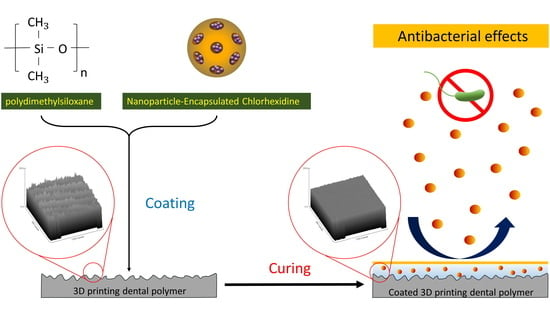
Antibacterial Drug-Release Polydimethylsiloxane Coating for 3D-Printing Dental Polymer: Surface Alterations and Antimicrobial Effects
Abstract
:1. Introduction
2. Results
2.1. Surface Characteristics
2.2. Antimicrobial Activity
3. Discussions
4. Materials and Methods
4.1. Synthesis of the Coating Material (CHX@MSN-Loaded PDMS)
4.2. Coating Procedure
4.3. Evaluation of Surface Microstructure
4.4. Measurement of Surface Wettability
4.5. Assessment of Antimicrobial Activities
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Stansbury, J.W.; Idacavage, M.J. 3D printing with polymers: Challenges among expanding options and opportunities. Dent. Mater. 2016, 32, 54–64. [Google Scholar] [CrossRef] [PubMed]
- Eleftheriadis, G.; Monou, P.K.; Andriotis, E.; Mitsouli, E.; Moutafidou, N.; Markopoulou, C.; Bouropoulos, N.; Fatouros, D. Development and Characterization of Inkjet Printed Edible Films for Buccal Delivery of B-Complex Vitamins. Pharmaceuticals 2020, 13, 203. [Google Scholar] [CrossRef] [PubMed]
- Tahayeri, A.; Morgan, M.; Fugolin, A.P.; Bompolaki, D.; Athirasala, A.; Pfeifer, C.S.; Ferracane, J.L.; Bertassoni, L.E. 3D printed versus conventionally cured provisional crown and bridge dental materials. Dent. Mater. 2018, 34, 192–200. [Google Scholar] [CrossRef] [PubMed]
- Tappa, K.; Jammalamadaka, U. Novel Biomaterials Used in Medical 3D Printing Techniques. J. Funct. Biomater. 2018, 9, 17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saratti, C.M.; Rocca, G.T.; Krejci, I. The potential of three-dimensional printing technologies to unlock the development of new ‘bio-inspired’ dental materials: An overview and research roadmap. J. Prosthodont. Res. 2019, 63, 131–139. [Google Scholar] [CrossRef]
- Park, J.-M.; Ahn, J.-S.; Cha, H.-S.; Lee, J.-H. Wear Resistance of 3D Printing Resin Material Opposing Zirconia and Metal Antagonists. Materials 2018, 11, 1043. [Google Scholar] [CrossRef] [Green Version]
- Mai, H.N.; Lee, K.B.; Lee, D.H. Fit of interim crowns fabricated using photopolymer-jetting 3D printing. J. Prosthet. Dent. 2017, 118, 208–215. [Google Scholar] [CrossRef]
- Boaro, L.C.C.; Campos, L.M.; Varca, G.H.C.; dos Santos, T.M.R.; Marques, P.A.; Sugii, M.M.; Saldanha, N.R.; Cogo-Müller, K.; Brandt, W.C.; Braga, R.R. Antibacterial resin-based composite containing chlorhexidine for dental applications. Dent. Mater. 2019, 35, 909–918. [Google Scholar] [CrossRef]
- Belter, J.T.; Dollar, A.M. Strengthening of 3D printed fused deposition manufactured parts using the fill compositing technique. PLoS ONE 2015, 10, e0122915. [Google Scholar] [CrossRef]
- Gonzalez-Henriquez, C.M.; Sarabia-Vallejos, M.A.; Hernandez, J.R. Antimicrobial Polymers for Additive Manufacturing. Int. J. Mol. Sci. 2019, 20, 1210. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Wu, R.; Fan, Y.; Liao, S.; Wang, Y.; Wen, Z.; Xu, X. Antibacterial dental composites with chlorhexidine and mesoporous silica. J. Dent. Res. 2014, 93, 1283–1289. [Google Scholar] [CrossRef]
- Yan, H.; Yang, H.; Li, K.; Yu, J.; Huang, C. Effects of Chlorhexidine-Encapsulated Mesoporous Silica Nanoparticles on the Anti-Biofilm and Mechanical Properties of Glass Ionomer Cement. Molecules 2017, 22, 1225. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Strydonck, D.A.; Slot, D.E.; Van der Velden, U.; Van der Weijden, F. Effect of a chlorhexidine mouthrinse on plaque, gingival inflammation and staining in gingivitis patients: A systematic review. J. Clin. Periodontol. 2012, 39, 1042–1055. [Google Scholar] [CrossRef] [PubMed]
- Anusavice, K.J.; Zhang, N.Z.; Shen, C. Controlled release of chlorhexidine from UDMA-TEGDMA resin. J. Dent. Res. 2006, 85, 950–954. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Priyadarshini, B.M.; Selvan, S.T.; Lu, T.B.; Xie, H.; Neo, J.; Fawzy, A.S. Chlorhexidine Nanocapsule Drug Delivery Approach to the Resin-Dentin Interface. J. Dent. Res. 2016, 95, 1065–1072. [Google Scholar] [CrossRef] [PubMed]
- Salim, N.; Moore, C.; Silikas, N.; Satterthwaite, J.D.; Rautemaa, R. Fungicidal amounts of antifungals are released from impregnated denture lining material for up to 28 days. J. Dent. 2012, 40, 506–512. [Google Scholar] [CrossRef] [PubMed]
- Al-Haddad, A.; Roudsari, R.V.; Satterthwaite, J.D. Fracture toughness of heat cured denture base acrylic resin modified with Chlorhexidine and Fluconazole as bioactive compounds. J. Dent. 2014, 42, 180–184. [Google Scholar] [CrossRef] [PubMed]
- Alghazzawi, T.F. Advancements in CAD/CAM technology: Options for practical implementation. J. Prosthodont. Res. 2016, 60, 72–84. [Google Scholar] [CrossRef]
- Azuma, A.; Akiba, N.; Minakuchi, S. Hydrophilic surface modification of acrylic denture base material by silica coating and its influence on Candida albicans adherence. J. Med. Dent. Sci. 2012, 59, 1–7. [Google Scholar]
- Fukunishi, M.; Inoue, Y.; Morisaki, H.; Kuwata, H.; Ishihara, K.; Baba, K. A Polymethyl Methacrylate-Based Acrylic Dental Resin Surface Bound with a Photoreactive Polymer Inhibits Accumulation of Bacterial Plaque. Int. J. Prosthodont. 2017, 30, 533–540. [Google Scholar] [CrossRef] [Green Version]
- Rokaya, D.; Srimaneepong, V.; Sapkota, J.; Qin, J.; Siraleartmukul, K.; Siriwongrungson, V. Polymeric materials and films in dentistry: An overview. J. Adv. Res. 2018, 14, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Cloutier, M.; Mantovani, D.; Rosei, F. Antibacterial Coatings: Challenges, Perspectives, and Opportunities. Trends Biotechnol. 2015, 33, 637–652. [Google Scholar] [CrossRef]
- Zhu, D.; Handschuh-Wang, S.; Zhou, X. Recent progress in fabrication and application of polydimethylsiloxane sponges. J. Mater. Chem. A 2017, 5, 16467–16497. [Google Scholar] [CrossRef]
- Raghu, P.K.; Bansal, K.K.; Thakor, P.; Bhavana, V.; Madan, J.; Rosenholm, J.M.; Mehra, N.K. Evolution of Nanotechnology in Delivering Drugs to Eyes, Skin and Wounds via Topical Route. Pharmaceuticals 2020, 13, 167. [Google Scholar] [CrossRef]
- Si, J.; Cui, Z.; Xie, P.; Song, L.; Wang, Q.; Liu, Q.; Liu, C. Characterization of 3D elastic porous polydimethylsiloxane (PDMS) cell scaffolds fabricated by VARTM and particle leaching. J. Appl. Polym. 2016, 133. [Google Scholar] [CrossRef]
- Palchesko, R.N.; Zhang, L.; Sun, Y.; Feinberg, A.W. Development of polydimethylsiloxane substrates with tunable elastic modulus to study cell mechanobiology in muscle and nerve. PLoS ONE 2012, 7, e51499. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hyun, D.C.; Jeong, U. Substrate thickness: An effective control parameter for polymer thin film buckling on PDMS substrates. J. Appl. Polym. Sci. 2009, 112, 2683–2690. [Google Scholar] [CrossRef]
- Yoshida, S.; Hagiwara, K.; Hasebe, T.; Hotta, A. Surface modification of polymers by plasma treatments for the enhancement of biocompatibility and controlled drug release. Surf. Coat. Technol. 2013, 233, 99–107. [Google Scholar] [CrossRef]
- Zhang, H.; Chiao, M. Anti-fouling coatings of poly (dimethylsiloxane) devices for biological and biomedical applications. J. Med. Biol. Eng. 2015, 35, 143–155. [Google Scholar] [CrossRef] [Green Version]
- Fornell, A.-C.; Sköld-Larsson, K.; Hallgren, A.; Bergstrand, F.; Twetman, S. Effect of a hydrophobic tooth coating on gingival health, mutans streptococci, and enamel demineralization in adolescents with fixed orthodontic appliances. Acta Odontol. Scand. 2002, 60, 37–41. [Google Scholar] [CrossRef]
- Aguayo, S.; Marshall, H.; Pratten, J.; Bradshaw, D.; Brown, J.S.; Porter, S.R.; Spratt, D.; Bozec, L. Early Adhesion of Candida albicans onto Dental Acrylic Surfaces. J. Dent. Res. 2017, 96, 917–923. [Google Scholar] [CrossRef] [PubMed]
- Mai, H.-N.; Hong, S.-H.; Kim, S.-H.; Lee, D.-H. Effects of different finishing/polishing protocols and systems for monolithic zirconia on surface topography, phase transformation, and biofilm formation. J. Adv. Prosthodont. 2019, 11, 81–87. [Google Scholar] [CrossRef] [PubMed]
- Erdemir, U.; Sancakli, H.S.; Yildiz, E. The effect of one-step and multi-step polishing systems on the surface roughness and microhardness of novel resin composites. Eur. J. Dent. 2012, 6, 198. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jefferies, S.R. Abrasive finishing and polishing in restorative dentistry: A state-of-the-art review. Dent. Clin. N. Am. 2007, 51, 379–397. [Google Scholar] [CrossRef]
- Rashid, H. The effect of surface roughness on ceramics used in dentistry: A review of literature. Eur. J. Dent. 2014, 8, 571. [Google Scholar] [CrossRef] [PubMed]
- Salamanca, C.H.; Yarce, C.J.; Roman, Y.; Davalos, A.F.; Rivera, G.R. Application of nanoparticle technology to reduce the anti-microbial resistance through β-lactam antibiotic-polymer inclusion nano-complex. Pharmaceuticals 2018, 11, 19. [Google Scholar] [CrossRef] [Green Version]
- Pereni, C.I.; Zhao, Q.; Liu, Y.; Abel, E. Surface free energy effect on bacterial retention. Colloid Surf. B Biointerfaces 2006, 48, 143–147. [Google Scholar] [CrossRef]
- Busscher, H.J.; Weerkamp, A.H.; van der Mei, H.C.; Van Pelt, A.; de Jong, H.P.; Arends, J. Measurement of the surface free energy of bacterial cell surfaces and its relevance for adhesion. Appl. Environ. Microbiol. 1984, 48, 980–983. [Google Scholar] [CrossRef] [Green Version]
- Schrader, M.E. Young-dupre revisited. Langmuir 1995, 11, 3585–3589. [Google Scholar] [CrossRef]
- Ye, X.; Cai, D.; Ruan, X.; Cai, A. Research on the selective adhesion characteristics of polydimethylsiloxane layer. AIP Adv. 2018, 8, 095004. [Google Scholar] [CrossRef] [Green Version]
- Colombo, A.P.V.; Tanner, A.C.R. The Role of Bacterial Biofilms in Dental Caries and Periodontal and Peri-implant Diseases: A Historical Perspective. J. Dent. Res. 2019, 98, 373–385. [Google Scholar] [CrossRef]
- Mai, H.N.; Kim, D.Y.; Hyun, D.C.; Park, J.H.; Lee, S.M.; Lee, D.H. A New Antibacterial Agent-Releasing Polydimethylsiloxane Coating for Polymethyl Methacrylate Dental Restorations. J. Clin. Med. 2019, 8, 1831. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laske, M.; Opdam, N.J.M.; Bronkhorst, E.M.; Braspenning, J.C.C.; Huysmans, M. Risk Factors for Dental Restoration Survival: A Practice-Based Study. J. Dent. Res. 2019, 98, 414–422. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Volinsky, A.A.; Gallant, N.D. Crosslinking effect on polydimethylsiloxane elastic modulus measured by custom-built compression instrument. J. Appl. Polym. Sci. 2014, 131. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Zong, G.; He, L.; Zhang, Y.; Liu, C.; Wang, L. Effects of fumed and mesoporous silica nanoparticles on the properties of sylgard 184 polydimethylsiloxane. Micromachines 2015, 6, 855–864. [Google Scholar] [CrossRef] [Green Version]
- Shim, S.E.; Isayev, A.I. Rheology and structure of precipitated silica and poly (dimethyl siloxane) system. Rheol. Acta 2004, 43, 127–136. [Google Scholar] [CrossRef]
- Banerjee, S.; Yang, R.; Courchene, C.E.; Conners, T.E. Scanning electron microscopy measurements of the surface roughness of paper. Ind. Eng. Chem. Res. 2009, 48, 4322–4325. [Google Scholar] [CrossRef]







| Group | Mean (SD) | ||
|---|---|---|---|
| SRI | CA | p-Value | |
| Noncoated | 39.25 (3.69) a | 120.22 (4.46) a | <0.001 |
| Coated | 15.41 (8.07) b | 91.88 (8.19) b | |
| Component * | Content (%) |
|---|---|
| α,α’-[(1-Methylethylidene)di-4,1-phenylene] bis [ω-[(2-methyl-1-oxo-2-propenyl) oxy] poly (oxy-1,2-ethanediyl) | 20–35 |
| 7,7,9(or 7,9,9)-Trimethyl-4,13-dioxo-3,14-dioxa-5,12-diazahexadecane1,16-diyl 2-methyl-2-propenoate | 20–28 |
| 2-Methyl-2-propenoic acid 1,2-ethanediylbis(oxy-2,1-ethanediyl) ester | 20–25 |
| Phenylbis (2,4,6-trimethylbenzoyl) phosphine oxide | 1–10 |
| Rutile (TiO2) | 0.1–5 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mai, H.-N.; Hyun, D.C.; Park, J.H.; Kim, D.-Y.; Lee, S.M.; Lee, D.-H. Antibacterial Drug-Release Polydimethylsiloxane Coating for 3D-Printing Dental Polymer: Surface Alterations and Antimicrobial Effects. Pharmaceuticals 2020, 13, 304. https://doi.org/10.3390/ph13100304
Mai H-N, Hyun DC, Park JH, Kim D-Y, Lee SM, Lee D-H. Antibacterial Drug-Release Polydimethylsiloxane Coating for 3D-Printing Dental Polymer: Surface Alterations and Antimicrobial Effects. Pharmaceuticals. 2020; 13(10):304. https://doi.org/10.3390/ph13100304
Chicago/Turabian StyleMai, Hang-Nga, Dong Choon Hyun, Ju Hayng Park, Do-Yeon Kim, Sang Min Lee, and Du-Hyeong Lee. 2020. "Antibacterial Drug-Release Polydimethylsiloxane Coating for 3D-Printing Dental Polymer: Surface Alterations and Antimicrobial Effects" Pharmaceuticals 13, no. 10: 304. https://doi.org/10.3390/ph13100304
APA StyleMai, H.-N., Hyun, D. C., Park, J. H., Kim, D.-Y., Lee, S. M., & Lee, D.-H. (2020). Antibacterial Drug-Release Polydimethylsiloxane Coating for 3D-Printing Dental Polymer: Surface Alterations and Antimicrobial Effects. Pharmaceuticals, 13(10), 304. https://doi.org/10.3390/ph13100304