Possibility of a New Indication for Amantadine in the Treatment of Bipolar Depression—Case Series Study
Abstract
:1. Introduction
2. Results
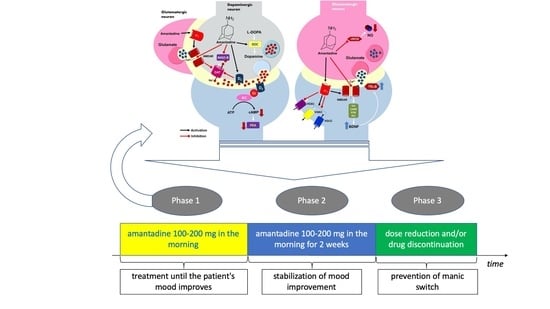
- A 51-year-old Caucasian male diagnosed with bipolar disorder since 2002, when he had his first episode of mania. Since then, the patient was treated with valproic acid in the dose of 1500 mg and lithium carbonate in the dose of 750 mg daily (serum concentration of 0.6 mmol/L). In 2016, he had a moderate episode of depression (16 points on HDRS). The clinical picture was dominated by anhedonia, apathy, lack of energy and motivation, anxiety, and indifference. Further attempts were made to treat depression with quetiapine 300 mg/day, olanzapine 10 mg together with fluoxetine 20 mg daily, and antidepressants: sertraline up to 200 mg daily and escitalopram up to 20 mg daily. However, despite treatment, each time for at least 6 weeks, none of these interventions resulted in improvement. The patient was then offered to add amantadine to lithium carbonate and valproic acid in an initial dose of 100 mg in the morning. After one week, the patient reported a slight improvement (15 points on the HDRS scale). With the patient’s consent, the dose was increased to 200 mg amantadine in the morning. After another week, the severity of depression in HDRS was 8 points. From the patient’s report, the improvement occurred 3–4 days after increasing the dose of amantadine. After another week, HDRS was 2 points (Figure 2). The patient continued treatment with amantadine for another 2 weeks, then reduced the dose to 100 mg in the morning and stopped the drug after three days. During the treatment, no side effects of amantadine were observed, no change in the manic phase, and no recurrence of depressive symptoms after drug discontinuation.
- A 56-year-old Caucasian male treated for bipolar disorder since 2016, when he experienced a two-month manic episode. He then received valproic acid in a dose of 1500 mg daily and olanzapine in a dose of 20 mg in the evening. His mania symptoms resolved and he was then treated only with valproic acid. In 2017, he reported significant depression (19 points on the HDRS scale). The clinical picture was dominated by tearfulness, sadness, lack of motivation, anhedonia, lack of energy, and difficulty with concentration. He received lamotrigine at the target dose of 150 mg/day, but after 4 months the depression was still there (15 points on the HDRS scale). He then received mirtazapine in a dose of 30 mg in the evening, but after another 2 months, the symptoms remained (16 points on the HDRS scale). The patient was then offered to add amantadine at an initial dose of 100 mg in the morning to valproic acid and lamotrigine. As there was no improvement after one week, the amantadine dose was increased to 200 mg in the morning. After another week, the patient felt better (HDRS 9 points). At the visit after 3 weeks of treatment with amantadine, the patient had an HDRS score of 4 (Figure 2). He continued treatment for another 2 weeks, then lowered the dose to 100 mg in the morning, and stopped taking the drug after 3 days. There were no adverse effects of amantadine and no change from the depressive phase to the manic one, and no relapse of depression after discontinuation of amantadine.
- A 50-year-old Caucasian man has had bipolar disorder since the age of 31. He started psychiatric treatment in 2013, when the manic episode was followed by an episode of depression that lasted 5 months (12 points on the HDRS scale at the first visit). The patient then received quetiapine, but was unable to function professionally due to sedation at a dose of 100 mg. The treatment was changed to lithium carbonate at a dose of 500 mg per day (serum concentration of 0.6 mmol/L). The patient felt better (6 points on the HDRS scale), but in 2014 there was another episode of depression (14 points on the HDRS scale). The patient then did not respond to 20 mg escitalopram for 6 weeks and then to 150 mg bupropion in the morning for 2 months. He was then offered to add amantadine to the lithium carbonate in an initial dose of 100 mg in the morning. The patient reported a slight improvement at the visit after one week of treatment (12 points on the HDRS scale). Due to incomplete improvement, the dose was increased to 200 mg amantadine in the morning. According to the patient, the improvement was achieved within 2 days (6 points on the HDRS scale after 2 weeks of treatment). After 3 weeks, the HDRS was 5 points (Figure 2). After another 2 weeks, the patient stopped taking amantadine within 3 days. There were no adverse effects of amantadine, no manic switch, and no recurrence of depression after discontinuation of amantadine.
- A 45-year-old Caucasian woman was diagnosed with BD-I in 2015. She had previously had a four-month manic episode, followed by a depressive episode for three months. She then received quetiapine at a target dose of 300 mg in the evening. The patient tolerated the drug well, but her mood improved only after 6 months of treatment. In 2017, there was another episode of depression (15 points on the HDRS scale), after an earlier short episode of hypomania. She received 30 mg of mirtazapine in the evening, but after 6 weeks there was no improvement and the patient gained 3 kg body weight. She was switched to 150 mg bupropion in the morning, but after another 2 months, there was still no improvement. The patient was then offered to add amantadine 100 mg in the morning to 300 mg of quetiapine. Improvement was observed after 7 days of taking amantadine (HDRS 8 points). After 2 weeks, HDRS was 5 points, and after 3 weeks, 1 point (Figure 2). The patient took 100 mg of amantadine for 2 weeks and then gradually, within 3 days, stopped taking it. As before, no adverse effects of amantadine, no change from the depressive phase to the manic phase, and no recurrence of depressive symptoms after discontinuation of amantadine were observed.
3. Discussion
4. Materials and Methods
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- McIntyre, R.S.; Calabrese, J.R. Bipolar depression: The clinical characteristics and unmet needs of a complex disorder. Curr. Med. Res. Opin. 2019, 35, 1993–2005. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leahy, R.L. Bipolar disorder: Causes, contexts, and treatments. J. Clin. Psychol. 2007, 63, 417–424. [Google Scholar] [CrossRef] [PubMed]
- Kraepelin, E. Manic Depressive Insanity and Paranoia; Livingstone: Edinburgh, UK, 1921. [Google Scholar]
- Tondo, L.; Vázquez, G.H.; Baldessarini, R.J. Depression and Mania in Bipolar Disorder. Curr. Neuropharmacol. 2017, 15, 353–358. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Post, R.M. Treatment of Bipolar Depression: Evolving Recommendations. Psychiatr. Clin. North Am. 2016, 39, 11–33. [Google Scholar] [CrossRef] [PubMed]
- Szmulewicz, A.G.; Angriman, F.; Samamé, C.; Ferraris, A.; Vigo, D.; Strejilevich, S.A. Dopaminergic agents in the treatment of bipolar depression: A systematic review and meta-analysis. Acta Psychiatrica Scandinavica 2017, 135, 527–538. [Google Scholar] [CrossRef]
- Raupp-Barcaro, I.F.; Vital, M.A.; Galduróz, J.C.; Andreatini, R. Potential antidepressant effect of amantadine: A review of preclinical studies and clinical trials. Braz. J. Psychiatry 2018, 40, 449–458. [Google Scholar] [CrossRef] [Green Version]
- Mizoguchi, K.; Yokoo, H.; Yoshida, M.; Tanaka, T.; Tanaka, M. Amantadine increases the extracellular dopamine levels in the striatum by re-uptake inhibition and by N-methyl-D-aspartate antagonism. Brain Res. 1994, 662, 255–258. [Google Scholar] [CrossRef]
- Blanpied, T.A.; Clarke, R.J.; Johnson, J.W. Amantadine inhibits NMDA receptors by accelerating channel closure during channel block. J. Neurosci. 2005, 25, 3312–3322. [Google Scholar] [CrossRef] [Green Version]
- Hosenbocus, S.; Chahal, R. Amantadine: A review of use in child and adolescent psychiatry. J. Can. Acad. Child Adolesc. Psychiatry 2013, 22, 55–60. [Google Scholar]
- Huber, T.J.; Dietrich, D.E.; Emrich, H.M. Possible use of amantadine in depression. Pharmacopsychiatry 1999, 32, 47–55. [Google Scholar] [CrossRef]
- Rogóż, Z.; Dlaboga, D.; Dziedzicka-Wasylewska, M.J. Effect of combined treatment with imipramine and amantadine on the central dopamine D2 and D3 receptors in rats. Physiol. Pharmacol. 2003, 54, 257–270. [Google Scholar]
- Fisher, A.; Starr, M.S. Opposite effects of glutamate antagonists and antiparkinsonian drugs on the activities of DOPA decarboxylase and 5-HTP decarboxylase in the rat brain. Brain Res. 2000, 868, 268–274. [Google Scholar] [CrossRef]
- Porter, R.H.; Greenamyre, J.T. Regional variations in the pharmacology of NMDA receptor channel blockers: Implications for therapeutic potential. J. Neurochem. 1995, 64, 614–623. [Google Scholar] [CrossRef] [PubMed]
- Tan, L.; Ge, H.; Tang, J.; Fu, C.; Duanmu, W.; Chen, Y.; Hu, R.; Sui, J.; Liu, X.; Feng, H. Amantadine preserves dopamine level and attenuates depression-like behawior induced by traumatic brain injury in rats. Behav. Brain Res. 2015, 279, 274–282. [Google Scholar] [CrossRef] [PubMed]
- Nikolaus, S.; Wittsack, H.J.; Beu, M.; Antke, C.; Hautzel, H.; Wickrath, F.; Müller-Lutz, A.; de Souza Silva, M.A.; Huston, J.P.; Antoch, G.; et al. Amantadine enhances nigrostriatal and mesolimbic dopamine function in the rat brain in relation to motor and exploratory activity. Pharmacol. Biochem. Behav. 2019, 179, 156–170. [Google Scholar] [CrossRef] [PubMed]
- Deutschenbaur, L.; Beck, J.; Kiyhankhadiv, A.; Mühlhauser, M.; Borgwardt, S.; Walter, M.; Hasler, G.; Sollberger, D.; Lang, U.E. Role of calcium, glutamate and NMDA in major depression and therapeutic application. Prog. Neuropsychopharmacol. Biol. Psychiatry 2016, 64, 325–333. [Google Scholar] [CrossRef] [PubMed]
- Newport, D.J.; Carpenter, L.L.; McDonald, W.M.; Potash, J.B.; Tohen, M.; Nemeroff, C.B. APA Council of Research Task Force on Novel Biomarkers and Treatments Ketamine and other NMDA antagonists: Early clinical trials and possible mechanisms in depression. Am. J. Psychiatry 2015, 172, 950–966. [Google Scholar]
- Mathew, S.J.; Keegan, K.; Smith, L. Glutamate modulators as novel interventions for mood disorders. Revista Brasiliera Psiquiatria 2005, 27, 243–248. [Google Scholar] [CrossRef] [Green Version]
- Page, G.; Peeters, M.; Maloteaux, J.M.; Hermans, E. Increased dopamine uptake in striatal synaptosomes after treatment of rats with amantadine. Eur. J. Pharmacol. 2000, 403, 75–80. [Google Scholar] [CrossRef]
- Dutta, A.; McKie, S.; Deakin, J.F. Ketamine and other potential glutamate antidepressants. Psychiatry Res. 2015, 225, 1–13. [Google Scholar] [CrossRef]
- Moryl, E.; Danysz, W.; Quack, G. Potential antidepressive properties of amantadine, memantine and bifemelane. Pharmacol. Toxicol. 1993, 72, 394–397. [Google Scholar] [CrossRef] [PubMed]
- Rogóż, Z.; Skuza, G. Effect of repeated co-treatment with fluoxetine and amantadine on the behavioral reactivity of the central dopamine and serotonin system in rats. Pharmacol. Rep. 2009, 61, 924–929. [Google Scholar] [CrossRef]
- Peeters, M.; Romieu, P.; Maurice, T.; Su, T.P.; Maloteaux, J.M.; Hermans, E. Involvement of the sigma 1 receptor in the modulation of dopaminergic transmission by amantadine. Eur. J. Neurosci. 2004, 19, 2212–2220. [Google Scholar] [CrossRef] [PubMed]
- Kornhuber, J.; Schoppmeyer, K.; Riederer, P. Affinity of 1-aminoadamantanes for the sigma binding site in post-mortem human frontal cortex. Neurosci. Lett. 1993, 163, 129–131. [Google Scholar] [CrossRef]
- Nuwayhid, S.J.; Werling, L.L. Sigma1 receptor agonist-mediated regulation of N-methyl-D-aspartate-stimulated [3H]dopamine release is dependent upon protein kinase C. J. Pharmacol. Exp. Ther. 2003, 304, 364–369. [Google Scholar] [CrossRef]
- Wang, J.; Mack, A.L.; Coop, A.; Matsumoto, R.R. Novel sigma (sigma) receptor agonists produce antidepressant-like effects in mice. Eur. Neuropsychopharmacol. 2007, 17, 708–716. [Google Scholar] [CrossRef] [Green Version]
- Narita, N.; Hashimoto, K.; Tomitaka, S.; Minabe, Y. Interactions of selective serotonin reuptake inhibitors with subtypes of sigma receptors in rat brain. Eur. J. Pharmacol. 1996, 307, 117–119. [Google Scholar] [CrossRef]
- Itzhak, Y.; Kassim, C.O. Clorgyline displays high affinity for σ-binding sites in C57BL/6 mouse brain. Eur. J. Pharmacol. 1990, 176, 107–108. [Google Scholar] [CrossRef]
- Castren, E.; Kojima, M. Brain-derived neurotrophic factor in mood disorders and antidepressant treatments. Neurobiol. Dis. 2017, 97, 119–126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rogóż, Z.; Skuza, G.; Legutko, B. Repeated co-treatment with fluoxetine and amantadine induces brain-derived neurotrophic factor gene expression in rats. Pharmacol. Rep. 2008, 60, 817–826. [Google Scholar] [PubMed]
- Rogóż, Z.; Skuza, G.; Legutko, B.J. Repeated co-treatment with imipramine and amantadine induces hippocampal brain-derived neurotrophic factor gene expression in rats. Physiol. Pharmacol. 2007, 58, 219–234. [Google Scholar]
- Amidfar, M.; Kim, Y.K.; Wiborg, O. Effectiveness of memantine on depression-like behavior, memory deficits and brain mRNA levels of BDNF and TrkB in rats subjected to repeated unpredictable stress. Pharmacol. Rep. 2018, 70, 600–606. [Google Scholar] [CrossRef]
- Walia, V.; Garg, C.; Garg, M. Amantadine exerts anxiolytic like effect in mice: Evidences for the involvement of nitrergic and GABAergic signaling pathways. Behav. Brain Res. 2020, 380, 112432. [Google Scholar] [CrossRef] [PubMed]
- Berk, M.; Dodd, S.; Kauer-Sant’anna, M.; Malhi, G.S.; Bourin, M.; Kapczinski, F.; Norman, T. Dopamine dysregulation syndrome: Implications for a dopamine hypothesis of bipolar disorder. Acta Psychiatrica Scandinavica Suppl. 2007, 434, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Van Rossum, I.; Tenback, D.; van Os, J. Bipolar disorder and dopamine dysfunction: An indirect approach focusing on tardive movement syndromes in a naturalistic setting. BMC Psychiatry 2009, 9, 16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vale, S.; Espejel, M.A.; Dominguez, J.C. Amantadine in depression. Lancet 1971, 2, 437. [Google Scholar] [CrossRef]
- Rizzo, M.; Biandrate, G.; Tognoni, G.; Morselli, P.L. Amantadine in depression: Relationship between behavioral effects and plasma levels. Eur. J. Clin. Pharmacol. 1973, 5, 226–228. [Google Scholar] [CrossRef]
- Rogóz, Z.; Skuza, G.; Daniel, W.A.; Wójcikowski, J.; Dudek, D.; Wróbel, A. Amantadine as an additive treatment in patients suffering from drug-resistant unipolar depression. Pharmacol. Rep. 2007, 59, 778–784. [Google Scholar]
- Stryjer, R.; Strous, R.D.; Shaked, G.; Bar, F.; Feldman, B.; Kotler, M.; Polak, L.; Rosenzcwaig, S.; Weizman, A. Amantadine as augmentation therapy in the management of treatment-resistant depression. Int. Clin. Psychopharmacol. 2003, 18, 93–96. [Google Scholar] [CrossRef]
- Dietrich, D.E.; Bode, L.; Spannhuth, C.W.; Lau, T.; Huber, T.J.; Brodhun, B.; Ludwig, H.; Emrich, H.M. Amantadine in depressive patients with Borna disease virus (BDV) infection: An open trial. Bipolar Disord. 2000, 2, 65–70. [Google Scholar] [CrossRef]
- Zarate, C.A.; Singh, J.B.; Carlson, P.J.; Brutsche, N.E.; Ameli, R.; Luckenbaugh, D.A.; Charney, D.A.; Manji, H.K. A Randomized Trial of an N-methyl-D-aspartate Antagonist in Treatment-Resistant Major Depression. Arch. Gen. Psychiatry 2006, 63, 856–864. [Google Scholar] [CrossRef] [PubMed]
- Wong, J.; Motulsky, A.; Eguale, T.; Buckeridge, D.L.; Abrahamowicz, M.; Tamblyn, R. Treatment indications for antidepressants prescribed in primary care in Quebec, Canada, 2006–2015. JAMA 2016, 315, 2230–2232. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Skånland, S.S.; Cieślar-Pobuda, A. Off-label uses of drugs for depression. Eur. J. Pharmacol. 2019, 865, 172732. [Google Scholar] [CrossRef] [PubMed]
- Sodré, L.A.; Bücker, J.; Zortéa, K.; Sulzbach-Vianna, M.F.; Gama, C.S. Mania switch induced by amantadine in bipolar disorder: Report of three cases. Braz. J. Psychiatry 2010, 32, 467–469. [Google Scholar] [CrossRef] [PubMed] [Green Version]



| Patients | 1-st Week | 2-nd Week | 3-rd Week |
|---|---|---|---|
| case | daily dose (improvement from baseline) | ||
| 1 (male) | 100 mg (6%) | 200 mg (50%) | 200 mg (87.5%) |
| 2 (male) | 100 mg (0%) | 200 mg (44%) | 200 mg (75%) |
| 3 (male) | 100 mg (14%) | 200 mg (57%) | 200 mg (64%) |
| 4 (female) | 100 mg (66%) | 100 mg (66%) | 100 mg (93%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krzystanek, M.; Pałasz, A. Possibility of a New Indication for Amantadine in the Treatment of Bipolar Depression—Case Series Study. Pharmaceuticals 2020, 13, 326. https://doi.org/10.3390/ph13100326
Krzystanek M, Pałasz A. Possibility of a New Indication for Amantadine in the Treatment of Bipolar Depression—Case Series Study. Pharmaceuticals. 2020; 13(10):326. https://doi.org/10.3390/ph13100326
Chicago/Turabian StyleKrzystanek, Marek, and Artur Pałasz. 2020. "Possibility of a New Indication for Amantadine in the Treatment of Bipolar Depression—Case Series Study" Pharmaceuticals 13, no. 10: 326. https://doi.org/10.3390/ph13100326
APA StyleKrzystanek, M., & Pałasz, A. (2020). Possibility of a New Indication for Amantadine in the Treatment of Bipolar Depression—Case Series Study. Pharmaceuticals, 13(10), 326. https://doi.org/10.3390/ph13100326