Synthesis, Antiproliferative Activity, and DNA Binding Studies of Nucleoamino Acid-Containing Pt(II) Complexes
Abstract
:1. Introduction
2. Results and Discussion
2.1. Preparation of the Nucleoamino Acid Ligand
2.2. Platination of the DAP(T)-Based Nucleoamino Acid
2.3. UV-Vis Analysis of T1 and T2 Platinum Complexes in Aqueous Solution
2.4. NMR Analysis of T1 and T2 Platinum Complexes in Aqueous Solution
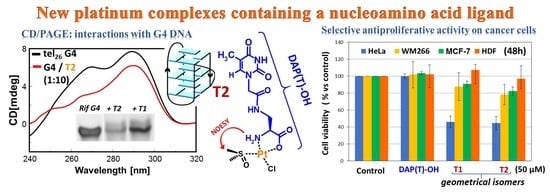
2.5. Antiproliferative Activity of the T1 and T2 Platinum Complexes
2.6. CD-Monitored Binding Experiments of the New Platinum Complexes with DNA Model Systems
2.7. PAGE-Monitored Binding Experiments of the New Platinum Complexes with DNA Model Systems
3. Materials and Methods
3.1. Reagents, Instruments, and General Methods
3.2. Synthesis of the DAP(T)–OH Nucleoaminoacid
3.2.1. Fmoc-DAP(T)–OH, (S)-2-[(9H-fluoren-9-yl)methoxycarbonylamino]-3-{2-[5-methyl-2,4-dioxo-3,4-dihydropyrimidin-1(2H)-yl]acetamido} propanoic acid (2)
3.2.2. DAP(T)–OH, (S)-2-amino-3-{2-[5-methyl-2,4-dioxo-3,4-dihydropyrimidin-1(2H)-yl]acetamido} propanoic acid (4)
3.3. Platination Reaction of DAP(T)–OH
3.3.1. T1 Characterization
3.3.2. T2 Characterization
3.4. MTT Assay
3.4.1. Cell Cultures
3.4.2. Cell Proliferation Experiments
3.5. UV-Vis Experiments
3.6. CD Experiments
3.7. PAGE Experiments
4. Conclusions
Supplementary Materials
Author Contributions
Funding

Conflicts of Interest
Abbreviations
References
- Dasari, S.; Bernard Tchounwou, P. Cisplatin in cancer therapy: Molecular mechanisms of action. Eur. J. Pharmacol. 2014, 740, 364–378. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Makovec, T. Cisplatin and beyond: Molecular mechanisms of action and drug resistance development in cancer chemotherapy. Radiol. Oncol. 2019, 53, 148–158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wexselblatt, E.; Yavin, E.; Gibson, D. Cellular interactions of platinum drugs. Inorg. Chim. Acta 2012, 393, 75–83. [Google Scholar] [CrossRef]
- Gąsior-Głogowska, M.; Malek, K.; Zajac, G.; Baranska, M. A new insight into the interaction of cisplatin with DNA: ROA spectroscopic studies on the therapeutic effect of the drug. Analyst 2016, 141, 291–296. [Google Scholar] [CrossRef]
- Mügge, C.; Liu, R.; Görls, H.; Gabbiani, C.; Michelucci, E.; Rüdiger, N.; Clement, J.H.; Messori, L.; Weigand, W. Novel platinum(II) compounds with O,S bidentate ligands: Synthesis, characterization, antiproliferative properties and biomolecular interactions. Dalton Trans. 2014, 43, 3072–3086. [Google Scholar] [CrossRef]
- Hardie, M.; Kava, H.; Murray, V. Cisplatin analogues with an increased interaction with DNA: Prospects for therapy. Curr. Pharm. Des. 2016, 22, 6645–6664. [Google Scholar] [CrossRef]
- Cucciolito, M.E.; D’Amora, A.; De Feo, G.; Ferraro, G.; Giorgio, A.; Petruk, G.; Monti, D.M.; Merlino, A.; Ruffo, F. Five-coordinate Platinum(II) compounds containing sugar ligands: Synthesis, characterization, cytotoxic activity, and interaction with biological macromolecules. Inorg. Chem. 2018, 57, 3133–3143. [Google Scholar] [CrossRef]
- Iakovidis, A.; Hadjiliadis, N. Complex compounds of platinum (II) and (IV) with amino acids, peptides and their derivatives. Coord. Chem. Rev. 1994, 135–136, 17–63. [Google Scholar] [CrossRef]
- Liu, R.; Li, H.; Gao, X.; Mi, Q.; Zhao, H.; Gao, Q. Mannose-conjugated platinum complexes reveals effective tumor targeting mediated by glucose transporter 1. Biochem. Biophys. Res. Commun. 2017, 487, 34–40. [Google Scholar] [CrossRef]
- Sengupta, P.; Basu, S.; Soni, S.; Pandey, A.; Roy, B.; Oh, M.S.; Chin, K.T.; Paraskar, A.S.; Sarangi, S.; Connor, Y.; et al. Cholesterol-tethered platinum II-based supramolecular nanoparticle increases antitumor efficacy and reduces nephrotoxicity. PNAS 2012, 109, 11294–11299. [Google Scholar] [CrossRef] [Green Version]
- Ratzon, E.; Najajreh, Y.; Salem, R.; Khamaisie, H.; Ruthardt, M.; Mahajna, J. Platinum (IV)-fatty acid conjugates overcome inherently and acquired Cisplatin resistant cancer cell lines: An in-vitro study. BMC Cancer 2016, 16, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Riccardi, C.; Capasso, D.; Rozza, G.M.; Platella, C.; Montesarchio, D.; Di Gaetano, S.; Marzo, T.; Pratesi, A.; Messori, L.; Roviello, G.N.; et al. Synthesis, DNA binding studies, and antiproliferative activity of novel Pt(II)-complexes with an L-alanyl-based ligand. J. Inorg. Biochem. 2020, 203, 110868. [Google Scholar] [CrossRef] [PubMed]
- D’Errico, S.; Oliviero, G.; Piccialli, V.; Amato, J.; Borbone, N.; D’Atri, V.; D’Alessio, F.; Di Noto, R.; Ruffo, F.; Salvatore, F.; et al. Solid-phase synthesis and pharmacological evaluation of novel nucleoside-tethered dinuclear platinum(II) complexes. Bioorg. Med. Chem. 2011, 21, 5835–5838. [Google Scholar] [CrossRef] [PubMed]
- D’Errico, S.; Oliviero, G.; Borbone, N.; Piccialli, V.; Pinto, B.; De Falco, F.; Maiuri, M.C.; Carnuccio, R.; Costantino, V.; Nici, F.; et al. Synthesis and pharmacological evaluation of modified adenosines joined to mono-functional platinum moieties. Molecules 2014, 19, 9339–9353. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.; Li, K.; Swavey, S.; Church, K.M. Synthesis, characterization and DNA binding activity of PtCl2[DMSO][N4[N-3(4-pyridylmethyl)thymidine]]. Inorg. Chim. Acta 2016, 444, 76–80. [Google Scholar] [CrossRef]
- Cincinelli, R.; Musso, L.; Dallavalle, S.; Artali, R.; Tinelli, S.; Colangelo, D.; Zunino, F.; De Cesare, M.; Beretta, G.L.; Zaffaroni, N. Design, modeling, synthesis and biological activity evaluation of camptothecin-linked platinum anticancer agents. Eur. J. Med. Chem. 2013, 63, 387–400. [Google Scholar] [CrossRef]
- Pastor-Anglada, M.; Pérez-Torras, S. Nucleoside transporter proteins as biomarkers of drug responsiveness and drug targets. Front. Pharmacol. 2015, 6, 1–14. [Google Scholar] [CrossRef]
- Inoue, K. Molecular basis of nucleobase transport systems in mammals. Biol. Pharm. Bull. 2017, 40, 1130–1138. [Google Scholar] [CrossRef] [Green Version]
- Jin, V.X.; Ranford, J.D. Complexes of platinum(II) or palladium(II) with 1,10-phenanthroline and amino acids. Inorg. Chim. Acta 2000, 304, 38–44. [Google Scholar] [CrossRef]
- Krylova, L.F.; Kovtunova, L.M.; Romanenko, G.V. Pt(II) and Pd(II) complexes with β-alanine. Bioinorg. Chem. Appl. 2008, 2008, 983725. [Google Scholar] [CrossRef] [Green Version]
- Moradell, S.; Lorenzo, J.; Rovira, A.; Robillard, M.S.; Avilés, F.X.; Moreno, V.; De Llorens, R.; Martinez, M.A.; Reedijk, J.; Llobet, A. Platinum complexes of diaminocarboxylic acids and their ethyl ester derivatives: The effect of the chelate ring size on antitumor activity and interactions with GMP and DNA. J. Inorg. Biochem. 2003, 96, 493–502. [Google Scholar] [CrossRef]
- Altman, J.; Wilchek, M.; Warshawsky, A. Platinum(II) complexes with 2,4-diaminobutyric acid, ornithine, lysine and 4,5-diaminovaleric acid. Inorg. Chim. Acta 1985, 107, 165–168. [Google Scholar] [CrossRef]
- Ziegler, C.J.; Sandman, K.E.; Liang, C.H.; Lippard, S.J. Toxicity of platinum(II) amino acid (N,O) complexes parallels their binding to DNA as measured in a new solid phase assay involving a fluorescent HMG1 protein construct readout. J. Biol. Inorg. Chem. 1999, 4, 402–411. [Google Scholar] [CrossRef] [PubMed]
- Moradell, S.; Lorenzo, J.; Rovira, A.; Van Zutphen, S.; Avilés, F.X.; Moreno, V.; De Llorens, R.; Martinez, M.A.; Reedijk, J.; Llobet, A. Water-soluble platinum(II) complexes of diamine chelating ligands bearing amino-acid type substituents: The effect of the linked amino acid and the diamine chelate ring size on antitumor activity, and interactions with 5′-GMP and DNA. J. Inorg. Biochem. 2004, 98, 1933–1946. [Google Scholar] [CrossRef] [PubMed]
- Roviello, G.N.; Musumeci, D.; Moccia, M.; Castiglione, M.; Sapio, R.; Valente, M.; Bucci, E.M.; Perretta, G.; Pedone, C. dabPNA: Design, synthesis, and DNA binding studies. Nucleosides Nucleotides Nucleic Acids 2007, 26, 1307–1310. [Google Scholar] [CrossRef]
- Roviello, G.N.; Musumeci, D.; Pedone, C.; Bucci, E.M. Synthesis, characterization and hybridization studies of an alternate nucleo-ε/γ-peptide: Complexes formation with natural nucleic acids. Amino Acids 2010, 38, 103–111. [Google Scholar] [CrossRef]
- Roviello, G.N.; Musumeci, D.; Bucci, E.M.; Pedone, C. Synthesis of a diaminopropanoic acid-based nucleoamino acid and assembly of cationic nucleopeptides for biomedical applications. Amino Acids 2012, 43, 2537–2543. [Google Scholar] [CrossRef]
- Roviello, G.N.; Musumeci, D.; D’Alessandro, C.; Pedone, C. Synthesis of a thymine-functionalized nucleoamino acid for the solid phase assembly of cationic nucleopeptides. Amino Acids 2013, 45, 779–784. [Google Scholar] [CrossRef]
- Roviello, G.N.; Musumeci, D. Synthetic approaches to nucleopeptides containing all four nucleobases, and nucleic acid-binding studies on a mixed-sequence nucleo-oligolysine. RSC Adv. 2016, 6, 63578–63585. [Google Scholar] [CrossRef] [Green Version]
- Musumeci, D.; Roviello, V.; Roviello, G.N. DNA- and RNA-binding ability of oligoDapt, a nucleobase-decorated peptide, for biomedical applications. Int. J. Nanomed. 2018, 13, 2613–2629. [Google Scholar] [CrossRef] [Green Version]
- Musumeci, D.; Mokhir, A.; Roviello, G.N. Synthesis and nucleic acid binding evaluation of a thyminyl L-diaminobutanoic acid-based nucleopeptide. Bioorg. Chem. 2020, 100, 103862. [Google Scholar] [CrossRef] [PubMed]
- D’Errico, S.; Borbone, N.; Piccialli, V.; Di Gennaro, E.; Zotti, A.; Budillon, A.; Vitagliano, C.; Piccialli, I.; Oliviero, G. Synthesis and evaluation of the antitumor properties of a small collection of PtII complexes with 7-deazaadenosine as scaffold. Eur. J. Org. Chem. 2017, 2017, 4935–4947. [Google Scholar] [CrossRef]
- Musumeci, D.; Roviello, G.N.; Valente, M.; Sapio, R.; Pedone, C.; Bucci, E.M. New synthesis of PNA-3′DNA linker monomers, useful building blocks to obtain PNA/DNA chimeras. Biopolym. Pept. Sci. Sect. 2004, 76, 535–542. [Google Scholar] [CrossRef] [PubMed]
- Avitabile, C.; Moggio, L.; Malgieri, G.; Capasso, D.; Di Gaetano, S.; Saviano, M.; Pedone, C.; Romanelli, A. γ sulphate PNA (PNA S): Highly selective DNA binding molecule showing promising antigene activity. PLoS ONE 2012, 7, e35774. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, T.J. MOLView: A program for analyzing and displaying atomic structures on the Macintosh personal computer. J. Mol. Graph. 1995, 13, 122–125. [Google Scholar] [CrossRef]
- Halgren, T.A. Merck molecular force field. I. Basis, form, scope, parameterization, and performance of MMFF94. J. Comput. Chem. 1996, 17, 490–519. [Google Scholar] [CrossRef]
- Cavaluzzi, M.J.; Borer, P.N. Revised UV extinction coefficients for nucleoside-5′-monophosphates and unpaired DNA and RNA. Nucleic Acids Res. 2004, 32, e13. [Google Scholar] [CrossRef] [Green Version]
- Musumeci, D.; Roviello, G.N.; Rigione, G.; Capasso, D.; Di Gaetano, S.; Riccardi, C.; Roviello, V.; Montesarchio, D. Benzodifuran derivatives as potential antiproliferative agents: Possible correlation between their bioactivity and aggregation properties. ChemPlusChem 2017, 82, 251–260. [Google Scholar] [CrossRef]
- Vicidomini, C.; Cioffi, F.; Broersen, K.; Roviello, V.; Riccardi, C.; Montesarchio, D.; Capasso, D.; Di Gaetano, S.; Roviello, G.N. Benzodifurans for biomedical applications: BZ4, a selective anti-proliferative and anti-amyloid lead compound. Future Med. Chem. 2019, 11, 285–302. [Google Scholar] [CrossRef]
- Platella, C.; Musumeci, D.; Arciello, A.; Doria, F.; Freccero, M.; Randazzo, A.; Amato, J.; Pagano, B.; Montesarchio, D. Controlled pore glass-based oligonucleotide affinity support: Towards high throughput screening methods for the identification of conformation-selective G-quadruplex ligands. Anal. Chim. Acta 2018, 1030, 133–141. [Google Scholar] [CrossRef]
- Platella, C.; Pirota, V.; Musumeci, D.; Rizzi, F.; Iachettini, S.; Zizza, P.; Biroccio, A.; Freccero, M.; Montesarchio, D.; Doria, F. Trifunctionalized naphthalene diimides and dimeric analogues as G-quadruplex-targeting anticancer agents selected by affinity chromatography. Int. J. Mol. Sci. 2020, 21, 1964. [Google Scholar] [CrossRef] [Green Version]
- Lipps, H.J.; Rhodes, D. G-quadruplex structures: In vivo evidence and function. Trends Cell Biol. 2009, 19, 414–422. [Google Scholar] [CrossRef] [PubMed]
- Balasubramanian, S.; Hurley, L.H.; Neidle, S. Targeting G-quadruplexes in gene promoters: A novel anticancer strategy? Nat. Rev. Drug Discov. 2011, 10, 261–275. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marzano, M.; Falanga, A.P.; Marasco, D.; Borbone, N.; D’Errico, S.; Piccialli, G.; Roviello, G.N.; Oliviero, G. Evaluation of an analogue of the marine ε-PLL peptide as a ligand of G-quadruplex DNA structures. Mar. Drugs 2020, 18, 49. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Petraccone, L.; Spink, C.; Trent, J.O.; Garbett, N.C.; Mekmaysy, C.S.; Giancola, C.; Chaires, J.B. Structure and stability of higher-order human telomeric quadruplexes. J. Am. Chem. Soc. 2011, 133, 20951–20961. [Google Scholar] [CrossRef] [Green Version]
- Petraccone, L.; Pagano, B.; Giancola, C. Studying the effect of crowding and dehydration on DNA G-quadruplexes. Methods 2012, 57, 76–83. [Google Scholar] [CrossRef]
- Musumeci, D.; Platella, C.; Riccardi, C.; Merlino, A.; Marzo, T.; Massai, L.; Messori, L.; Montesarchio, D. A first-in-class and a fished out anticancer platinum compound: Cis-[PtCl2(NH3)2] and cis-[PtI2(NH3)2] compared for their reactivity towards DNA model systems. Dalton Trans. 2016, 45, 8587–8600. [Google Scholar] [CrossRef]
- Mügge, C.; Musumeci, D.; Michelucci, E.; Porru, F.; Marzo, T.; Massai, L.; Messori, L.; Weigand, W.; Montesarchio, D. Elucidating the reactivity of Pt(II) complexes with (O,S) bidentate ligands towards DNA model systems. J. Inorg. Biochem. 2016, 160, 198–209. [Google Scholar] [CrossRef]
- Farina, B.; De Paola, I.; Russo, L.; Capasso, D.; Liguoro, A.; Del Gatto, A.; Saviano, M.; Pedone, P.V.; Di Gaetano, S.; Malgieri, G.; et al. A combined NMR and computational approach to determine the RGDechi-hCit-αvβ3 integrin recognition mode in isolated cell membranes. Chemistry 2016, 22, 681–693. [Google Scholar] [CrossRef]
- D’Errico, S.; Falanga, A.P.; Capasso, D.; Di Gaetano, S.; Marzano, M.; Terracciano, M.; Roviello, G.N.; Piccialli, G.; Oliviero, G.; Borbone, N. Probing the DNA reactivity and the anticancer properties of a novel tubercidin-Pt (II) complex. Pharmaceutics 2020, 12, 627. [Google Scholar] [CrossRef]
- Di Gaetano, S.; Bedini, E.; Landolfi, A.; Pedone, E.; Pirone, L.; Saviano, M.; Traboni, S.; Capasso, D.; Iadonisi, A. Synthesis of diglycosylated (di)sulfides and comparative evaluation of their antiproliferative effect against tumor cell lines: A focus on the nature of sugar-recognizing mediators involved. Carbohydr. Res. 2019, 482, 107740. [Google Scholar] [CrossRef] [PubMed]
- Musumeci, D.; Rozza, L.; Merlino, A.; Paduano, L.; Marzo, T.; Massai, L.; Messori, L.; Montesarchio, D. Interaction of anticancer Ru(III) complexes with single stranded and duplex DNA model systems. Dalton Trans. 2015, 44, 13914–13925. [Google Scholar] [CrossRef] [PubMed]
- Riccardi, C.; Musumeci, D.; Trifuoggi, M.; Irace, C.; Paduano, L.; Montesarchio, D. Anticancer ruthenium (III) complexes and Ru(III) containing nanoformulations: An update on the mechanism of action and biological activity. Pharmaceuticals 2019, 12, 146. [Google Scholar] [CrossRef] [PubMed] [Green Version]







| 1H-NMR | 13C-NMR | ||||||
|---|---|---|---|---|---|---|---|
| ppm | DAP(T)–OH | T1 | T2 | ppm | DAP(T)–OH | T1 | T2 |
| CH3-5(T) | 1.75 | 1.75 | 1.75 | CH3-5(T) | 11.87 | 11.93/11.91 | 11.94 |
| H-3 (T) | 11.25 | 11.29/11.28 | 11.30 | C-2 (T) | 151.05 | 151.05 | 151.07 |
| H-6 (T) | 7.41 | 7.44/7.43 | 7.43 | C-4 (T) | 164.36 | 164.44/164.42 | 164.47 |
| A | 4.31 | 4.35 | 4.34 | C-5 (T) | 108.01 | 108.10/107.98 | 108.16 |
| C | 8.35 | 8.58/8.48 | 8.43 | C-6 (T) | 142.31 | 142.24 | 142.23 |
| D1 | 3.53 | 3.64/3.56 | 3.55 | A | 49.16 | 49.22 | 49.27 |
| D2 | 3.33 | 3.49/3.28 | 3.29 | B | 167.77 | 168.86/168.31 | 168.44 |
| E | 3.27 | 3.90/3.52 | 3.51 | D | 39.62 | 41.66/38.58 | 41.69 |
| G1 | - | 6.29 | 6.25 | E | 54.04 | 56.78/52.40 | 56.90 |
| G2 | - | 5.68 | 5.63 | F | 168.82 | 181.33 | 181.38 |
| CH3 (DMSO-Pt) | 3.16 | 3.16 | CH3 (DMSO-Pt) | 49.02 | 48.61 | ||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Riccardi, C.; Capasso, D.; Coppola, A.; Platella, C.; Montesarchio, D.; Di Gaetano, S.; Roviello, G.N.; Musumeci, D. Synthesis, Antiproliferative Activity, and DNA Binding Studies of Nucleoamino Acid-Containing Pt(II) Complexes. Pharmaceuticals 2020, 13, 284. https://doi.org/10.3390/ph13100284
Riccardi C, Capasso D, Coppola A, Platella C, Montesarchio D, Di Gaetano S, Roviello GN, Musumeci D. Synthesis, Antiproliferative Activity, and DNA Binding Studies of Nucleoamino Acid-Containing Pt(II) Complexes. Pharmaceuticals. 2020; 13(10):284. https://doi.org/10.3390/ph13100284
Chicago/Turabian StyleRiccardi, Claudia, Domenica Capasso, Angela Coppola, Chiara Platella, Daniela Montesarchio, Sonia Di Gaetano, Giovanni N. Roviello, and Domenica Musumeci. 2020. "Synthesis, Antiproliferative Activity, and DNA Binding Studies of Nucleoamino Acid-Containing Pt(II) Complexes" Pharmaceuticals 13, no. 10: 284. https://doi.org/10.3390/ph13100284
APA StyleRiccardi, C., Capasso, D., Coppola, A., Platella, C., Montesarchio, D., Di Gaetano, S., Roviello, G. N., & Musumeci, D. (2020). Synthesis, Antiproliferative Activity, and DNA Binding Studies of Nucleoamino Acid-Containing Pt(II) Complexes. Pharmaceuticals, 13(10), 284. https://doi.org/10.3390/ph13100284