Oral Treatment of Central Serous Chorioretinopathy Patients Using Propranolol Tablets
Abstract
:1. Introduction
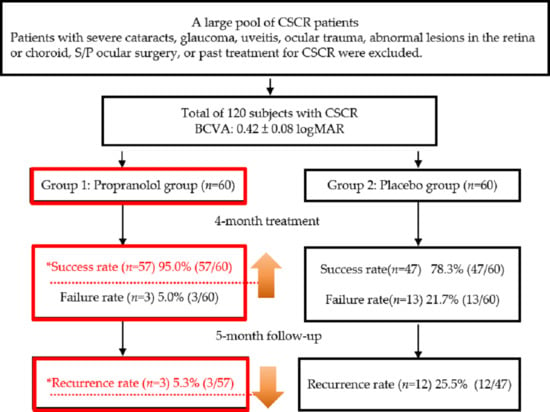
2. Results
3. Discussion
4. Materials and Methods
4.1. Patients and Study Design
4.2. Patients and Criteria
4.3. Experimental Design
4.4. Termination of Treatment
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Matet, A.; Daruich, A.; Zola, M.; Behar-Cohen, F. Risk factors for recurrence of central serious chorioretinopathy. Retina 2018, 38, 1403–1414. [Google Scholar] [CrossRef] [PubMed]
- Hamimovici, R.; Koh, S.; Gagnon, D.R.; Todd, L.; Sarah, W. Risk factors for central serous chorioretinopathy: A case-control study. Ophthalmology 2004, 111, 244–249. [Google Scholar] [CrossRef] [PubMed]
- Ghasemi, A. Normality test for statistically analysis: A guide for non-statisticians. Int. J. Endocrinol. Metab. 2012, 10, 486–489. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Türkcü, F.M.; Yüksel, H.; Yüksel, H.; Şahin, A.; Cinar, Y.; Cingü, A.K.; Şahin, M.; Çaça, İ. Serum dehydroepiandrosterone sulphate, total antioxidant capacity, and total oxidant status in central serous chorioretinopathy. Graefes Arch. Clin. Exp. Ophthalmol. 2014, 252, 17–21. [Google Scholar] [CrossRef]
- Baran, N.V.; Guriu, V.P.; Esgin, H. Long-term macular function in eyes with central serous chorioretinopathy. Clin. Exp. Ophthalmol. 2005, 33, 369–372. [Google Scholar] [CrossRef]
- Yu, J.; Jiang, C.; Xu, G. Study of subretinal exudation and consequent changes in acute central serous chorioretinopathy by optic coherence tomography. Am. J. Ophthalmol. 2014, 158, 752–756. [Google Scholar] [CrossRef]
- Daruich, A.; Matet, A.; Dirani, A.; Bousquet, E.; Zhao, M.; Nicolette Farman, N.; Jaisser, F.; Behar-Cohen, F. Central serous chorioretinopathy: Recent findings and new physiopathology hypothesis. Prog. Retin. Eye Res. 2015, 48, 82–118. [Google Scholar] [CrossRef] [Green Version]
- Levine, R.; Brucker, A.J.; Robinson, F. Long-term follow-up of idiopathic central serous chorioretinopathy by fluorescein angiography. Ophthalmology 1989, 96, 854–859. [Google Scholar] [CrossRef]
- Hanumunthadu, D.; Tan, A.S.C.; Singh, S.R.; Sahu, N.K.; Chhablani, J. Management of chronic central serous chorioretinopathy. Ind. J. Ophthalmol. 2018, 66, 1704–1714. [Google Scholar]
- Loo, R.H.; Scott, I.U.; Flynn, H.W., Jr.; Gass, J.D.M.; Murray, T.G.; Lewis, L.; Rosenfeld, P.J.; Smiddy, W.E. Factors associated with reduced visual acuity during long-term follow-up of patients with idiopathic central serous chorioretinopathy. Retina 2002, 22, 19–24. [Google Scholar] [CrossRef]
- Daruich, A.; Matet, A.; Marchionni, L.; De Azevedo, J.D.; Ambresin, A.; Mantel, I.; Behar-Cohen, F. Acute central serous chorioretinopathy. Retina 2017, 37, 1905–1915. [Google Scholar] [CrossRef] [Green Version]
- Maruko, I.; Iida, T.; Ojima, A.; Sekiryu, T. Subretinal dot-like precipitates and yellow materials in central serous chorioretinopathy. Retina 2011, 31, 759–765. [Google Scholar] [CrossRef]
- Bousquet, E.; Beydoun, T.; Zhao, M.; Hassan, L. Mineralcortocoid receptor antagonism in the treatment of chronic central serous chorioretinopathy: A pilot study. Retina 2013, 33, 2096–2102. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.S.; Sander, B.; Larsen, M. Retinal atrophy idiopathic central serous chorioretinopathy. Am. J. Ophthalmol. 2002, 133, 787–793. [Google Scholar] [CrossRef]
- Ünlü, G.; Erdogan, G.; Aydogan, T.; Akcay, B.S. Intravitreal bevacizumab for treatment of central serous chorioretinopathy. J. Ophthalmic Vis. Res. 2016, 11, 61–65. [Google Scholar] [PubMed]
- Gramajo, A.L.; Marquez, G.E.; Torres, V.E.; Juárez1, C.P.; Rosenstein, R.E.; Luna, J.D. Therapeutic benefit of melatonin in refractory central serous chorioretinopathy. Eye 2015, 29, 1036–1045. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ambiya, V.; Khodani, M.; Goud, A.; Narayanan, R.; Tyagi, M.; Rani, P.K.; Chhablani, J. Early foal laser photocoagulation in acute central serous chorioretinopathy: A prospective, randomized study. Ophthalmic Surg. Lasers Imaging Retina 2017, 8, 564–571. [Google Scholar] [CrossRef] [PubMed]
- Erikitola, O.C.; Crosby-Nwaobi, R.; Lotery, A.J.; Sivaprasad, S. Photodynamic therapy for central serous chorioretinopathy. Eye 2014, 28, 944–957. [Google Scholar] [CrossRef] [Green Version]
- Michael, J.C.; Pak, J.; Pulido, J.; Venecia, G.D. Central serous chorioretinopathy associated with administration of sympathomimetric agents. Am. J. Ophthalmol. 2003, 136, 182–185. [Google Scholar] [CrossRef]
- Tyrre, P. Current status of beta-blocking drugs in the treatment of anxiety disorders. Drugs 1998, 36, 773–783. [Google Scholar] [CrossRef]
- Sahoo, N.K.; Singh, S.R.; Rajendran, A.; Shukla, D.; Chhablani, J. Masqueraders of central serous chorioretinopathy. Surv. Ophthalmol. 2019, 64, 30–44. [Google Scholar] [CrossRef] [PubMed]
- Mansour, A.M.; Koaik, M.; Lima, L.H.; Casella, A.M.B.; Uwaydat, S.H.; Shahin, M.; Tamim, H.; JoseSanchez-Ruiz, M.; Mansour, H.A.; Dodwell, D. Physiologic and psychologic risk factors in central serous chorioretinopathy. Ophthalmology 2017, 124, 1718–1722. [Google Scholar] [CrossRef] [PubMed]
- Shima, C.; Sakaguchi, H.; Gomi, F.; Kamei, M.; Iknuo, Y.; Osima, Y.; Sawa, M.; Tsujikawa, M.; Kusaka, S.; Tano, Y. Complications in patients after intravitreal injection of bevacizumab. Acta Ophthalmol. 2008, 86, 372–376. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-C.; Chou, Y.-B.; Lin, C.-K.; Tsai, C.-C.; Hsu, T.-K.; Chang, Y.-F.; Chao, H.-M.; Tung, T.-H.; Chen, S.-J.; Liu, J.-H. Characterization and functional correlation of multiple imaging modalities with focal choroidal excavation. J. Chin. Med. Assoc. 2018, 81, 487–495. [Google Scholar] [CrossRef]
- Goldhagen, B.E.; Goldhardt, R. Diagnosed a patient with central serous chorioretinopathy? Now What? Management of Central Serous Chorioretinopathy. Curr. Ophthalmol. Rep. 2017, 5, 141–148. [Google Scholar] [CrossRef]
- Schantz, H.; Madeira, D.; Johnson, R.N.; McDonald, R. Central serous chorioretinopathy occurring in patients 60 years of agents and older. Ophthalmology 1992, 99, 63–67. [Google Scholar] [CrossRef]
- Mathur, V.; Parihar, J.K.S.; Maggon, R.; Mishra, S.K. Role of tranpupillary thermotherapy in central serous chorioretinopathy. Med. J. Armed Forces India 2009, 65, 323–327. [Google Scholar] [CrossRef] [Green Version]
- Sun, J.; Tan, J.; Wang, Z.; Jong, Y.; Xuefei, Z.; Lingli, L. Effect of catecholamine on central serous chorioretinopathy. J. Huazhong Univ. Sci. Tech. 2003, 23, 313–316. [Google Scholar]
- Jeuune, C.L.L.; Hugues, F.C.; Dufier, J.L.; Munera, Y.; Bringer, L. Bronchial cardiovascular effects of ocular topical β-antagonist in asthmatic subjects: Comparison of timolol, carteolol and metipranolol. J. Clin. Pharmacol. 1989, 29, 97–101. [Google Scholar] [CrossRef]
- Afarid, M.; Sarvestani, A.S.; Rahat, F.; Azimi, L. Intravitreal injection of bevacizumab: Review of our previous experience. Iran. J. Pharm. Res. 2018, 17, 1093–1098. [Google Scholar]
- Chhablani, J.; Anantharaman, G.; Behar-Cohen, F.; Boon, C.; Manayath, G.; Singh, R. Management of central serous chorioretinopathy. Indian J. Ophthalmol. 2018, 12, 1700–1703. [Google Scholar] [CrossRef] [PubMed]
- Fabianová, J.; Porubská, M.; Cepilová, Z. Central serous chorioretinopathy treatment with beta-blockers. Cesk. Slov. Oftalmol. 1998, 54, 401–404. [Google Scholar] [PubMed]
- Chrapek, O.; Spacková, K.; Rehák, J. Treatment of central serous chorioretinopathy with beta-blockers. Cesk. Slov. Oftalmol. 2002, 58, 382–386. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Browing, D.J. Nadolol in the treatment of central serous retinopathy. Am. J. Ophthalmol. 1993, 116, 770–771. [Google Scholar] [CrossRef]
- Tatham, A.; MaCfarlane, A. The use of propranolol to treat central serous choriretinopathy: An evaluation by serial OCT. J. Ocul. Pharmacol. Ther. 2006, 22, 145–149. [Google Scholar] [CrossRef]
- Chrapek, O.; Jirkova, B.; Kandmal, V.; Rehak, J.; Sin, M. Treatment of central serous chorioretinopathy with beta-blocker metipranolol. Biomed. Pap. Med. Fac. Univ. Palacký. Olomouc. Czech. Repub. 2015, 159, 120–123. [Google Scholar] [CrossRef] [Green Version]
- Matulla, B.; Streit, G.; Pieh, S.; Findl, O.; Entlicher, J.; Graselli, U.; Eichler, H.G.; Wolzt, M.; Schmetterer, L. Effects of losartan on cerebral and ocular circulation in healthy subjects. Br. J. Clin. Pharmacol. 1997, 44, 369–375. [Google Scholar] [CrossRef] [Green Version]
- Wilkinson-Berka, J.L.; Tan, G.; Jaworski, K.; Harbig, H.; Miller, A.G. Identification of a retinal aldosterone system and the protective effects of mineralocorticoid receptor antagonism on retinal vascular pathology. Circ. Res. 2009, 104, 124–133. [Google Scholar] [CrossRef] [Green Version]
- Yancopoulos, G.D.; Davis, S.; Gale, N.W.; Rudge, J.S.; Wiegand, S.J.; Holash, J. Vascular-specific growth factors and blood vessel formation. Nature 2000, 407, 242–248. [Google Scholar] [CrossRef]
- Shao, Y.; Xu, T.T.; Zhou, Q.; Liu, R.Q.; Yang, Q.C.; Ye, L.; Lv, J.L. Clinical findings associated with propranolol and fenofibrate on acute central serous chorioretinopathy. Int. J. Clin. Exp. Med. 2017, 10, 638–647. [Google Scholar]
- Kazani, S.; Israel, E. What doesn’t not kill may not make you stronger: Beta-blockers for asthma. Am. J. Respir. Crit. Care Med. 2013, 187, 1281. [Google Scholar] [CrossRef] [PubMed]



| Group | Group 1 (n = 60) Propranolol Treatment | Group 2 (n = 60) Placebo Treatment | p-Value | |
|---|---|---|---|---|
| Parameter | ||||
| Mean complete remission time | * 1.9 months | 3.5 months | 0.008 | |
| Success rate | * 95.0% (57/60) | * 78.3% (47/60) | 0.001 | |
| Mean BCVA | * 0.09 ± 0.01 logMAR | 0.19 ± 0.03 logMAR | 0.032 | |
| Rate of recurrence | * 5.3% (3/57) | 25.5% (12/47) | 0.14 | |
| Group | Group 1 (Propranolol Group) | Group 2 (Placebo Group) | |
|---|---|---|---|
| Parameter | |||
| Eyes involved | 60 patients (60 eyes) | 60 patients (60 eyes) | |
| The drug for treatment | Propranolol | Vitamin C | |
| The given dose | 2 × 20 mg/day | 100 mg/day | |
| Mean age (years) | 42.5 ± 2.6 | 43.8 ± 3.4 | |
| Male/female ratio | 50:10 | 47:13 | |
| Parameters | Drugs | Doses | Eyes | Results and Outcomes | |
|---|---|---|---|---|---|
| Research Group | |||||
| Fabianová et al. [18] | Trimepranol Vasocardin | 2 × 10 mg/day 2 × 50 mg/day | 21 30 | 1. The average remission time was 4.5–4.8 weeks. 2. No difference in selective or non-selective blockers. | |
| Chrapek et al. [19] | Trimepranol | 2 × 5 mg/day | 13 | 1. Success rate: 84.6%. 2. Failure rate: 15.4%. 3. Complete remission time: 8.8 weeks. 4. Trimepranol was not reliable. | |
| Browing [20] | Nadolol | 40 mg/day | 4 | 1. Failure rate: 100%. 2. Nadolol had adverse effects. | |
| Tatham et al. [23] | Propranolol | 2 × 40 mg/day | 2 | 1. VA recovery and OCT became flat after 72 days. In addition, successful for both eyes. 2. Recurrence after 2 months likely. | |
| Chrapek et al. [21] | Metipranolol | 1 × 10 mg/day | 23 | 1. No significant difference between metipranolol and placebo therapy. 2. No effect on CSCR. | |
| Chen et al. (our study) | Propranolol | 2 × 20 mg/day | 60 | 1. Remission: 1.9 months. 2. Success rate: 95%. 3. Recurrence: 5.3%. | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, L.-C.; Ma, J.-W.; Shieh, P.-C.; Horng, C.-T. Oral Treatment of Central Serous Chorioretinopathy Patients Using Propranolol Tablets. Pharmaceuticals 2020, 13, 336. https://doi.org/10.3390/ph13110336
Chen L-C, Ma J-W, Shieh P-C, Horng C-T. Oral Treatment of Central Serous Chorioretinopathy Patients Using Propranolol Tablets. Pharmaceuticals. 2020; 13(11):336. https://doi.org/10.3390/ph13110336
Chicago/Turabian StyleChen, Li-Chai, Jui-Wen Ma, Po-Chuen Shieh, and Chi-Ting Horng. 2020. "Oral Treatment of Central Serous Chorioretinopathy Patients Using Propranolol Tablets" Pharmaceuticals 13, no. 11: 336. https://doi.org/10.3390/ph13110336
APA StyleChen, L. -C., Ma, J. -W., Shieh, P. -C., & Horng, C. -T. (2020). Oral Treatment of Central Serous Chorioretinopathy Patients Using Propranolol Tablets. Pharmaceuticals, 13(11), 336. https://doi.org/10.3390/ph13110336