Antiviral Activities of Eucalyptus Essential Oils: Their Effectiveness as Therapeutic Targets against Human Viruses
Abstract
:1. Introduction
2. Literature Search Strategy and Study Selection Criteria
3. Results and Discussion
3.1. Chemical Composition of Eucalyptus Essential Oil for Medicinal Use
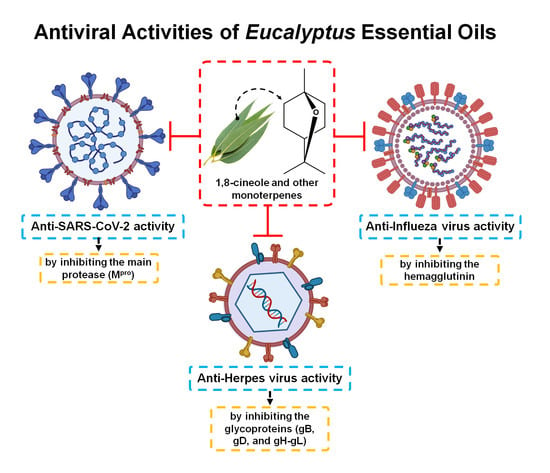
3.2. Antiviral Activity of Eucalyptus Essential Oil
3.2.1. Herpes Simplex Virus
3.2.2. Influenza Virus
3.2.3. SARS-CoV-2 (COVID-19)
3.2.4. Other Viruses
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chandorkar, N.; Tambe, S.; Amin, P.; Madankar, C. A systematic and comprehensive review on current understanding of the pharmacological actions, molecular mechanisms, and clinical implications of the genus Eucalyptus. Phyto. Plus 2021, 1, 100089. [Google Scholar] [CrossRef]
- Valenzuela, C.E.; Ballesta, P.; Ahmar, S.; Fiaz, S.; Heidari, P.; Maldonado, C.; Mora-Poblete, F. Haplotype- and SNP-Based GWAS for Growth and Wood Quality Traits in Eucalyptus cladocalyx Trees under Arid Conditions. Plants 2021, 10, 148. [Google Scholar] [CrossRef]
- Ballesta, P.; Serra, N.; Guerra, F.P.; Hasbún, R.; Mora, F. Genomic Prediction of Growth and Stem Quality Traits in Eucalyptus globulus Labill. at Its Southernmost Distribution Limit in Chile. Forests 2018, 9, 779. [Google Scholar] [CrossRef] [Green Version]
- Mora, F.; Ballesta, P.; Serra, N. Bayesian analysis of growth, stem straightness and branching quality in full-sib families of Eucalyptus globulus. Bragantia 2019, 78, 328–336. [Google Scholar] [CrossRef] [Green Version]
- Ballesta, P.; Bush, D.; Silva, F.F.; Mora, F. Genomic Predictions Using Low-Density SNP Markers, Pedigree and GWAS Information: A Case Study with the Non-Model Species Eucalyptus cladocalyx. Plants 2020, 9, 99. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maldonado, C.; Mora-Poblete, F.; Contreras-Soto, R.I.; Ahmar, S.; Chen, J.T.; do Amaral Júnior, A.T.; Scapim, C.A. Genome-Wide Prediction of Complex Traits in Two Outcrossing Plant Species Through Deep Learning and Bayesian Regularized Neural Network. Front. Plant Sci. 2020, 11, 593897. [Google Scholar] [CrossRef]
- Mora-Poblete, F.; Ballesta, P.; Lobos, G.A.; Molina-Montenegro, M.; Gleadow, R.; Ahmar, S.; Jiménez-Aspee, F. Genome-wide association study of cyanogenic glycosides, proline, sugars, and pigments in Eucalyptus cladocalyx after 18 consecutive dry summers. Physiol. Plant. 2021, 172, 1550–1569. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Zhu, Y.; Xu, J.; Lu, Z.; Chen, G.; Song, P.; Guo, W. Genetic variation and genetic gain for energy production, growth traits and wood properties in Eucalyptus hybrid clones in China. Aust. For. 2017, 80, 57–65. [Google Scholar] [CrossRef]
- Salehi, B.; Sharifi-Rad, J.; Quispe, C.; Llaique, H.; Villalobos, M.; Smeriglio, A.; Trombetta, D.; Ezzat, Z.M.; Salem, M.A.; Zayed, A.; et al. Insights into Eucalyptus genus chemical constituents, biological activities and health-promoting effects. Trends Food Sci. Technol. 2019, 91, 609–624. [Google Scholar] [CrossRef]
- Shao, J.; Yin, Z.; Wang, Y.; Yang, Y.; Tang, Q.; Zhang, M. Effects of different doses of Eucalyptus oil from Eucalyptus globulus Labill on respiratory tract immunity and immune function in healthy Rats. Front. Pharmacol. 2020, 11, 1287. [Google Scholar] [CrossRef]
- da Silva, J.K.R.; Figueiredo, P.L.B.; Byler, K.G.; Setzer, W.N. Essential Oils as Antiviral Agents. Potential of Essential Oils to Treat SARS-CoV-2 Infection: An In−Silico Investigation. Int. J. Mol. Sci. 2020, 21, 3426. [Google Scholar] [CrossRef]
- Fitriani, I.N.; Utami, W.; Zikri, A.T.; Santoso, P. In Silico Approach of Potential Phytochemical Inhibitor from Moringa oleifera, Cocos nucifera, Allium cepa, Psidium guajava, and Eucalyptus globulus for the Treatment of COVID-19 by Molecular Docking. Res. Sq. 2020. Available online: https://doi.org/10.21203/rs.3.rs-42747/v1 (accessed on 30 June 2021).
- Panikar, S.; Shoba, G.; Arun, M.; Sahayarayan, J.J.; Raja Nanthini, A.U.; Chinnathambi, A.; Alharbi, S.A.; Nasif, O.; Kim, H.J. Essential oils as an effective alternative for the treatment of COVID-19: Molecular interaction analysis of protease (Mpro) with pharmacokinetics and toxicological properties. J. Infect. Public Health 2021, 14, 601–610. [Google Scholar] [CrossRef]
- Sagaya Jansi, R.; Khusro, A.; Agastian, P.; Alfarhan, A.; Al-Dhabi, N.A.; Arasu, M.V.; Rajagopal, R.; Barcelo, D.; Al-Tamimi, A. Emerging paradigms of viral diseases and paramount role of natural resources as antiviral agents. Sci. Total Environ. 2021, 10, 143539. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.H. Anti–SARS-CoV-2 Natural Products as Potentially Therapeutic Agents. Front. Pharmacol. 2021, 12, 590509. [Google Scholar] [CrossRef] [PubMed]
- Silveira, D.; Prieto-garcia, J.M.; Boylan, F.; Estrada, O.; Fonseca-bazzo, Y.M.; Jamal, C.M.; Magalhães, P.O.; Oliveira, E.; Tomczyk, M.; Heinrich, M. COVID-19: Is there evidence for the use of herbal medicines as adjuvant symptomatic therapy? Front Pharmacol. 2020, 11, 1479. [Google Scholar] [CrossRef]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gotzsche, P.C.; Ioannidis, J.P.A.; Clarke, M.; Devereauz, P.J.; Kleijnen, J.; Moher, D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: Explanation and elaboration. Brit. Med. J. 2009, 339, e1–e34. [Google Scholar] [CrossRef] [Green Version]
- Elaissi, A.; Rouis, Z.; Salem, N.A.B.; Mabrouk, S.; Salem, Y.B.; Salah, K.B.H.; Aouni, M.; Farhat, F.; Chemli, R.; Skhiri, F.H.; et al. Chemical composition of 8 Eucalyptus species essential oils and the evaluation of their antibacterial, antifungal and antiviral activities. BMC Complement. Altern. 2012, 12, 81. [Google Scholar] [CrossRef] [Green Version]
- Mubarak, E.E.; Landa, Z.A.; Ahmed, I.F.A.; Ahmed, A.B.A.; Taha, R. Essential Oil Compositions and Cytotoxicity from Various Organs of Eucalyptus camaldulensis. Int. J. Agric. Biol. 2015, 17, 1560–8530. [Google Scholar]
- Chalchat, J.C.; Muhayimana, A.; Habimana, J.B.; Chabard, J.L. Aromatic Plants of Rwanda. II. Chemical Composition of Essential Oils of Ten Eucalyptus Species Growing in Ruhande Arboretum. Butare, Rwanda. J. Essent. Oil Res. 1997, 9, 159–165. [Google Scholar] [CrossRef]
- Hardel, D.; Laxmidhar, S. A review on phytochemical and pharmacological of Eucalyptus globulus: A multipurpose tree. Int. J. Res. Ayurveda Pharm. 2011, 2, 1527–1530. [Google Scholar]
- Setzer, W.N. Essential oils as complementary and alternative medicines for the treatment of influenza. Am. J. Essent. Oil Nat. Prod. 2016, 4, 16–22. [Google Scholar]
- Li, Y.; Xu, Y.; Lai, Y.; Liao, S.; Liu, N.; Xu, P. Intranasal co-administration of 1,8-cineole with influenza vaccine provide cross-protection against influenza virus infection. Phytomedicine 2017, 34, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Dhakad, A.K.; Pandey, V.V.; Beg, S.; Rawat, J.M.; Singh, A. Biological, medicinal and toxicological significance of Eucalyptus leaf essential oil: A review. J. Sci. Food Agric. 2017, 98, 833–848. [Google Scholar] [CrossRef] [PubMed]
- Juergens, L.J.; Worth, H.; Juergens, U.R. New Perspectives for Mucolytic, Anti-inflammatory and Adjunctive Therapy with 1,8-Cineole in COPD and Asthma: Review on the New Therapeutic Approach. Adv. Ther. 2020, 37, 1737–1753. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ogunwande, I.A.; Olawore, N.O.; Adeleke, K.A.; Konig, W.A. Chemical Composition of the Essential Oils from the Leaves of Three Eucalyptus Species Growing in Nigeria. J. Essent. Oil Res. 2003, 15, 297–301. [Google Scholar] [CrossRef]
- Maciel, M.V.; Morais, S.M.; Bevilaqua, C.M.L.; Silva, R.A.; Barros, R.S.; Sousa, R.N.; Sousa, L.C.; Brito, E.S.; Souza-Neto, M.A. Chemical composition of Eucalyptus spp. essential oils and their insecticidal effects on Lutzomyia longipalpis. Vet. Parasitol. 2010, 167, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Juan, L.W.; Lucia, A.; Zerba, E.N.; Harrand, L.; Marco, M.; Masuh, H.M. Chemical Composition and Fumigant Toxicity of the Essential Oils From 16 Species of Eucalyptus Against Haematobia irritans (Diptera: Muscidae) Adults. J. Econ. Entomol. 2011, 104, 1087–1092. [Google Scholar] [CrossRef]
- Sebei, K.; Sakouhi, F.; Herchi, W.; Khouja, M.L.; Boukhchina, S. Chemical composition and antibacterial activities of seven Eucalyptus species essential oils leaves. Biol. Res. 2015, 48, 1–5. [Google Scholar] [CrossRef] [Green Version]
- Aleksic Sabo, V.; Knezevic, P. Antimicrobial activity of Eucalyptus camaldulensis Dehn. plant extracts and essential oils: A review. Ind. Crop. Prod. 2019, 132, 413–429. [Google Scholar] [CrossRef]
- Gavanji, S.; Sayedipour, S.S.; Larki, B.; Bakhtari, A. Antiviral activity of some plant oils against herpes simplex virus type 1 in Vero cell culture. J. Acute Med. 2015, 5, 62–68. [Google Scholar] [CrossRef] [Green Version]
- Tagde, P.; Tagde, S.; Tagde, P.; Bhattacharya, T.; Monzur, S.M.; Rahman, M.H.; Otrisal, P.; Behl, T.; ul Hassan, S.S.; Abdel-Daim, M.M.; et al. Nutraceuticals and Herbs in Reducing the Risk and Improving the Treatment of COVID-19 by Targeting SARS-CoV-2. Biomedicines 2021, 9, 1266. [Google Scholar] [CrossRef]
- Schnitzler, P. Essential Oils for the Treatment of Herpes Simplex Virus Infections. Chemotherapy 2019, 64, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Reichling, J. Antiviral and Virucidal Properties of Essential Oils and Isolated Compounds—A Scientific Approach. Planta Med. 2021. Available online: https://doi.org/10.1055/a-1382-2898 (accessed on 30 June 2021). [CrossRef] [PubMed]
- van de Sand, L.; Bormann, M.; Schmitz, Y.; Heilingloh, C.S.; Witzke, O.; Krawczyk, A. Antiviral Active Compounds Derived from Natural Sources against Herpes Simplex Viruses. Viruses 2021, 13, 1386. [Google Scholar] [CrossRef]
- Bourne, K.Z.; Bourne, N.; Reising, S.F.; Stanberry, L.R. Plant products as topical microbicide candidates: Assessment of in vitro and in vivo activity against herpes simplex virus type 2. Antivir. Res. 1999, 42, 219–226. [Google Scholar] [CrossRef]
- Schnitzler, P.; Schön, K.; Reichling, J. Antiviral activity of Australian tea tree oil and Eucalyptus oil against herpes simplex virus in cell culture. Pharmazie 2001, 56, 343–347. [Google Scholar] [PubMed]
- Minami, M.; Kita, M.; Nakaya, T.; Yamamoto, T.; Kuriyama, H.; Imanishi, J. The Inhibitory Effect of Essential Oils on Herpes Simplex Virus Type-1 Replication In Vitro. Microbiol. Immunol. 2003, 47, 681–684. [Google Scholar] [CrossRef]
- Astani, A.; Reichling, J.; Schnitzler, P. Comparative study on the antiviral activity of selected monoterpenes derived from essential oils. Phytother. Res. 2010, 24, 557–559. [Google Scholar] [CrossRef] [PubMed]
- Usachev, E.V.; Pyankov, O.V.; Usacheva, O.V.; Agranovski, I.E. Antiviral activity of tea tree and Eucalyptus oil aerosol and vapour. J. Aerosol Sci. 2013, 59, 22–30. [Google Scholar] [CrossRef]
- Vimalanathan, S.; Hudson, J. Anti-influenza virus activity of essential oils and vapors. Am. J. Essent. Oil Nat. Prod. 2014, 2, 47–53. [Google Scholar]
- Li, Y.; Lai, Y.; Wang, Y.; Liu, N.; Zhang, F.; Xu, P. 1,8-Cineol Protect Against Influenza-Virus-Induced Pneumonia in Mice. Inflammation 2016, 39, 1582–1593. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.D.; Kaur, I. Eucalyptol (1,8 cineole) from Eucalyptus Essential Oil a Potential Inhibitor of COVID 19 Corona Virus Infection by Molecular Docking Studies. Not. Sci. Biol. 2020, 12, 536–545. [Google Scholar] [CrossRef]
- Cermelli, C.; Fabio, A.; Fabio, G.; Quaglio, P. Effect of Eucalyptus essential oil on respiratory bacteria and viruses. Curr. Microbiol. 2008, 56, 89–92. [Google Scholar] [CrossRef]
- El-Baz, F.K.; Mahmoud, K.; El-Senousy, W.M.; Darwesh, O.M.; ElGohary, A.E. Antiviral, antimicrobial and schistosomicidal activities of Eucalyptus camaldulensis essential oils. Int. J. Pharm. Sci. Res. 2015, 31, 262–268. [Google Scholar]
- Karasneh, G.A.; Shukla, D. Herpes simplex virus infects most cell types in vitro: Clues to its success. Virol. J. 2011, 8, 481. [Google Scholar] [CrossRef] [Green Version]
- Lussignol, M.; Esclatine, A. Herpesvirus and Autophagy: “All Right, Everybody Be Cool, This Is a Robbery!”. Viruses 2017, 9, 372. [Google Scholar] [CrossRef] [Green Version]
- Choi, H. Chemical Constituents of Essential Oils Possessing Anti-Influenza A/WS/33 Virus Activity. Osong. Public Health Res. Perspect. 2018, 9, 348–353. [Google Scholar] [CrossRef]
- Najar, B.; Nardi, V.; Stincarelli, M.A.; Patrissi, S.; Pistelli, L.; Giannecchini, S. Screening of the essential oil effects on human H1N1 influenza virus infection: An in vitro study in MDCK cells. Nat. Prod. Res. 2021, 1–4. Available online: https://doi.org/10.1080/14786419.2021.1944137 (accessed on 30 June 2021). [CrossRef]
- Pyankov, O.V.; Usachev, E.V.; Pyankova, O.; Agranovski, I.E. Inactivation of Airborne Influenza Virus by Tea Tree and Eucalyptus Oils. Aerosol Sci. Technol. 2012, 46, 1295–1302. [Google Scholar] [CrossRef]
- Wani, A.R.; Yadav, K.; Khursheed, A.; Rather, M.A. An updated and comprehensive review of the antiviral potential of essential oils and their chemical constituents with special focus on their mechanism of action against various influenza and coronaviruses. Microb. Pathog. 2020, 152, 104620. [Google Scholar] [CrossRef] [PubMed]
- Gavliakova, S.; Dolak, T.; Licha, H.; Krizova, S.; Plevkova, J. Cineole, thymol and camphor nasal challenges and their effect on nasal symptoms and cough in an animal model. Acta Med. Int. 2013, 13, 5–13. [Google Scholar] [CrossRef] [Green Version]
- Worth, H.; Dethlefsen, U. Patients with Asthma Benefit from Concomitant Therapy with Cineole: A Placebo-Controlled, Double-Blind Trial. J. Asthma 2012, 49, 849–853. [Google Scholar] [CrossRef] [PubMed]
- Worth, H.; Schacher, C.; Dethlefsen, U. Concomitant therapy with Cineole (Eucalyptole) reduces exacerbations in COPD: A placebo-controlled double-blind trial. Respir. Res. 2009, 10, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kehrl, W.; Sonnemann, U.; Dethlefsen, U. Therapy for Acute Nonpurulent rhinosinusitis with Cineole: Results of a double-blind, randomized, placebo-controlled trial. Laryngoscope 2004, 114, 738–742. [Google Scholar] [CrossRef]
- Müller, J.; Greiner, J.F.W.; Zeuner, M.; Brotzmann, V.; Schafermann, J.; Wieters, F.; Widera, D.; Sudhoff, H.; Kaltschmidt, B.; Kaltschmidt, C. 1,8-Cineole potentiates IRF3-mediated antiviral response in human stem cells and in an ex vivo model of rhinosinusitis. Clin. Sci. 2016, 130, 1339–1352. [Google Scholar] [CrossRef]
- Neumann, G.; Noda, T.; Kawaoka, Y. Emergence and pandemic potential of swine-origin H1N1 influenza virus. Nature 2009, 459, 931–939. [Google Scholar] [CrossRef] [Green Version]
- Shi, Y.; Wu, Y.; Zhang, W.; Qi, J.; Gao, G.F. Enabling the “host jump”: Structural determinants of receptor-binding specificity in influenza A viruses. Nat. Rev. Microbiol. 2014, 12, 822–831. [Google Scholar] [CrossRef]
- Nadjib, B.M. Effective Antiviral Activity of Essential Oils and their Characteristic Terpenes against Coronaviruses: An Update. J. Pharmacol. Clin. Toxicol. 2020, 8, 1138. [Google Scholar]
- Abbass, H.S. Eucalyptus essential oil; an off-label use to protect the world from COVID-19 pandemic: Review-based hypotheses. Univers. J. Pharm. Res. 2020, 5, 57–60. [Google Scholar] [CrossRef]
- Doroftei, B.; Ciobica, A.; Ilie, O.D.; Maftei, R.; Ilea, C. Mini-Review Discussing the Reliability and Efficiency of COVID-19 Vaccines. Diagnostics 2021, 11, 579. [Google Scholar] [CrossRef] [PubMed]
- Asif, M.; Saleem, M.; Saadullah, M.; Yaseen, H.S.; Al Zarzour, R. COVID-19 and therapy with essential oils having antiviral, anti-inflammatory, and immunomodulatory properties. Inflammopharmacology 2020, 28, 1153–1161. [Google Scholar] [CrossRef] [PubMed]
- Colalto, C. Volatile molecules for COVID-19: A possible pharmacological strategy? Drug Dev. Res. 2020, 81, 950–968. [Google Scholar] [CrossRef]
- V’kovski, P.; Kratzel, A.; Steiner, S.; Stalder, H.; Thiel, V. Coronavirus biology and replication: Implications for SARS-CoV-2. Nat. Rev. Microbiol. 2020, 19, 155–170. [Google Scholar] [CrossRef] [PubMed]
- Alvarez Costa, A.; Naspi, C.V.; Lucia, A.; Masuh, H.M. Repellent and Larvicidal Activity of the Essential Oil from Eucalyptus nitens Against Aedes aegypti and Aedes albopictus (Diptera: Culicidae). J. Med. Entomol. 2017, 54, 670–676. [Google Scholar] [CrossRef]
- Manh, H.D.; Hue, D.T.; Hieu, N.T.T.; Tuyen, D.T.T.; Tuyet, O.T. The Mosquito Larvicidal Activity of Essential Oils from Cymbopogon and Eucalyptus Species in Vietnam. Insects 2020, 11, 128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lucia, A.; Juan, L.W.; Zerba, E.N.; Harrand, L.; Marcó, M.; Masuh, H.M. Validation of models to estimate the fumigant and larvicidal activity of Eucalyptus essential oils against Aedes aegypti (Diptera: Culicidae). Parasitol. Res. 2011, 110, 1675–1686. [Google Scholar] [CrossRef]
- Madreseh-Ghahfarokhi, S.; Dehghani-Samani, A.; Pirali, Y.; Dehghani-Samani, A. Zingiber officinalis and Eucalyptus globulus, Potent Lethal/Repellent Agents against Rhipicephalus bursa, Probable Carrier for Zoonosis. J. Arthropod-Borne Dis. 2019, 13, 214–223. [Google Scholar] [CrossRef]
- Yao, Z.-W.; Liu, H.; Zhou, R.; Feng, M.-Y.; Wang, F.; Qin, X.-J.; Chen, X.-X.; Zheng, C.-B.; Luo, R.-H.; Yang, L.-M.; et al. Non-volatile acylphloroglucinol components from Eucalyptus robusta inhibit Zika virus by impairing RdRp activity of NS5. Bioorg. Chem. 2021, 116, 105303. [Google Scholar] [CrossRef]




| Species | Essential Oil Constituents (%) | References |
|---|---|---|
| Eucalyptus polybractea | 1,8-cineole (85.01), p-cymene (4.12), terpinen-4-ol (1.48), limonene (1.00), α-terpineol (0.70), minor constituents (7.69) | [28] |
| Eucalyptus smithii | 1,8-cineole (84.27), limonene (2.86), α-terpinyl acetate (1.51), p-cymene (1.27), α-pinene (1.02), minor constituents (9.07) | [20] |
| Eucalyptus globulus | 1,8-cincole (83.89), limolene (8.16), ᾳ-pinene (4.15), o-cymene (2.93), minor constituents (0.87) | [27] |
| Eucalyptus maidenii | 1,8-cineole (83.59), globulol (3.61), trans-pinocarveol (3.40), pinocarvone (1.28), α-pinene (1.27%), minor constituents (6.85) | [29] |
| Eucalyptus bicostata | 1,8-cineole (81.29), trans-pinocarveol (4.49), pinocarvone (3.93), α-pinene (2.16), globulol (1.81), minor constituents (6.32) | [29] |
| Eucalyptus sideroxylon | 1,8-cineole (80.75), α-pinene (5.81), limonene (3.32), α-terpineol (2.45), α-terpinyl acetate (2.30), trans-pinocarveol (1.00), minor constituents (4.62) | [29] |
| Eucalyptus cinerea | 1,8-cineole (79.18), α-terpinyl acetate (5.43), α-pinene (4.08), α-terpineol (2.20), trans-pinocarveol (2.07), minor constituents (7.04) | [29] |
| Eucalyptus leucoxylon | 1,8-cineole (77.76), α-pinene (5.85), trans-pinocarveol (3.23), globulol (1.42), limonene (1.33), pinocarvone (1.15), minor constituents (7.26) | [29] |
| Eucalyptus caesia | 1,8-cineole (40.18), p-cymene (14.11), γ-terpinene (12.43), α-pinene (7.70), terpinen-4-ol (5.62), α-terpineol (1.53), minor constituents (18.43) | [31] |
| Eucalyptus camaldulensis | γ-terpinene (72.50), o-cymene (14.60), terpinen-4-ol (6.70), 1,8-cineole (0.90), minor constituents (5.30) | [19] |
| Eucalyptus tereticornis | β-pinene (39.40), α-pinene (21.40), limolene (8.00), α-phellandrene (5.00), p-cymene (4.10), γ-terpinene (2.40), minor constituents (19.70) | [26] |
| Eucalyptus grandis | α-pinene (30.40), terpen-4-ol (10.70), (E)-β-ocimene (9.40), terpinen-4-ol (8.40), α-terpineol (8.00), α-humulene (3.20), β-eudesmol (2.20), minor constituents (27.70) | [26] |
| Treatment | Type of Study | Active against | Main Findings | IC50/IC100 | Mechanism of Action/Viral Target | Reference |
|---|---|---|---|---|---|---|
| 1,8-cineole | In vivo (murine model (females) of genital infection) | HSV-2 | At an absolute concentration (100%), 1,8-cineole produced a 44% reduction in viral infection. | - | Protection prior to the viral infection challenge | [36] |
| Essential oil (E. caesia) | In vitro (plaque reduction assay in RC-37 cells) | HSV-1 and HSV-2 | At a concentration of 0.03% in medium, E. caesia essential oil reduced virus titers by 57.9% for HSV-1 and 75.4% for HSV-2. Significant inhibitory effect on the HSV-1 and HSV-2 plaque formation. | IC50 of 0.009% for HSV-1 and 0.008% for HSV-2. | Direct binding to free virus | [37] * |
| Essential oil (E. globulus) | In vitro (plaque reduction assay in Vero cells) | HSV-1 | Significant inhibitory effect on the HSV-1 plaque formation. | IC100 of 1%. | Direct binding to free virus | [38] * |
| Essential oil (E. globulus) and individual monoterpenes (1,8-cineole, α-pinene, p-cymene, γ-terpinene, α-terpineol, and terpinen-4-ol) | In vitro (plaque reduction assay in RC-37 cells) | HSV-1 (cepa KOS) | Significant inhibitory effect on the HSV-1 plaque formation. | IC50: E. globulus essential oil (55 μg/mL), 1,8-cineole (1.20 mg/mL), α-pinene (4.5 μg/mL), γ-terpinene (7.0 μg/mL), p-cymene. (16.0 μg/mL), α-terpineol (22.0 μg/mL), terpinen-4-ol (60.0 μg/mL). | Direct binding to free virus | [39] * |
| Essential oil (E. caesia) | In vitro (plaque reduction assay in Vero cells) | HSV-1 | Significant inhibitory effect on the HSV-1 plaque formation. | IC50 of 0.004%. | Direct binding to free virus | [31] * |
| Essential oil (E. polybractea) | In vitro (plaque reduction assay in MDCK cells) | Influenza virus A (NWS/G70C/H11N9) | Exposure to the aerosol (15 s; 125 μg/L of air) achieved 100% inactivation of IFV-A in the air. One day of exposure to oil vapor (saturated) reduced viral infection by IFV-A by 86%. | - | Direct binding to free virus | [40] |
| Essential oil (E. globulus) | In vitro (plaque reduction assay in MDCK cells and hemagglutinin and neuraminidase inhibition assays) | Influenza virus A (Denver/1/57/H1N1) | Significant inhibitory effect on the Influenza virus A plaque formation. Exposure to steam (250 µL of essential oil at 100%) for 10 min produced a 94% reduction in viral infection. Exposure to steam (1/160 dilution of essential oil) for 10 min inhibited hemagglutinin activity but not neuraminidase activity. | IC100 of 50 μL/mL. | Binding with the virus surface hemagglutinin protein (responsible for the binding of the virus to the host cell) | [41] * |
| 1,8-cineole | In vivo (murine model of influenza infection) | Influenza virus A (FM/47/H1N1) | Treatments of 60 and 120 mg/kg prolonged the survival time of the mice. Treatment decreased IL-4, IL-5, IL-10, and MCP-1 levels in nasal lavage fluids and IL-1β, IL-6, TNF-α, and IFN-γ levels in lung tissue of mice infected with the virus. The expression of NF-kB p65, ICAM-1, and VCAM)-1 decreased. | - | Attenuate pulmonary inflammatory responses caused by IFV-A | [42] |
| 1,8-cineole | In vivo (murine model immunized and then challenged with the virus) | Influenza virus A (FM/47/H1N1) | The coadministration of the vaccine with 1,8-cineole (12.5 mg/kg) increased the serum production of specific antibodies against influenza (IgG2a), the secretory response of IgA in the nasal cavity mucosa, the expression of intraepithelial lymphocytes in the upper respiratory tract, the maturation of dendritic cells and the expression of costimulatory molecules cluster of differentiation (CD) 40, CD80 and CD86 in peripheral blood. | - | Cross-protection against influenza virus | [23] |
| 1,8-cineole | In silico (molecular docking: Mpro -1,8-cineole interaction) | SARS-CoV-2 | The free energy of binding was −6.04 kcal/mol within the amino acids of the active site of Mpro. The interaction of 1,8-cineole in the binding pocket of the active site of Mpro was mediated by two hydrophobic interactions through MET6, PHE8, ASP295, and ARG298. | - | Interaction with the active site of Mpro | [43] |
| 1,8-cineole, α-pinene α-terpineol, limonene and o-cymene | In silico (molecular docking: Mpro -monoterpene interaction) | SARS-CoV-2 | The binding of monoterpenes to the active site of Mpro was investigated. The following binding free energies were obtained: 1,8-cineole (−5.86 kcal/mol) > α-pinene (−5.6 kcal/mol) > α-terpineol (−5.43 kcal/mol), > limonene (−5.18 kcal/mol), >o-cymene (−4.99 kcal/mol). | - | Interaction with the active site of Mpro | [13] |
| Essential oil (E. globulus) | In vitro (plaque reduction assay in Vero cells) | Mumps virus | Treatment with 0.25 μg/mL reduced virus plaque formation by nearly 33%. | - | Direct binding to free virus | [44] |
| Essential oil (E. maidenii, E. cinerea, and E. bicostata) | In vitro (Vero cell protection assay) | Coxsackievirus B3 strain Nancy | Significant reduction in viral infectivity. | IC50: E. bicostata (0.7 µg/µL), E. cinerea (102.0 µg/µL), E. maidenii (136.5 µg/µL). | Direct binding to free virus | [18] * |
| Essential oil (E. camaldulensis) | In vitro (plaque reduction assay in MA104, BGM, and Vero cells) | Rotavirus strain Wa, Coxsackievirus B4, and HSV-1 | A 1/10 dilution of 100 μL of oil reduced Rotavirus strain Wa, Coxsackievirus B4, and HSV-1 plaque formation by 50%, 53.3%, and 90%, respectively. | - | Direct binding to free virus | [45] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mieres-Castro, D.; Ahmar, S.; Shabbir, R.; Mora-Poblete, F. Antiviral Activities of Eucalyptus Essential Oils: Their Effectiveness as Therapeutic Targets against Human Viruses. Pharmaceuticals 2021, 14, 1210. https://doi.org/10.3390/ph14121210
Mieres-Castro D, Ahmar S, Shabbir R, Mora-Poblete F. Antiviral Activities of Eucalyptus Essential Oils: Their Effectiveness as Therapeutic Targets against Human Viruses. Pharmaceuticals. 2021; 14(12):1210. https://doi.org/10.3390/ph14121210
Chicago/Turabian StyleMieres-Castro, Daniel, Sunny Ahmar, Rubab Shabbir, and Freddy Mora-Poblete. 2021. "Antiviral Activities of Eucalyptus Essential Oils: Their Effectiveness as Therapeutic Targets against Human Viruses" Pharmaceuticals 14, no. 12: 1210. https://doi.org/10.3390/ph14121210
APA StyleMieres-Castro, D., Ahmar, S., Shabbir, R., & Mora-Poblete, F. (2021). Antiviral Activities of Eucalyptus Essential Oils: Their Effectiveness as Therapeutic Targets against Human Viruses. Pharmaceuticals, 14(12), 1210. https://doi.org/10.3390/ph14121210