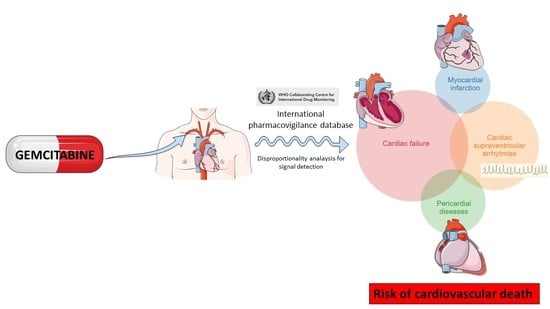
Cardiotoxicity Associated with Gemcitabine: Literature Review and a Pharmacovigilance Study
Abstract
:1. Introduction
2. Results
2.1. Literature Review
2.2. Pharmacovigilance Study
3. Discussion
4. Materials and Methods
4.1. Literature Review
4.2. Pharmacovigilance Study
4.2.1. Study Design and Data Sources
4.2.2. Procedures
4.2.3. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mini, E.; Nobili, S.; Caciagli, B.; Landini, I.; Mazzei, T. Cellular pharmacology of gemcitabine. Ann. Oncol. 2006, 17 (Suppl. S5), v7–v12. [Google Scholar] [CrossRef]
- Eli Lilly and Company. GEMZAR (Gemcitabine) [package insert]. U.S. Food and Drug Administration Website. Revised May 2014. Available online: www.accessdata.fda.gov/drugsatfda_docs/label/2014/020509s077lbl.pdf (accessed on 28 September 2020).
- Humphreys, B.D.; Sharman, J.P.; Henderson, J.M.; Clark, J.W.; Marks, P.W.; Rennke, H.G.; Zhu, A.X.; Magee, C.C. Gemcitabine-associated thrombotic microangiopathy. Cancer 2004, 100, 2664–2670. [Google Scholar] [CrossRef] [PubMed]
- Roychowdhury, D.F.; Cassidy, C.A.; Peterson, P.; Arning, M. A report on serious pulmonary toxicity associated with gemcitabine-based therapy. Investig. New Drugs 2002, 20, 311–315. [Google Scholar] [CrossRef] [PubMed]
- Mertz, P.; Lebrun-Vignes, B.; Salem, J.E.; Arnaud, L. Characterizing drug-induced capillary leak syndromes using the World Health Organization VigiBase. J. Allergy Clin. Immunol. 2019, 143, 433–436. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Agency, E.M. Gemzar. Available online: https://www.ema.europa.eu/en/documents/referral/gemzar-article-30-referral-annex-i-ii-iii_en.pdf (accessed on 28 September 2020).
- Ozturk, B.; Tacoy, G.; Coskun, U.; Yaman, E.; Sahin, G.; Buyukberber, S.; Yildiz, R.; Kaya, A.O.; Topal, S.; Ozdemir, M.; et al. Gemcitabine-induced acute coronary syndrome: A case report. Med. Princ. Pract. 2009, 18, 76–80. [Google Scholar] [CrossRef] [PubMed]
- Bdair, F.M.; Graham, S.P.; Smith, P.F.; Javle, M.M. Gemcitabine and acute myocardial infarction—A case report. Angiology 2006, 57, 367–371. [Google Scholar] [CrossRef]
- Kalapura, T.; Krishnamurthy, M.; Reddy, C.V. Acute myocardial infarction following gemcitabine therapy—A case report. Angiology 1999, 50, 1021–1025. [Google Scholar] [CrossRef]
- Santini, D.; Tonini, G.; Abbate, A.; Di Cosimo, S.; Gravante, G.; Vincenzi, B.; Campisi, C.; Patti, G.; Di Sciascio, G. Gemcitabine-induced atrial fibrillation: A hitherto unreported manifestation of drug toxicity. Ann. Oncol. 2000, 11, 479–481. [Google Scholar] [CrossRef]
- Ferrari, D.; Carbone, C.; Codeca, C.; Fumagalli, L.; Gilardi, L.; Marussi, D.; Tartaro, T.; Oldani, S.; Zannier, F.; Foa, P. Gemcitabine and atrial fibrillation: A rare manifestation of chemotherapy toxicity. Anti-Cancer Drugs 2006, 17, 359–361. [Google Scholar] [CrossRef]
- Tavil, Y.; Arslan, U.; Okyay, K.; Sen, N.; Boyaci, B. Atrial fibrillation induced by gemcitabine treatment in a 65-year-old man. Oncol. Res. Treat. 2007, 30, 253–255. [Google Scholar] [CrossRef] [PubMed]
- Ciotti, R.; Belotti, G.; Facchi, E.; Cantu, A.; D’Amico, A.; Gatti, C. Sudden cardio-pulmonary toxicity following a single infusion of gemcitabine. Ann. Oncol. 1999, 10, 997. [Google Scholar] [CrossRef] [PubMed]
- Tayer-Shifman, O.E.; Rottenberg, Y.; Shuvy, M. Gemcitabine-induced supraventricular tachycardia. Tumori J. 2009, 95, 547–549. [Google Scholar] [CrossRef]
- Khan, M.F.; Gottesman, S.; Boyella, R.; Juneman, E. Gemcitabine-induced cardiomyopathy: A case report and review of the literature. J. Med. Case Rep. 2014, 8, 220. [Google Scholar] [CrossRef] [Green Version]
- Yajima, T.; Furukawa, Y.; Ishii, Y.; Hattori, Y.; Matsumoto, N.; Yamamoto, M.; Yamaoka, Y.; Fujihara, M.; Fujita, M.; Kuniki, H. Two cases of advanced pancreatic cancer responding to gemcitabine with long survival of 2 years. Gan Kagaku Ryoho 2004, 31, 953–957. [Google Scholar]
- Alam, S.; Illo, C.; Ma, Y.T.; Punia, P. Gemcitabine-Induced Cardiotoxicity in Patients Receiving Adjuvant Chemotherapy for Pancreatic Cancer: A Case Series. Case Rep. Oncol. 2018, 11, 221–227. [Google Scholar] [CrossRef] [PubMed]
- Mohebali, D.; Matos, J.; Chang, J.D. Gemcitabine induced cardiomyopathy: A case of multiple hit cardiotoxicity. ESC Heart Fail. 2017, 4, 71–74. [Google Scholar] [CrossRef] [PubMed]
- Vogl, D.T.; Glatstein, E.; Carver, J.R.; Schuster, S.J.; Stadtmauer, E.A.; Luger, S.; Nasta, S.D.; Porter, D.L.; Elstrom, R.; Tsai, D.E. Gemcitabine-induced pericardial effusion and tamponade after unblocked cardiac irradiation. Leuk. Lymphoma 2005, 46, 1313–1320. [Google Scholar] [CrossRef]
- Kido, H.; Morizane, C.; Tamura, T.; Hagihara, A.; Kondo, S.; Ueno, H.; Okusaka, T. Gemcitabine-induced pleuropericardial effusion in a patient with pancreatic cancer. Jpn. J. Clin. Oncol. 2012, 42, 845–850. [Google Scholar] [CrossRef] [Green Version]
- Konstantinopoulos, P.A.; Cheng, S.C.; Hendrickson, A.E.W.; Penson, R.T.; Schumer, S.T.; Doyle, L.A.; Lee, E.K.; Kohn, E.C.; Duska, L.R.; Crispens, M.A.; et al. Berzosertib plus gemcitabine versus gemcitabine alone in platinum-resistant high-grade serous ovarian cancer: A multicentre, open-label, randomised, phase 2 trial. Lancet Oncol. 2020, 21, 957–968. [Google Scholar] [CrossRef]
- Conroy, T.; Hammel, P.; Hebbar, M.; Ben Abdelghani, M.; Wei, A.C.; Raoul, J.L.; Chone, L.; Francois, E.; Artru, P.; Biagi, J.J.; et al. FOLFIRINOX or Gemcitabine as Adjuvant Therapy for Pancreatic Cancer. N. Engl. J. Med. 2018, 379, 2395–2406. [Google Scholar] [CrossRef]
- Melisi, D.; Garcia-Carbonero, R.; Macarulla, T.; Pezet, D.; Deplanque, G.; Fuchs, M.; Trojan, J.; Oettle, H.; Kozloff, M.; Cleverly, A.; et al. Galunisertib plus gemcitabine vs. gemcitabine for first-line treatment of patients with unresectable pancreatic cancer. Br. J. Cancer 2018, 119, 1208–1214. [Google Scholar] [CrossRef] [Green Version]
- Middleton, G.; Palmer, D.H.; Greenhalf, W.; Ghaneh, P.; Jackson, R.; Cox, T.; Evans, A.; Shaw, V.E.; Wadsley, J.; Valle, J.W.; et al. Vandetanib plus gemcitabine versus placebo plus gemcitabine in locally advanced or metastatic pancreatic carcinoma (ViP): A prospective, randomised, double-blind, multicentre phase 2 trial. Lancet Oncol. 2017, 18, 486–499. [Google Scholar] [CrossRef]
- Neoptolemos, J.P.; Palmer, D.H.; Ghaneh, P.; Psarelli, E.E.; Valle, J.W.; Halloran, C.M.; Faluyi, O.; O’Reilly, D.A.; Cunningham, D.; Wadsley, J.; et al. Comparison of adjuvant gemcitabine and capecitabine with gemcitabine monotherapy in patients with resected pancreatic cancer (ESPAC-4): A multicentre, open-label, randomised, phase 3 trial. Lancet 2017, 389, 1011–1024. [Google Scholar] [CrossRef]
- Evans, T.R.J.; Van Cutsem, E.; Moore, M.J.; Bazin, I.S.; Rosemurgy, A.; Bodoky, G.; Deplanque, G.; Harrison, M.; Melichar, B.; Pezet, D.; et al. Phase 2 placebo-controlled, double-blind trial of dasatinib added to gemcitabine for patients with locally-advanced pancreatic cancer. Ann. Oncol. 2017, 28, 354–361. [Google Scholar] [CrossRef]
- Rougier, P.; Riess, H.; Manges, R.; Karasek, P.; Humblet, Y.; Barone, C.; Santoro, A.; Assadourian, S.; Hatteville, L.; Philip, P.A. Randomised, placebo-controlled, double-blind, parallel-group phase III study evaluating aflibercept in patients receiving first-line treatment with gemcitabine for metastatic pancreatic cancer. Eur. J. Cancer 2013, 49, 2633–2642. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, A.; Gilabert, M.; François, E.; Dahan, L.; Perrier, H.; Lamy, R.; Re, D.; Largillier, R.; Gasmi, M.; Tchiknavorian, X.; et al. BAYPAN study: A double-blind phase III randomized trial comparing gemcitabine plus sorafenib and gemcitabine plus placebo in patients with advanced pancreatic cancer. Ann. Oncol. 2012, 23, 2799–2805. [Google Scholar] [CrossRef]
- Loehrer, P.J., Sr.; Feng, Y.; Cardenes, H.; Wagner, L.; Brell, J.M.; Cella, D.; Flynn, P.; Ramanathan, R.K.; Crane, C.H.; Alberts, S.R.; et al. Gemcitabine alone versus gemcitabine plus radiotherapy in patients with locally advanced pancreatic cancer: An Eastern Cooperative Oncology Group trial. J. Clin. Oncol. 2011, 29, 4105–4112. [Google Scholar] [CrossRef]
- Colucci, G.; Labianca, R.; Di Costanzo, F.; Gebbia, V.; Cartenì, G.; Massidda, B.; Dapretto, E.; Manzione, L.; Piazza, E.; Sannicolò, M.; et al. Randomized phase III trial of gemcitabine plus cisplatin compared with single-agent gemcitabine as first-line treatment of patients with advanced pancreatic cancer: The GIP-1 study. J. Clin. Oncol. 2010, 28, 1645–1651. [Google Scholar] [CrossRef]
- Richards, D.A.; Kuefler, P.R.; Becerra, C.; Wilfong, L.S.; Gersh, R.H.; Boehm, K.A.; Zhan, F.; Asmar, L.; Myrand, S.P.; Hozak, R.R.; et al. Gemcitabine plus enzastaurin or single-agent gemcitabine in locally advanced or metastatic pancreatic cancer: Results of a phase II, randomized, noncomparative study. Investig. New Drugs 2011, 29, 144–153. [Google Scholar] [CrossRef]
- Spano, J.P.; Chodkiewicz, C.; Maurel, J.; Wong, R.; Wasan, H.; Barone, C.; Létourneau, R.; Bajetta, E.; Pithavala, Y.; Bycott, P.; et al. Efficacy of gemcitabine plus axitinib compared with gemcitabine alone in patients with advanced pancreatic cancer: An open-label randomised phase II study. Lancet 2008, 371, 2101–2108. [Google Scholar] [CrossRef]
- Herrmann, R.; Bodoky, G.; Ruhstaller, T.; Glimelius, B.; Bajetta, E.; Schüller, J.; Saletti, P.; Bauer, J.; Figer, A.; Pestalozzi, B.; et al. Gemcitabine plus capecitabine compared with gemcitabine alone in advanced pancreatic cancer: A randomized, multicenter, phase III trial of the Swiss Group for Clinical Cancer Research and the Central European Cooperative Oncology Group. J. Clin. Oncol. 2007, 25, 2212–2217. [Google Scholar] [CrossRef] [PubMed]
- Van Cutsem, E.; van de Velde, H.; Karasek, P.; Oettle, H.; Vervenne, W.L.; Szawlowski, A.; Schoffski, P.; Post, S.; Verslype, C.; Neumann, H.; et al. Phase III trial of gemcitabine plus tipifarnib compared with gemcitabine plus placebo in advanced pancreatic cancer. J. Clin. Oncol. 2004, 22, 1430–1438. [Google Scholar] [CrossRef] [PubMed]
- Sederholm, C. Gemcitabine versus gemcitabine/carboplatin in advanced non-small cell lung cancer: Preliminary findings in a phase III trial of the Swedish Lung Cancer Study Group. Semin. Oncol. 2002, 29, 50–54. [Google Scholar] [CrossRef] [PubMed]
- Cappuzzo, F.; Novello, S.; De Marinis, F.; Selvaggi, G.; Scagliotti, G.V.; Barbieri, F.; Maur, M.; Papi, M.; Pasquini, E.; Bartolini, S.; et al. A randomized phase II trial evaluating standard (50 mg/min) versus low (10 mg/min) infusion duration of gemcitabine as first-line treatment in advanced non-small-cell lung cancer patients who are not eligible for platinum-based chemotherapy. Lung Cancer 2006, 52, 319–325. [Google Scholar] [CrossRef]
- Sederholm, C.; Hillerdal, G.; Lamberg, K.; Kölbeck, K.; Dufmats, M.; Westberg, R.; Gawande, S.R. Phase III trial of gemcitabine plus carboplatin versus single-agent gemcitabine in the treatment of locally advanced or metastatic non-small-cell lung cancer: The Swedish Lung Cancer Study Group. J. Clin. Oncol. 2005, 23, 8380–8388. [Google Scholar] [CrossRef]
- Aapro, M.S.; Martin, C.; Hatty, S. Gemcitabine—A safety review. Anti-Cancer Drugs 1998, 9, 191–201. [Google Scholar] [CrossRef] [PubMed]
- Semb, K.A.; Aamdal, S.; Oian, P. Capillary protein leak syndrome appears to explain fluid retention in cancer patients who receive docetaxel treatment. J. Clin. Oncol. 1998, 16, 3426–3432. [Google Scholar] [CrossRef]
- Piccart, M.J.; Klijn, J.; Paridaens, R.; Nooij, M.; Mauriac, L.; Coleman, R.; Bontenbal, M.; Awada, A.; Selleslags, J.; Van Vreckem, A.; et al. Corticosteroids significantly delay the onset of docetaxel-induced fluid retention: Final results of a randomized study of the European Organization for Research and Treatment of Cancer Investigational Drug Branch for Breast Cancer. J. Clin. Oncol. 1997, 15, 3149–3155. [Google Scholar] [CrossRef]
- Siddall, E.; Khatri, M.; Radhakrishnan, J. Capillary leak syndrome: Etiologies, pathophysiology, and management. Kidney Int. 2017, 92, 37–46. [Google Scholar] [CrossRef] [Green Version]
- Accordino, M.K.; Neugut, A.I.; Hershman, D.L. Cardiac effects of anticancer therapy in the elderly. J. Clin. Oncol. 2014, 32, 2654–2661. [Google Scholar] [CrossRef] [Green Version]
- Burris, H.A., 3rd; Hurtig, J. Radiation recall with anticancer agents. Oncologist 2010, 15, 1227–1237. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shrum, K.J.; Gill, S.E.; Thompson, L.K.; Blackhurst, D.W.; Puls, L.E. New-onset congestive heart failure with gemcitabine in ovarian and other solid cancers. Am. J. Clin. Oncol. 2014, 37, 364–368. [Google Scholar] [CrossRef] [PubMed]
- Salem, J.E.; Manouchehri, A.; Moey, M.; Lebrun-Vignes, B.; Bastarache, L.; Pariente, A.; Gobert, A.; Spano, J.P.; Balko, J.M.; Bonaca, M.P.; et al. Cardiovascular toxicities associated with immune checkpoint inhibitors: An observational, retrospective, pharmacovigilance study. Lancet Oncol. 2018, 19, 1579–1589. [Google Scholar] [CrossRef]
- Zamorano, J.L.; Lancellotti, P.; Munoz, D.R.; Aboyans, V.; Asteggiano, R.; Galderisi, M.; Habib, G.; Lenihan, D.J.; Lip, G.Y.H.; Lyon, A.R.; et al. 2016 ESC Position Paper on cancer treatments and cardiovascular toxicity developed under the auspices of the ESC Committee for Practice Guidelines: The Task Force for cancer treatments and cardiovascular toxicity of the European Society of Cardiology (ESC). Eur. Heart J. 2016, 37, 2768–2801. [Google Scholar] [CrossRef] [PubMed]
- Biagi, C.; Montanaro, N.; Buccellato, E.; Roberto, G.; Vaccheri, A.; Motola, D. Underreporting in pharmacovigilance: An intervention for Italian GPs (Emilia-Romagna region). Eur. J. Clin. Pharmacol. 2013, 69, 237–244. [Google Scholar] [CrossRef]
- Tandon, V.R.; Mahajan, V.; Khajuria, V.; Gillani, Z. Under-reporting of adverse drug reactions: A challenge for pharmacovigilance in India. Indian J. Pharmacol. 2015, 47, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Grouthier, V.; Lebrun-Vignes, B.; Glazer, A.M.; Touraine, P.; Funck-Brentano, C.; Pariente, A.; Courtillot, C.; Bachelot, A.; Roden, D.M.; Moslehi, J.J.; et al. Increased long QT and torsade de pointes reporting on tamoxifen compared with aromatase inhibitors. Heart 2018, 104, 1859–1863. [Google Scholar] [CrossRef] [Green Version]
- Salem, J.E.; Yang, T.; Moslehi, J.J.; Waintraub, X.; Gandjbakhch, E.; Bachelot, A.; Hidden-Lucet, F.; Hulot, J.S.; Knollmann, B.C.; Lebrun-Vignes, B.; et al. Androgenic Effects on Ventricular Repolarization: A Translational Study From the International Pharmacovigilance Database to iPSC-Cardiomyocytes. Circulation 2019, 140, 1070–1080. [Google Scholar] [CrossRef]
- Lindquist, M. VigiBase, the WHO Global ICSR Database System: Basic Facts. Drug Inf. J. 2008, 42, 409–419. [Google Scholar] [CrossRef]
- Chang, L.C.; Mahmood, R.; Qureshi, S.; Breder, C.D. Patterns of use and impact of standardised MedDRA query analyses on the safety evaluation and review of new drug and biologics license applications. PLoS ONE 2017, 12, e0178104. [Google Scholar] [CrossRef]
- Rothman, K.J.; Lanes, S.; Sacks, S.T. The reporting odds ratio and its advantages over the proportional reporting ratio. Pharmacoepidemiol. Drug Saf. 2004, 13, 519–523. [Google Scholar] [CrossRef] [PubMed]
- Bate, A.; Lindquist, M.; Edwards, I.R.; Olsson, S.; Orre, R.; Lansner, A.; De Freitas, R.M. A Bayesian neural network method for adverse drug reaction signal generation. Eur. J. Clin. Pharmacol. 1998, 54, 315–321. [Google Scholar] [CrossRef] [PubMed]
- Noren, G.N.; Hopstadius, J.; Bate, A. Shrinkage observed-to-expected ratios for robust and transparent large-scale pattern discovery. Stat. Methods Med. Res. 2013, 22, 57–69. [Google Scholar] [CrossRef] [PubMed]


| Author, Year, Reference | Cardiovascular Adverse events | Age, Gender Type of Cancer | Cardiovascular Risk Factors and Medical History | Gemcitabine Dosing/Cycle Cumulative Doses | Time to Onset after 1st Intake; after Infusion | Concurrent Suspected Drugs | Management | Outcome | Rechallenge |
|---|---|---|---|---|---|---|---|---|---|
| Ozturk et al., 2009, [7] | Acute myocardial infarction | 59, Female Leiomyosarcoma | HTN, Dyslip, diabetes, CAD | 900 mg/m2 D1-8-21 (D1 = D21); 1800 mg/m2 | 8 days; 30 min | Docetaxel | Aspirin, clopidogrel, BB-, heparin, nitrate revascularization | Discharged D2, Complete recovery | No |
| Bdair et al., 2006, [8] | Acute myocardial infarction; cardiac arrest (ventricular tachycardia) | 43, Female Lung cancer | HTN, smoker Postpartum cardiomyopathy, stroke, CAD | 1000 mg/m2 D1-8-21 (D1 = D21); 4000 mg/m2 | 42 days; 72 h | No | Aspirin, BB-heparin, nitrate, glycoprotein IIb/IIIa inhibitors | Discharged D4, Complete recovery | No |
| Kalapura et al., 1999, [9] | Acute myocardial infarction; HF (LVEF:45%) | 54, Male Pancreatic cancer | None | NA; 9500 mg | 60 days; 6 h | No | Aspirin, heparin, BB- | Discharged D7, Partial recovery | Yes, recurred on nitrate and BB- |
| Santini et al., 2000, [10] | AF | 78, Male Pancreatic cancer | Paroxysmal AF | NA D1-8-15-28 (D1 = D28); NA | 18 h; 18 h | No | Propafenone | Discharged within D, Complete recovery | Yes, recurred on propafenone |
| Ferrari et al., 2006, [11] | AF | 72, Female Lung cancer | None | 1200 mg/m2 D1-8-21 (D1 = D21); 1200 mg/m2 | 18 h; 18 h | No | Amiodarone | Discharged D1, Complete recovery | No |
| Ferrari et al., 2006, [11] | AF | 73, Female Lung cancer | None | 1200 mg/m2 D1-8-21 (D1 = D21); 7200 mg/m2 | 42 days; 12 h | No | Digoxin | Discharged D5, Partial recovery (AF rate control) | No |
| Tavil et al., 2007, [12] | AF | 65, Male Lung cancer | None | 1200 mg/m2 D1-8-21 (D1 = D21); 2400 mg/m2 | 8 days; 7 h | Cisplatin | Propafenone, verapamil | Discharged D2, Complete recovery | No |
| Ciotti et al., 1999, [13] | AF | 70, Male Pancreatic cancer | None | NA; NA | 6 days; 6 days | No | Digoxin | Complete recovery after 12 days | Yes, recurred |
| Tayer-shifman et al., 2009, [14] | Junctional tachycardia (nodal reentrant) | 67, Female Breast cancer | None | 1000 mg/m2 D1-8-21 (D1 = D21); 3000 mg/m² | 21 days; few hours | No | Adenosine, verapamil, BB- | Discharged D5, Complete recovery | No |
| Khan et al., 2014, [15] | HF (LVEF: 20%) | 56, Male Pancreatic cancer | None | 1000 mg/m2 D1-8-15-28 (D1 = D28); 6000 mg/m2 | 56 days; NA | No | Furosemide, BB-, ACE | Discharged D2, Partial recovery (LVEF 40% few months later) | Yes, recurred |
| Yajima et al., 2004, [16] | HF | 82, Female Pancreatic cancer | NA | NA; 16,800 mg | 2 years; NA | No | NA | Partial recovery | No |
| Alam et al., 2018, [17] | HF (LVEF: 40%) Myocardial ischaemia | 62, MalePancreatic cancer | CAD, HTN | 1000 mg/m2 D1-8-15-28 (D1 = D28); 13,000 mg/m2 | 112 days; NA | No | Diuretics | Discharged after weeks, Partial recovery (LVEF:40%) | No |
| Alam et al., 2018, [17] | HF (LVEF: 38%) | 63, Male Pancreatic cancer | None | 1000 mg/m2 D1-8-15-28 (D1 = D28); 7000 mg/m2 | 56 days; NA | No | Diuretics | Complete recovery (LVEF:67%) | No |
| Alam et al., 2018, [17] | HF (LVEF: 60%) | 72, Female Pancreatic and lung cancer | HTN, diabetes, Dyslip, ex-smoker | 1000 mg/m2, NA | 28 days; NA | No | Diuretics | Complete recovery after few months | No |
| Mohebali et al. 2017, [18] | HF (LVEF: 20%) | 67, Female Lymphoma | Dyslip | NA; NA | 30 days; NA | Rituximab Oxaliplatin | Diuretics, ACE, BB- | Partial recovery at 6 months (LVEF:40%) | No |
| Hilmi et al., 2020, [NA] | HF (LVEF: 35%) Pericardial effusion | 67, Female Carcinoma of Vater’s papilla | HTN, Dyslip | 800-1000 mg/m2 D1-8-15-28 (D1 = D28); 14,800 mg/m2 | 170 days; 2 days | No | Furosemide, amlodipine, ACE, Pericardial tap | Discharged D9, Partial recovery at 1 year (LVEF:40%) | No |
| Hilmi et al., 2020, [NA] | HF (LVEF:20%) Pericardial effusion | 47, Male Pancreatic cancer | Cardiac XR, previously treated with anthracyclines | 1000 mg/m2 D1-8-15-28 (D1 = D28); 18,000 mg/m2 | 175 days; 7 days | No | Furosemide, BB-, ACE | Discharged D7, Complete recovery at 1 year | No |
| Hilmi et al., 2020, [NA] | Pericardial effusion CLS | 71, Female Pancreatic cancer | None | 1000 mg/m2 D1-8-15-28 (D1 = D28); 24,000 mg/m2 | 246 days; 6 days | No | Glucocorticoid, furosemide | Discharged D10, Complete recovery at 1 year | No |
| Vogl et al., 2005, [19] | Pericardial effusion | 26, Female Lymphoma | Cardiac XR, and previously cisplatin/cytarabine | 750 mg/m2; 750 mg/m2 | 1 day; 1 day | Rituximab Vincristine | Pericardial surgery (pericardial window) | Not recovered (constriction) | Yes |
| Vogl et al., 2005, [19] | Cardiac tamponade | 36, Male Lymphoma | Cardiac XR, and previously AC | 1000 mg/m2; 1000 mg/m2 | 3 days; 3 days | Rituximab Vincristine | Pericardial surgery (pericardial window) | Complete recovery 2 months later | No |
| Vogl et al., 2005, [19] | Pericardial effusion | 53, Male Lymphoma | Cardiac XR, and previously AC | 750 mg/m2 D1-14 (D1 = D14), 4500 mg/m2 | 70 days; NA | No | Glucocorticoid | Not recovered (constriction) | Yes |
| Vogl et al., 2005, [19] | Constrictive pericarditis | 31, Female Lymphoma | Cardiac XR, and previously AC | 1000 mg/m2 D1-14 (D1 = D14), 4000 mg/m2 | 30 days; NA | No | NA | Not recovered (constriction) | Yes |
| Kido et al., 2012, [20] | Pericardial effusion, CLS HF (LVEF:59%) | 56, Female Pancreatic cancer | None | NA | 120 days; NA | No | Furosemide, pericardial surgery (pericardiocentesis) | Discharged D20, Complete recovery (LVEF:76%) | No |
| Study | Type of Study | Number of Patients in the Gemcitabine Monotherapy Arm | Type of Cancer | Median Follow-up | Number of Previous Chemotherapies | Cardiovascular Adverse Drug Reaction (CV-ADR) |
|---|---|---|---|---|---|---|
| Konstantinopoulos et al. [21] | II | 36 | Ovary | 13.3 months | >1 | Myocardial infarction: 1 grade 3 |
| Conroy et al. [22] | III | 169 | Pancreas | 26.6 months | 0 | Heart failure: 1 death |
| Melisi et al. [23] | II | 52 | Pancreas | Not available | 0 | Pericardial effusion: 1 death |
| Middleton et al. [24] | II | 70 | Pancreas | 24.9 months | 0 | Myocardial infarction: 1 death |
| Neoptolemos et al. [25] | III | 366 | Pancreas | 43.2 months | 0 | Cardiac disorders: 1 death, 1 grade 3, 4 grade 2 |
| Evans et al. [26] | II | 102 | Pancreas | Not available | >1 | Cardiac arrest: 3 deaths |
| Rougier et al. [27] | III | 275 | Pancreas | 7.9 months | 0 | Heart failure: 1 grade 3 |
| Gonçalves et al. [28] | III | 52 | Pancreas | 27.7 months | 0 or more | Cardiac disorders: 6 grade 3/4 |
| Loehrer et al. [29] | II | 35 | Pancreas | Not available | 0 | Myocardial infarction: 1 death |
| Colucci et al. [30] | III | 199 | Pancreas | 38.2 months | 0 | Arrhythmia: 1 grade 3 |
| Richards et al. [31] | II | 39 | Pancreas | Not available | 0 | Myocardial infarction: 1 death |
| Spano et al. [32] | II | 31 | Pancreas | Not available | 0 | Myocardial infarction: 1 grade 3 |
| Herrmann et al. [33] | III | 156 | Pancreas | Not available | 0 or 1 | Myocardial infarction: 1 death |
| Van Cutsem et al. [34] | III | 347 | Pancreas | Not available | 0 or 1 | Heart failure: 2 grade 2 Pericardial effusion: 1 death Myocardial infarction: 1 death |
| Sederholm et al. [35] | III | 170 | Lung | Not available | 0 | Heart failure: 2 grade 3 |
| Cappuzzo et al. [36] | II | 117 | Lung | 6 months | 0 | Myocardial infarction: 1 death |
| Sederholm et al. [37] | III | 170 | Lung | 10.5 months | 0 | Heart failure: 1 grade 3 |
| Cardiovascular Adverse Events | MedDRA Preferred Term Level | ICSR Reported with Gemcitabine (n = 46,898) | ICSR Reported in Full Database (n = 18,908,940) | IC (IC025) | ROR (95% CI) |
|---|---|---|---|---|---|
| Myocardial ischemia | Acute myocardial infarction | 69 | 16,348 | 0.76 (0.40) | 1.71 (1.35–2.17) |
| n = 119 | Myocardial ischemia | 32 | 7855 | 0.70 (0.16) | 1.65 (1.17–2.34) |
| Acute coronary syndrome | 19 | 4087 | 0.87 (0.15) | 1.88 (1.21–2.95) | |
| Cardiac supraventricular arrhythmias n = 308 | Atrial flutter | 36 | 4457 | 1.66 (1.15) | 3.28 (2.36–4.55) |
| Supraventricular tachycardia | 51 | 7729 | 1.39 (0.97) | 2.67 (2.03–3.52) | |
| Arrhythmia supraventricular | 12 | 1478 | 1.59 (0.66) | 3.29 (1.86–5.81) | |
| Atrial tachycardia | 9 | 982 | 1.69 (0.60) | 3.72 (1.93–7.17) | |
| Atrial fibrillation | 199 | 51,662 | 0.63 (0.43) | 1.56 (1.36–1.79) | |
| Tachyarrhythmia | 12 | 1771 | 1.35 (0.42) | 2.74 (1.55–4.83) | |
| Supraventricular extrasystoles | 12 | 2095 | 1.13 (0.20) | 2.32 (1.32–4.09) | |
| Pericardial diseases | Pericardial effusion | 151 | 11,040 | 2.44 (2.20) | 5.59 (4.76–6.57) |
| n = 164 | Cardiac tamponade | 28 | 2016 | 2.37 (1.79) | 5.67 (3.90–8.23) |
| Pericarditis constrictive | 6 | 171 | 2.81 (1.44) | 14.63 (6.48–33.04) | |
| Heart failure | Cardiac failure | 229 | 40,801 | 1.17 (0.98) | 2.28 (2.01–2.60) |
| n = 484 | Cardiac failure acute | 15 | 1914 | 1.56 (0.74) | 3.18 (1.91–5.29) |
| Ventricular hypokinesia | 10 | 1131 | 1.67 (0.64) | 3.59 (1.93–6.69) | |
| Cardiomegaly | 42 | 8196 | 1.03 (0.56) | 2.08 (1.54–2.82) | |
| Systolic dysfunction | 4 | 226 | 2.09 (0.35) | 7.18 (2.67–19.30) | |
| Ventricular dysfunction | 9 | 1269 | 1.38 (0.29) | 2.87 (1.49–5.53) | |
| Cardiac failure congestive | 207 | 63,389 | 0.40 (0.19) | 1.32 (1.15–1.51) | |
| Others | Sinus tachycardia | 62 | 8435 | 1.54 (1.16) | 2.98 (2.32–3.83) |
| Atrial thrombosis | 7 | 997 | 1.34 (0.08) | 2.84 (1.35–5.97) |
| Clinical Characteristics | Myocardial Ischaemia n = 119 | Supraventricular Arrhythmias n = 308 | Pericardial Diseases n = 164 | Heart Failure n = 484 | p | |
|---|---|---|---|---|---|---|
| Reporting regions | America | 67/119 (56%) | 195/308 (63%) | 110/164 (67%) | 260/484 (54%) | 0.0003 |
| Europe | 39/119 (33%) | 98/308 (32%) | 38/164 (23%) | 198/484 (41%) | ||
| Africa | 1/119 (1%) | 2/308 (1%) | 1/164 (1%) | 0/484 (0%) | ||
| Australia | 0/119 (0%) | 3/308 (1%) | 1/164 (1%) | 0/484 (0%) | ||
| Asia | 12/119 (10%) | 10/308 (3%) | 14/164 (8%) | 26/484 (5%) | ||
| Reporting year | 2015–2019 | 33/119 (28%) | 65/308 (21%) | 42/164 (26%) | 121/484 (25%) | <0.0001 |
| 2009–2014 | 55/119 (46%) | 94/308 (30%) | 39/164 (24%) | 142/484 (29%) | ||
| 2003–2008 | 26/119 (22%) | 95/308 (31%) | 54/164 (33%) | 121/484 (25%) | ||
| 1997–2002 | 5/119 (4%) | 54/308 (18%) | 29/164 (18%) | 100/484 (21%) | ||
| Reporters | N available | 102/119 (86%) | 254/308 (82%) | 135/164 (82%) | 420/484 (87%) | |
| Health care professional | 99/102 (97%) | 236/254 (93%) | 122/135 (90%) | 394/420 (94%) | 0.22 | |
| Other | 3/102 (3%) | 18/254 (7%) | 13/135 (10%) | 26/420 (6%) | ||
| Report type | Standard of care | 109/119 (91%) | 273/308 (89%) | 155/164 (94%) | 452/484 (93%) | 0.06 |
| Clinical trials | 10/119 (9%) | 35/308 (11%) | 9/164 (6%) | 32/484 (7%) | ||
| Sex | N available | 105/119 (88%) | 295/308 (96%) | 152/164 (93%) | 451/484 (93%) | |
| Male | 65/105 (62%) | 184/295 (62%) | 57/152 (38%) | 235/451 (52%) | <0.0001 | |
| Female | 40/105 (38%) | 111/295 (38%) | 95/152 (62%) | 216/451 (48%) | ||
| Age at onset, years | N available | 91/119 (76%) | 253/308 (82%) | 125/164 (76%) | 397/484 (82%) | |
| Mean (min-max) | 65 (23–85) | 68 (32–85) | 55 (16–81) | 64 (20-89) | <0.0001 | |
| Standard deviation | 11.5 | 9.4 | 15.6 | 11.7 | ||
| Suspected drugs | Gemcitabine alone | 33/119 (28%) | 178/308 (55%) | 124/164 (76%) | 292/484 (60%) | <0.0001 |
| Gemcitabine and ≥1 other | 86/119 (72%) | 130/308 (45%) | 40/164 (24%) | 192/484 (40%) | ||
| Other concomitant or suspected drugs | Taxanes | 29/119 (24%) | 122/308 (40%) | 34/164 (21%) | 99/484 (21%) | <0.0001 |
| Vinca alkaloids | 7/119 (6%) | 18/308 (6%) | 9/164 (5%) | 36/484 (7%) | 0.74 | |
| Anthracyclines | 8/119 (7%) | 8/308 (3%) | 5/164 (3%) | 20/484 (4%) | 0.22 | |
| Topoisomerase I inhibitors | 2/119 (2%) | 7/308 (2%) | 4/164 (2%) | 5/484 (1%) | 0.22 | |
| Platins | 55/119 (46%) | 80/308 (26%) | 25/164 (15%) | 104/484 (21%) | <0.0001 | |
| Antimetabolites | 10/119 (8%) | 32/308 (10%) | 13/164 (8%) | 36/484 (7%) | 0.94 | |
| Mustard gas derivative | 2/119 (2%) | 3/308 (1%) | 3/164 (2%) | 11/484 (2%) | 0.63 | |
| Angiogenesis inhibitors | 16/119 (14%) | 28/308 (9%) | 10/164 (6%) | 39/484 (8%) | 0.16 | |
| Human epidermal growth factor receptor 2 blockers | 4/119 (3%) | 4/308 (1%) | 20/164 (1%) | 29/484 (6%) | 0.02 | |
| Epidermal growth factor receptor blockers | 19/119 (16%) | 22/308 (7%) | 11/164 (7%) | 26/484 (5%) | 0.001 | |
| Immune checkpoint inhibitors | 0/119 (0%) | 3/308 (1%) | 5/164 (3%) | 0/484 (0%) | 0.001 | |
| Duration of administration, days | N available | 47/119 (40%) | 135/308 (44%) | 47/164 (29%) | 213/484 (44%) | <0.0001 |
| Median | 28 | 16 | 60 | 91 | ||
| Interquartile range | 7-97 | 1-57 | 7-146 | 20–176 | ||
| Time to onset, days | N available | 46/119 (39%) | 142/308 (46%) | 62/164 (38%) | 202/484 (42%) | <0.001 |
| Median | 29 | 14 | 55 | 75 | ||
| Interquartile range | 6-80 | 4-52 | 13–144 | 22–166 | ||
| Severe adverse events * | 113/119 (94%) | 240/308 (78%) | 129/164 (79%) | 369/484 (76%) | <0.0001 | |
| Recovery | N available | 51/119 (43%) | 132/308 (43%) | 55/164 (34%) | 163/484 (34%) | 0.02 |
| Recovered | 35/51 (69%) | 107/132 (81%) | 48/55 (87%) | 115/163 (70%) | ||
| Not recovered or sequelae | 16/51 (31%) | 25/132 (19%) | 7/55 (13%) | 48/163 (30%) | ||
| Indications | N available | 93/119 (78%) | 228/308 (74%) | 97/164 (59%) | 293/484 (61%) | <0.0001 |
| Pancreatic cancer | 31/93 (33%) | 64/228 (28%) | 24/97 (25%) | 115/293 (39%) | ||
| Lymphoma | 4/93 (4%) | 17/228 (7%) | 19/97 (20%) | 16/293 (6%) | ||
| Lung cancer | 24/93 (26%) | 83/228 (36%) | 23/97 (24%) | 70/293 (24%) | ||
| Urothelial cancer | 13/93 (14%) | 14/228 (8%) | 6/97 (6%) | 24/293 (8%) | ||
| Breast cancer | 5/93 (5%) | 19/228 (8%) | 8/97 (8%) | 36/293 (12%) | ||
| Ovarian cancer | 6/93 (6%) | 8/228 (3%) | 8/97 (8%) | 12/293 (4%) | ||
| Bile duct cancer | 8/93 (9%) | 21/228 (9%) | 2/97 (2%) | 13/293 (5%) | ||
| Sarcoma | 2/93 (2%) | 2/228 (1%) | 7/97 (7%) | 7/293 (2%) | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hilmi, M.; Ederhy, S.; Waintraub, X.; Funck-Brentano, C.; Cohen, A.; Vozy, A.; Lebrun-Vignes, B.; Moslehi, J.; Nguyen, L.S.; Salem, J.-E. Cardiotoxicity Associated with Gemcitabine: Literature Review and a Pharmacovigilance Study. Pharmaceuticals 2020, 13, 325. https://doi.org/10.3390/ph13100325
Hilmi M, Ederhy S, Waintraub X, Funck-Brentano C, Cohen A, Vozy A, Lebrun-Vignes B, Moslehi J, Nguyen LS, Salem J-E. Cardiotoxicity Associated with Gemcitabine: Literature Review and a Pharmacovigilance Study. Pharmaceuticals. 2020; 13(10):325. https://doi.org/10.3390/ph13100325
Chicago/Turabian StyleHilmi, Marc, Stéphane Ederhy, Xavier Waintraub, Christian Funck-Brentano, Ariel Cohen, Aurore Vozy, Bénédicte Lebrun-Vignes, Javid Moslehi, Lee S. Nguyen, and Joe-Elie Salem. 2020. "Cardiotoxicity Associated with Gemcitabine: Literature Review and a Pharmacovigilance Study" Pharmaceuticals 13, no. 10: 325. https://doi.org/10.3390/ph13100325
APA StyleHilmi, M., Ederhy, S., Waintraub, X., Funck-Brentano, C., Cohen, A., Vozy, A., Lebrun-Vignes, B., Moslehi, J., Nguyen, L. S., & Salem, J.-E. (2020). Cardiotoxicity Associated with Gemcitabine: Literature Review and a Pharmacovigilance Study. Pharmaceuticals, 13(10), 325. https://doi.org/10.3390/ph13100325