G. Diurnal and annual exchanges of mass and energy between an aspen-hazelnut forest and the atmosphere: testing the mathematical model Ecosys with data from the BOREAS experiment. J. Geophys. Res. 1999, 27, 27699–27718. [Google Scholar]
- Wilson, K.B.; Baldocchi, D.D.; Hanson, P.J. Spatial and seasonal variability of photosynthetic parameters and their relationship to leaf nitrogen in a deciduous forest. Tree Physi. 2000, 20, 565–578. [Google Scholar]
- Wilson, K.B.; Baldocchi, D.D.; Hanson, P.J. Leaf age affects the seasonal pattern photosynthetic capacity and net ecosystem exchange of carbon in a deciduous forest. Plant Cell Environ. 2001, 24, 571–583. [Google Scholar]
- Warren, C.R.; Adams, M.A. Distribution of N. Rubisco and photosynthesis in Pinus pinaster and acclimation to light. Plant Cell Environ. 2001, 24, 597–609. [Google Scholar]
- Govind, A.; Chen, J.M.; Margolis, H.; Ju, W.; Sonnentag, O.; Giasson, M.-A. A spatially explicit hydro-ecological modeling framework (BEPS-TerrainLab V2.0): model description and test in a boreal ecosystem in Eastern North America. J. Hydro. 2009, 367, 200–216. [Google Scholar]
- Creed, I.F.; Band, L.E. Exploring functional similarity in the export of Nitrate-N from forested catchments: a mechanistic modeling approach. Water Resour. Res. 1998, 34, 3079–3093. [Google Scholar]
- Band, L.E.; Tague, C.L.; Groffman, P.; Belt, K. Forest ecosystem processes at the watershed scale: hydrological and ecological controls of Nitrogen export. Hydro. Processes 2001, 15, 2013–2028. [Google Scholar]
- Porporato, A.; Rodriguez-Iturbe, I. Ecohydrology—a challenging multidisciplinary research perspective. Hydro. Sci. 2002, 47, 811–821. [Google Scholar]
- Porporato, A.; D'odorico, P.; Laio, F.; Rodriguez-Iturbe, I. Hydrologic Controls on Soil Carbon and Nitrogen Cycles. I. Modeling Scheme. Adv. Water Resour. 2003, 26, 45–58. [Google Scholar]
- Daly, E.; Porporato, A.; Rodriguez-Iturbe, I. Coupled dynamics of photosynthesis, transpiration, and soil water balance. Part I: upscaling from hourly to daily level. J. Hydrometeo. 2004, 5, 546–558. [Google Scholar]
- Chen, J.M.; Chen, X.Y.; Ju, W.M.; Geng, X.Y. Distributed hydrological model for mapping evapotranspiration using remote sensing inputs. J. Hydrol. 2005, 305, 15–39. [Google Scholar]
- Kite, G.W.; Pietroniro, A. Remote sensing applications in hydrological modelling. Hydro. Sci. J. 1996, 41, 563–591. [Google Scholar]
- Engman, E.T. Remote sensing applications to hydrology: future impact. Hydro. Sci. J. 1996, 41, 637–647. [Google Scholar]
- Melesse, A.M.; Vijay, N.; Jon, H. Analysis of energy fluxes and land surface parameters in grassland ecosystem: remote sensing perspective. Int. J. Remot. Sens. 2008, 29, 3325–3341. [Google Scholar]
- Plummer, S.E. Perspectives on combining ecological process models and remotely sensed data. Ecol. Model. 2000, 129, 169–186. [Google Scholar]
- Running, S.W.; Coughlan, J.C. A general model of forest ecosystem processes for regional applications I. Hydrological balance, canopy gas exchange and primary production processes. Ecol. Model. 1998, 42, 125–154. [Google Scholar]
- Chiesi, M.; Maselli, F.; Bindi, M.; Fibbi, L.; Bonora, L.; Raschi, A.; Tognetti, R.; Cermak, J.; Nadezhdina, N. Calibration and application of FOREST-BGC in a Mediterranean area by the use of conventional and remote sensing data. Ecol. Model. 2002, 154, 251–262. [Google Scholar]
- Loiselle, S.; Bracchini, L.; Bonechi, C.; Rossi, C. Modelling energy fluxes in remote wetland ecosystems with the help of remote sensing. Ecol. Model. 2001, 145, 243–261. [Google Scholar]
- Goetz, S.J.; Prince, S.D.; Goward, S.N.; Thawley, M.M.; Small, J. Satellite remote sensing of primary production: an improved production efficiency modelling approach. Ecol. Model. 1999, 122, 239–255. [Google Scholar]
- Seaquist, J.W.; Olsson, L.; Ardo, J. A remote sensing-based primary production model for grassland biomes. Ecol. Model. 2003, 169, 131–155. [Google Scholar]
- Bergen, K.M.; Dobson, M.C. Integration of remotely sensed radar imagery in modeling and mapping forest biomass and net primary production. Ecol. Model. 1999, 122, 257–274. [Google Scholar]
- Maas, S.J. Use of remotely sensed information in agricultural crop growth models. Ecol. Model. 1988, 41, 247–268. [Google Scholar]
- Kurth, W. Morphological models of plant growth: possibilities and ecological relevance. Ecol. Model. 1994, 75, 299–308. [Google Scholar]
- Boegh, E.; Thorsen, M.; Butts, M.B.; Hansen, S.; Christiansen, J.S.; Abrahamsen, P.; Hasager, C.B.; Jensen, N.O.; van der Keur, P.; Refsgaard, J.C.; Schelde, K.; Soegaard, H.; Thomsen, A. Incorporating remote sensing data in physically based distributed agro-hydrological modelling. J. Hydro. 2004, 287, 279–299. [Google Scholar]
- Wigmosta, M.S.; Vail, L.W.; Lettenmaier, D.P. A distributed hydrology-vegetation model for complex terrain. Water Resour. Res. 1994, 30, 1665–1679. [Google Scholar]
- Govind, A.; Chen, J.M.; Ju, W. Spatially explicit simulation of hydrologically controlled carbon and nitrogen cycles and associated feedback mechanisms in a boreal ecosystem. J. Geophys. Res. 2009, 114, G02006. [Google Scholar] [CrossRef]
- Montzka, C.; Canty, M.; Kunkel, R.; Menz, G.; Vereecken, H.; Wendland, F. Modelling the water balance of a mesoscale catchment basin using remotely sensed land cover data. J. Hydro. 2008, 353, 322–334. [Google Scholar]
- Stisen, S.; Jensen, K.H.; Sandholt, I.; Grimes, D.I.F. A remote sensing driven distributed hydrological model of the Senegal River basin. J. Hydro. 2008, 354, 131–148. [Google Scholar]
- Ritchie, J.C.; Rango, A. Remote sensing applications to hydrology: introduction. Hydro. Sci. J. 1996, 41, 429–431. [Google Scholar]
- Schultz, G.A. Remote sensing applications to hydrology: runoff. Hydro. Sci. J. 1996, 41, 453–475. [Google Scholar]
- Melesse, A.M.; Weng, Q.; Thenkabail, P.; Senay, G. Remote sensing sensors and applications in environmental resources mapping and modeling. Sensors 2007, 7, 3209–3241. [Google Scholar]
- Schmugge, J.S.; Kustas, W.P.; Ritchie, J.C.; Jackson, T.J.; Rango, A. Remote sensing in hydrology. Adv. Water Resour. 2002, 25, 1367–1385. [Google Scholar]
- Jain, M.K.; Kothyari, U.C.; Raju, K.G. A GIS based distributed rainfall–runoff model. J. Hydro. 2004, 299, 107–135. [Google Scholar]
- Pietroniro, A.; Leconte, R. A review of Canadian remote sensing and hydrology, 1999-2003. Hydro. Proc. 2005, 19, 285–301. [Google Scholar]
- French, R.H.; Miller, J.J.; Dettling, C.; Carr, J. Use of remotely sensed data to estimate the flow of water to a playa lake. J. Hydro. 2006, 325, 67–81. [Google Scholar]
- Engman, E.T. Remote sensing applications to hydrology: future impact. Hydro. Sci. J. 1996, 41, 637–647. [Google Scholar]
- Wang, J.; Price, K.P.; Rich, P.M. Spatial patterns of NDVI in response to precipitation and temperature in the central Great Plains. Int. J. Remote Sens. 2001, 22, 3827–3844. [Google Scholar]
- Jackson, T.J. Measuring surface soil moisture using passive microwave remote sensing. Hydr. Proc. 1993, 7, 139–152. [Google Scholar]
- Hollenbeck, K.J.; Schmugge, T.J.; Homberger, G.M.; Wang, J.R. Identifying soil hydraulic heterogeneity by detection of relative change in passive microwave remote sensing observations. Water Resour. Res. 1996, 32, 139–148. [Google Scholar]
- Kim, G.; Barros, A.P. Space-time characterization of soil moisture from passive microwave remotely sensed imagery and ancillary data. Remote Sens. Environ. 2002, 81, 393–403. [Google Scholar]
- Koster, R.D.; Guo, Z.C.; Dirmeyer, P.A.; Bonan, G.; Chan, E.; Cox, P.; Davies, H.; Gordon, C.T.; Kanae, S.; Kowalczyk, E. Glace: the global land-atmosphere coupling experiment. Part I: overview. J. Hydrometeor. 2006, 7, 590–610. [Google Scholar]
- Tans, P.P.; Fung, I.Y.; Takahashi, T. Observation Constraints on the global atmospheric CO2 budget. Science 1990, 247, 1431–1438. [Google Scholar]
- Enting, I.G.; Trudinger, C.M.; Francey, R.J. A synthesis inversion of the concentration and δ13C of atmospheric CO2. Tellus B 1995, 47, 35–52. [Google Scholar]
- Fan, S.; Gloor, M.; Mahlman, J.; Pacala, S.; Sarmiento, J. A large terrestrial carbon sink in North America implied by atmospheric and oceanic carbon dioxide data and models. Science 1998, 282, 442–446. [Google Scholar]
- Bousquet, P.; Ciais, P.; Peylin, P.; Ramonet, M.; Monfray, P. Inverse modeling of annual atmospheric CO2 sources and sinks 1. method and control inversion. J. Geophys. Res. 1999, 104, 26161–26178. [Google Scholar]
- Gurney, K.R.; Law, R.M.; Denning, A.S.; Rayner, P.J.; Baker, D. Towards robust regional estimates of CO2 sources and sinks using atmospheric transport models. Nature 2002, 415, 626–630. [Google Scholar]
- Gurney, K.R. TransCom 3 CO2 Inversion Intercomparison: 1. Annual mean control results and sensitivity to transport and prior flux information. Tellus B 2003, 55, 555–579. [Google Scholar]
- Gurney, K.R. Transcom 3 inversion intercomparison: model mean results for the estimation of seasonal carbon sources and sinks. Global Biogeochem. Cycles 2004, 18, GB1010. [Google Scholar] [CrossRef]
- Gurney, K.R.; Chen, Y.-H.; Maki, T.; Kawa, S.R.; Andrews, A.; Zhu, Z. Sensitivity of atmospheric CO2 inversions to seasonal and interannual variations in fossil fuel emissions. J. Geophys. Res. 2005, 110, D10308. [Google Scholar] [CrossRef]
- Gurney, K.R.; Baker, D.; Rayner, P.; Denning, S. Interannual variations in continental-scale net carbon exchange and sensitivity to observing networks estimated from atmospheric CO2 inversions for the period 1980 to 2005. Global Biogeochem. Cycles 2008, 22, GB3025. [Google Scholar] [CrossRef]
- Gerbig, C.; Lin, J.; Wofsy, S.C.; Daube, B.C.; Andrews, A.E. Towards constraining regional scale fluxes of CO2 with atmospheric observations over a continent: Observed spatial variability from airborne platforms. J. Geophys. Res. 2003, 108, D4756. [Google Scholar] [CrossRef]
- Crevoisier, C.; Gloor, M.; Gloaguen, E.; Horowitz, L.W.; Sarmiento, J.; Sweeney, C.; Tans, P. A direct carbon budgeting approach to infer carbon sources and sinks: Design and synthetic application to complement the NACP observation network. Tellus B 2006, 58, 366–375. [Google Scholar]
- Levy, P.E.; Grelle, A.; Lindroth, A.; Mölder, M.; Jarvis, P.G.; Kruijt, B.; Moncrieff, J.B. Regional-scale CO2 fluxes over central Sweden by a boundary layer budget method. Agric. For. Meteor. 1999, 98-99, 169–180. [Google Scholar]
- Gloor, M.; Fan, S.M.; Pacala, S.; Sarmiento, J.; Ramonet, M.A. Model-based evaluation of inversions of atmospheric transport, using annual mean mixing ratios, as a tool to monitor fluxes of nonreactive trace substances like CO2 on a continental scale. J. Geophys. Res. 1999, 104, 14245–14260. [Google Scholar]
- De Pury, D.G.G.; Farquhar, G.D. Simple scaling of photosynthesis from leaves to canopies without the errors of big-leaf models. Plant Cell Environ. 1997, 20, 537–557. [Google Scholar]
- Norman, J.M. Interfacing Leaf and Canopy Light Interception Models. In Predicting Photosynthesis for Ecosystem Models; Hesketh, J.D., Jones, J.W., Eds.; CRC: Boca Raton, FL, USA, 1980; pp. 49–68. [Google Scholar]
- Tarantola, A. Inverse Problem: Theory Methods for Data Fitting and Parameter Estimation.; Elsevier: New York, NY, USA, 1987; pp. 155–178. [Google Scholar]





© 2009 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Chen, B.; Coops, N.C. Understanding of Coupled Terrestrial Carbon, Nitrogen and Water Dynamics—An Overview. Sensors 2009, 9, 8624-8657. https://doi.org/10.3390/s91108624
Chen B, Coops NC. Understanding of Coupled Terrestrial Carbon, Nitrogen and Water Dynamics—An Overview. Sensors. 2009; 9(11):8624-8657. https://doi.org/10.3390/s91108624
Chicago/Turabian StyleChen, Baozhang, and Nicholas C. Coops. 2009. "Understanding of Coupled Terrestrial Carbon, Nitrogen and Water Dynamics—An Overview" Sensors 9, no. 11: 8624-8657. https://doi.org/10.3390/s91108624
APA StyleChen, B., & Coops, N. C. (2009). Understanding of Coupled Terrestrial Carbon, Nitrogen and Water Dynamics—An Overview. Sensors, 9(11), 8624-8657. https://doi.org/10.3390/s91108624