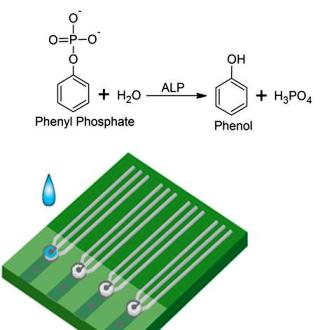
The Detection of Alkaline Phosphatase Using an Electrochemical Biosensor in a Single-Step Approach
Abstract
:1. Introduction
2. Results and Discussion
2.1. Temperature effect on the ALP biosensor performance
2.2. Evaluation of potential interference of other components in the test medium
2.3. Calibration of the ALP Biosensor in Bovine Serum
3. Experimental Section
3.1. Chemicals and Solutions
3.2. Instrumentation and Electrochemical Sensor
3.3. Testing Procedure
- Evaluation of the potential effects of the chemical species involved in the enzymatic reaction.As shown in Scheme 1b and other information, the preliminary studied enzymatic reaction was carried out first in a phosphate buffer solution (PBS) at pH = 7.0 in order to determine its feasibility for ALP detection by quantifying the produced phenol. This reaction involved MgCl2 and phenyl phosphate which were needed to activate the ALP enzymatic reaction and served as the reaction substrate, respectively. It was necessary to assess if any of these basic materials, the PBS, the MgCl2 and the phenyl phosphate may contribute to the sensor output in ALP detection. Cyclic voltammetric measurements were carried out in this experimental evaluation.
- Sensitivity of phenol detection using the biosensor prototype.It would be essential that this iridium nano-catalyst contained biosensor prototype could be used effectively for the detection of phenol as a means of quantifying ALP in a surrogate fluid. This assessment was first undertaken in PBS at a fixed pH value. Both cyclic voltammetric and amperometric studies were carried out. The cyclic voltammetric study assessed the appropriate oxidation potential of phenol, whereas the amperometric measurements aided in the assessment of the relationship between the sensor output (in current) and the ALP concentration level in a test medium. The ALP concentration range of 0 to 300 IU/L used covered the important physiological range of ALP in a biological system.
- Effect of operating temperature on the performance of the ALP biosensor.The operating temperature can affect the performance and the sensor output of the ALP biosensor directly. It was anticipated that at a higher operating temperature the sensor current would increase. However, a biosensor operating at a temperature higher than ambient temperature will require an additional heating element. This would complicate the single use, disposable ALP biosensor design. In this study, the temperature effect on the performance of the ALP biosensor was experimentally assessed at three temperatures, ambient temperature (approximately 23–25 °C), 32 and 37 °C. As mentioned, a constant temperature water bath was used in this study to maintain temperature.
- Evaluation of potential interference by the biological species.Selected biological species, such as glucose, ascorbic acid and uric acid can affect ALP detection. In this study, a stock solution containing 25 mM/L of glucose was prepared and added to a test solution containing ALP in the range of 10–300 IU/L. Similarly, a stock solution of 15 mg/L of ascorbic acid solution, and uric acid stock solution of 400 mg/L were prepared for the potential interference studies. The concentration levels of these stock solutions chosen were based on the maximum reported values for interfering species found in physiological fluid.
- Evaluation of the performance of this ALP biosensor in bovine serum.In order to assess the sensor's ability to detect ALP in bovine serum, test samples containing varying concentrations of ALP were tested with the biosensor and compared to measurements obtained by a Dimension® RxL Max™ spectrophotometer (Siemens Healthcare Diagnostics, Inc., Tarrytown, NY). This was done to establish a comparison between the traditional method of detection and the sensor's performance. Testing samples containing shrimp alkaline phosphatase (USB, Cleveland, OH) were diluted in bovine serum over the concentration range of 0 to 300 IU/L. The ALP levels of the samples were then measured by both the spectrophotometer and our electrochemical based biosensor. The samples were stored at –20 °C when they were not used. Electrochemical testing of the ALP biosensors was conducted following the following steps:
- 75 μL of the ALP contained bovine serum was added into a 0.6 mL microtube along with 75 μL of pH 10 phosphate buffer solution containing 150 mM of phenyl phosphate.
- The tube was vortexed for 5–10 seconds to ensure a complete mixing.
- 5 μL of the combined solution was pipetted onto the surface of the ALP biosensor.
- The potential was set at +0.45 V vs Ag/AgCl reference electrode, for a time of 600 seconds and was operated at 25 °C.
4. Conclusions
Acknowledgments
References and Notes
- Fishman, W.H.; Stigbrand, T. Human alkaline phosphatases. Proceedings of International Society for Oncodevelopmental Biology and Medicine Annual Meeting, Umea, Sweden, September 16–18, 1983; pp. 361–364.
- Favus, M.J. Primer on the metabolic bone diseases and disorders of mineral metabolism; American Society for Bone and Mineral Research: Washington, DC, USA, 2006. [Google Scholar]
- McComb, R.B.; Bowers, G.N.; Posen, S. Alkaline phosphatase; Plenum: New York, NY, USA, 1979. [Google Scholar]
- Ewen, L.M.; Griffiths, J. Patterns of enzyme activity following myocardial infarction and ischemia. Am. J. Clin. Pathol. 1971, 56, 614–622. [Google Scholar]
- Raftery, M.J.; Saldanha, R.G.; Geczy, C.L.; Kumar, R.K. Mass spectrometric analysis of electrophoretically separated allergens and proteases in grass pollen diffusates. Respir. Res. 2003, 4, 10. [Google Scholar]
- Lee, S.W.; Berger, S.J.; Martinovic, S.; Pasa-Tolic, L.; Anderson, G.A.; Shen, Y.; Zhao, R.; Smith, R.D. Direct mass spectrometric analysis of intact proteins of the yeast large ribosomal subunit using capillary LC/FTICR. Proc. Natl. Acad. Sci. USA 2002, 99, 5942–5947. [Google Scholar]
- Ukeda, H.; Maeda, S.; Ishii, T.; Sawamura, M. Spectrophotometric assay for superoxide dismutase based on tetrazolium salt 3′-{1-[(phenylamino)-carbonyl]-3,4-tetrazolium}-bis(4-methoxy-6-nitro)benzenesulfonic acid hydrate reduction by xanthine-xanthine oxidase. Anal. Biochem. 1997, 251, 206–209. [Google Scholar]
- Thompson, R.; Baroneiii, G.; Halsall, H.; Heineman, W. Comparison of methods for following alkaline phosphatase catalysis: Spectrophotometric versus amperometric detection. Anal. Biochem. 1991, 192, 90–95. [Google Scholar]
- Jiao, K.; Sun, W.; Wang, H.Y. A new voltammetric enzyme immunoassay system for the detection of alkaline phosphatase. Chin. Chem. Lett. 2002, 13, 69–70. [Google Scholar]
- Ho, W.O.; Athey, D.; McNeil, C.J. Amperometric detection of alkaline phosphatase activity at a horseradish peroxidase enzyme electrode based on activated carbon: potential application to electrochemical immunoassay. Biosens. Bioelectron. 1995, 10, 683–691. [Google Scholar]
- Nistor, C.; Emneus, J.; Gorton, L.; Ciucu, A. Improved stability and altered selectivity of tyrosinase based graphite electrodes for detection of phenolic compounds. Anal. Chim. Acta 1999, 387, 309–326. [Google Scholar]
- Gouda, M.D.; Kumar, M.A.; Thakur, M.S.; Karanth, N.G. Enhancement of operational stability of an enzyme biosensor for glucose and sucrose using protein based stabilizing agents. Biosens. Bioelectron. 2002, 17, 503–507. [Google Scholar]
- Guntupalli, R.; Lakshmanan, R.; Wan, J.; Kim, D.J.; Huang, T.; Vodyanoy, V.; Chin, B. Analytical performance and characterization of antibody immobilized magnetoelastic biosensors. Sens. Instrum Food Qual. Saf. 2008, 2, 27–33. [Google Scholar]
- Ito, S.; Yamazaki, S.; Kano, K.; Ikeda, T. Highly sensitive electrochemical detection of alkaline phosphatase. Anal. Chim. Acta 2000, 424, 57–63. [Google Scholar]
- Bauer, C.G.; Eremenko, A.V.; Ehrentreich-Forster, E.; Bier, F.F.; Makower, A.; Halsall, H.B.; Heineman, W.R.; Scheller, F.W. Zeptomole-detecting biosensor for alkaline phosphatase in an electrochemical immunoassay for 2,4-dichlorophenoxyacetic acid. Anal. Chem. 1996, 68, 2453–2458. [Google Scholar]
- Fang, L.; Wang, S.H.; Liu, C.C. An electrochemical biosensor of the ketone 3-[beta]-hydroxybutyrate for potential diabetic patient management. Sens. Actuat. B 2008, 129, 818–825. [Google Scholar]
- Shen, J.; Dudik, L.; Liu, C.C. An iridium nanoparticles dispersed carbon based thick film electrochemical biosensor and its application for a single use, disposable glucose biosensor. Sens. Actuat. B 2007, 125, 106–113. [Google Scholar]
- Bartling, B.; Li, L.; Liu, C.C. Determination of total bile acid levels using a thick-film screen-printed Ir/C sensor for the detection of liver disease. Analyst 2009, 134, 973–979. [Google Scholar]
- Liao, W.Y.; Liu, C.C.; Wang, C. Detection of lipoprotein-associated phospholipase A2 using a nano-iridium particle catalyst-based biosensor. Sens. Actuat. B 2008, 134, 993–999. [Google Scholar]
- Bishop, M.L.; Duben-Engelkirk, J.L.; Fody, E.P. Clinical Chemistry: Principles, Procedures, Correlations; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2000. [Google Scholar]







© 2009 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Wang, J.H.; Wang, K.; Bartling, B.; Liu, C.-C. The Detection of Alkaline Phosphatase Using an Electrochemical Biosensor in a Single-Step Approach. Sensors 2009, 9, 8709-8721. https://doi.org/10.3390/s91108709
Wang JH, Wang K, Bartling B, Liu C-C. The Detection of Alkaline Phosphatase Using an Electrochemical Biosensor in a Single-Step Approach. Sensors. 2009; 9(11):8709-8721. https://doi.org/10.3390/s91108709
Chicago/Turabian StyleWang, Joanne H., Kevin Wang, Brandon Bartling, and Chung-Chiun Liu. 2009. "The Detection of Alkaline Phosphatase Using an Electrochemical Biosensor in a Single-Step Approach" Sensors 9, no. 11: 8709-8721. https://doi.org/10.3390/s91108709
APA StyleWang, J. H., Wang, K., Bartling, B., & Liu, C. -C. (2009). The Detection of Alkaline Phosphatase Using an Electrochemical Biosensor in a Single-Step Approach. Sensors, 9(11), 8709-8721. https://doi.org/10.3390/s91108709