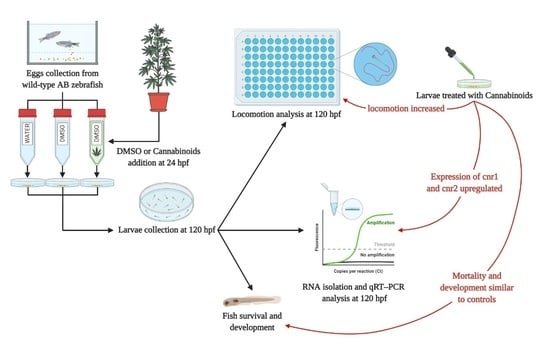
In Vivo Evaluation of Cannabis sativa Full Extract on Zebrafish Larvae Development, Locomotion Behavior and Gene Expression
Abstract
:1. Introduction
2. Results
2.1. Embryo Development and Survival
2.2. Locomotion Behavior
2.3. Analysis of Cannabinoid Receptors Expressions
3. Discussion
4. Materials and Methods
4.1. Plant Material and Preparation of the Cannabis Extract
4.2. Fish Management
4.3. Experimental Design
4.4. Measurements and Analysis
4.5. qRT-PCR
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Thornton, C.; Dickson, K.E.; Carty, D.R.; Ashpole, N.M.; Willett, K.L. Cannabis constituents reduce seizure behavior in chemically-induced and scn1a-mutant zebrafish. Epilepsy Behav. 2020, 110, 107152. [Google Scholar] [CrossRef]
- Della Rocca, G.; Di Salvo, A. Hemp in Veterinary Medicine: From Feed to Drug. Front. Vet. Sci. 2020, 7, 387. [Google Scholar] [CrossRef]
- Ahmed, K.T.; Amin, M.R.; Shah, P.; Ali, D.W. Motor neuron development in zebrafish is altered by brief (5-hr) exposures to THC (∆9-tetrahydrocannabinol) or CBD (cannabidiol) during gastrulation. Sci. Rep. 2018, 8, 10518. [Google Scholar] [CrossRef] [Green Version]
- Citti, C.; Pacchetti, B.; Vandelli, M.A.; Forni, F.; Cannazza, G. Analysis of cannabinoids in commercial hemp seed oil and decarboxylation kinetics studies of cannabidiolic acid (CBDA). J. Pharm. Biomed. Anal. 2018, 149, 532–540. [Google Scholar] [CrossRef]
- Andre, C.M.; Hausmanand, J.F.; Guerriero, G. Cannabis sativa: The Plant of the Thousand and One Molecules. Front. Plant Sci. 2016, 7, 19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pertwee, R.G. Handbook of Cannabis; Oxford University Press: New York, NY, USA, 2014. [Google Scholar]
- Maroon, J.; Bost, J. Review of the neurological benefits of phytocannabinoids. Surg. Neurol. Int. 2018, 9, 91. [Google Scholar] [CrossRef] [PubMed]
- Jensen, H.M.; Korbut, R.; Kania, P.W.; Buchmann, K. Cannabidiol effects on behaviour and immune gene expression in zebrafish (Danio rerio). PLoS ONE 2018, 13, e0200016. [Google Scholar] [CrossRef]
- Goodwin, R.D.; Kim, J.H.; Cheslack-Postava, K.; Weinberger, A.H.; Wu, M.; Wyka, K.; Kattan, M. Trends in cannabis use among adults with children in the home in the United States, 2004–2017: Impact of state-level legalization for recreational and medical use. Addiction 2021, 116, 2770–2778. [Google Scholar] [CrossRef]
- Devinsky, O.; Cross, J.H.; Wright, S. Trial of Cannabidiol for Drug-Resistant Seizures in the Dravet Syndrome. N. Engl. J. Med. 2017, 377, 699–700. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ingram, G.; Pearson, O.R. Cannabis and multiple sclerosis. Pract. Neurol. 2019, 19, 310–315. [Google Scholar] [CrossRef]
- Kerr, A.; Walston, V.; Wong, V.S.S.; Kellogg, M.; Ernst, L. Marijuana use among patients with epilepsy at a tertiary care center. Epilepsy Behav. 2019, 97, 144–148. [Google Scholar] [CrossRef] [PubMed]
- Kosiba, J.D.; Maisto, S.A.; Ditre, J.W. Patient-reported use of medical cannabis for pain, anxiety, and depression symptoms: Systematic review and meta-analysis. Soc. Sci. Med. 2019, 233, 181–192. [Google Scholar] [CrossRef]
- Hamilton, I.; Monaghan, M. Cannabis and Psychosis: Are We any Closer to Understanding the Relationship? Curr. Psychiatry Rep. 2019, 21, 48. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boehnke, K.F.; Scott, J.R.; Litinas, E.; Sisley, S.; Williams, D.A.; Clauw, D.J. Pills to Pot: Observational Analyses of Cannabis Substitution Among Medical Cannabis Users with Chronic Pain. J. Pain 2019, 20, 830–841. [Google Scholar] [CrossRef]
- MacMillan, K.; MacMillan, K.M.; Keddy, A.; Furlong, J. Cannabis and glaucoma: A literature review. Dalhous. Med. J. 2019, 46. [Google Scholar] [CrossRef]
- Habib, G.; Avisar, I. The consumption of Cannabis by fibromyalgia patients in Israel. Pain Res. Treat. 2018, 2018, 1–5. [Google Scholar] [CrossRef] [Green Version]
- Guzmán, M. Cannabis for the Management of Cancer Symptoms: THC Version 2.0? Cannabis Cannabinoid Res. 2018, 3, 117–119. [Google Scholar] [CrossRef] [Green Version]
- Bonini, S.A.; Premoli, M.; Tambaro, S.; Kumar, A.; Maccarinelli, G.; Memo, M.; Mastinu, A. Cannabis sativa: A comprehensive ethnopharmacological review of a medicinal plant with a long history. J. Ethnopharmacol. 2018, 5, 300–315. [Google Scholar] [CrossRef]
- Bailoni, L.; Bacchin, E.; Trocino, A.; Arango, S. Hemp (Cannabis sativa L.) Seed and Co-Products Inclusion in Diets for Dairy Ruminants: A Review. Animals 2021, 11, 856. [Google Scholar] [CrossRef]
- Klir, Ž.; Novoselec, J.; Antunović, Z. An Overview on the Use of Hemp (Cannabis Sativa L.) in Animal Nutrition. Poljoprivreda 2019, 25, 52–61. [Google Scholar] [CrossRef]
- Vispute, M.M.; Sharma, D.; Mandal, A.B.; Rokade, J.J.; Tagyi, P.K.; Yadav, A.S. Effect of dietary supplementation of hemp (Cannabis sativa) and dill seed (Anethum graveolens) on performance, serum biochemicals and gut health of broiler chickens. J. Anim. Physiol. Anim. Nutr. 2019, 103, 525–533. [Google Scholar] [CrossRef]
- Archie, S.R.; Cucullo, L. Harmful Effects of Smoking Cannabis: A Cerebrovascular and Neurological Perspective. Front. Pharmacol. 2019, 10, 1481. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akhtar, M.T.; Ali, S.; Rashidi, H.; van der Kooy, F.; Verpoorte, R.; Richardson, M.K. Developmental effects of cannabinoids on zebrafish larvae. Zebrafish 2013, 10, 283–293. [Google Scholar] [CrossRef]
- Amin, M.R.; Ahmed, K.T.; Ali, D.W. Early Exposure to THC Alters M-Cell Development in Zebrafish Embryos. Biomedicines 2020, 8, 5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, H.C.; Mackie, K. An Introduction to the Endogenous Cannabinoid System. Biol. Psychiatry 2016, 79, 516–525. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kyriakatos, A.; El Manira, A. Long-term plasticity of the spinal locomotor circuitry mediated by endocannabinoid and nitric oxide signaling. J. Neurosci. 2007, 27, 12664–12674. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carty, D.R.; Thornton, C.; Gledhill, J.H.; Willett, K.L. Developmental Effects of Cannabidiol and Δ9-Tetrahydrocannabinol in Zebrafish. Toxicol. Sci. 2018, 162, 137–145. [Google Scholar] [CrossRef] [Green Version]
- Oltrabella, F.; Melgoza, A.; Nguyen, B.; Guo, S. Role of the endocannabinoid system in vertebrates: Emphasis on the zebrafish model. Dev. Growth Differ. 2017, 59, 194–210. [Google Scholar] [CrossRef]
- Clarke, T.L.; Johnson, R.L.; Simone, J.J.; Carlone, R.L. The Endocannabinoid System and Invertebrate Neurodevelopment and Regeneration. Int. J. Mol. Sci. 2021, 22, 2103. [Google Scholar] [CrossRef]
- Krug, R.G.; Clark, K.J. Elucidating cannabinoid biology in zebrafish (Danio rerio). Gene 2015, 570, 168–179. [Google Scholar] [CrossRef] [Green Version]
- Fuerte-Hortigón, A.; Gonçalves, J.; Zeballos, L.; Masa, R.; Gómez-Nieto, R.; López, D.E. Distribution of the Cannabinoid Receptor Type 1 in the Brain of the Genetically Audiogenic Seizure-Prone Hamster GASH/Sal. Front. Behav. Neurosci. 2021, 15, 613798. [Google Scholar] [CrossRef] [PubMed]
- Kendall, D.A.; Yudowski, G.A. Cannabinoid Receptors in the Central Nervous System: Their Signaling and Roles in Disease. Front. Cell. Neurosci. 2017, 10, 294. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Di Marzo, V.; Stella, N.; Zimmer, A. Endocannabinoid signalling and the deteriorating brain. Nat. Rev. Neurosci. 2014, 16, 30–42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chahardehi, A.M.; Arsad, H.; Lim, V. Zebrafish as a Successful Animal Model for Screening Toxicity of Medicinal Plants. Plants 2020, 9, 1345. [Google Scholar] [CrossRef]
- Licitra, R.; Marchese, M.; Brogi, L.; Fronte, B.; Pitto, L.; Santorelli, F.M. Nutraceutical Screening in a Zebrafish Model of Muscular Dystrophy: Gingerol as a Possible Food Aid. Nutrients 2021, 13, 998. [Google Scholar] [CrossRef]
- Naef, V.; Marchese, M.; Ogi, A.; Fichi, G.; Galatolo, D.; Licitra, R.; Doccini, S.; Verri, T.; Argenton, F.; Morani, F.; et al. Efficient Neuroprotective Rescue of Sacsin-Related Disease Phenotypes in Zebrafish. Int. J. Mol. Sci. 2021, 22, 8401. [Google Scholar] [CrossRef] [PubMed]
- More, S.M.; Layar, A.; Darade, S.; Shahu, A.; Kushwaha, N.; Kharwade, R.S.; Mahajan, U.N. Zebrafish: A New Emerging Model of Experimental Pharmacology. Int. J. Cur. Res. Rev. 2021, 13, 53–58. [Google Scholar] [CrossRef]
- King, A. Researchers Find Their Nemo. Cell 2009, 139, 843–846. [Google Scholar] [CrossRef] [Green Version]
- Naef, V.; Mero, S.; Fichi, G.; D’Amore, A.; Ogi, A.; Gemignani, F.; Santorelli, F.M.; Marchese, M. Swimming in Deep Water: Zebrafish Modeling of Complicated Forms of Hereditary Spastic Paraplegia and Spastic Ataxia. Front. Neurosci. 2019, 13, 1311. [Google Scholar] [CrossRef]
- Jørgensen, L.V.G. Zebrafish as a Model for Fish Diseases in Aquaculture. Pathogens 2020, 9, 609. [Google Scholar] [CrossRef]
- Benvenutti, R.; Marcon, M.; Gallas-Lopes, M.; de Mello, A.J.; Herrmann, A.P.; Piato, A. Swimming in the maze: An overview of maze apparatuses and protocols to assess zebrafish behavior. Neurosci. Biobehav. Rev. 2021, 127, 761–778. [Google Scholar] [CrossRef] [PubMed]
- Choi, T.Y.; Choi, T.I.; Lee, Y.R.; Choe, S.K.; Kim, C.H. Zebrafish as an animal model for biomedical research. Exp. Mol. Med. 2021, 53, 310–317. [Google Scholar] [CrossRef] [PubMed]
- Ogi, A.; Licitra, R.; Naef, V.; Marchese, M.; Fronte, B.; Gazzano, A.; Santorelli, F.M. Social Preference Tests in Zebrafish: A Systematic Review. Front. Vet. Sci. 2021, 7, 590057. [Google Scholar] [CrossRef]
- Kanyo, R.; Amin, M.R.; Locskai, L.F.; Bouvier, D.D.; Olthuis, A.M.; Allison, W.T.; Ali, D.W. Medium-throughput zebrafish optogenetic platform identifies deficits in subsequent neural activity following brief early exposure to cannabidiol and Δ9-tetrahydrocannabinol. Sci. Rep. 2021, 11, 11515. [Google Scholar] [CrossRef]
- Luchtenburg, F.J.; Schaaf, M.J.M.; Richardson, M.K. Functional characterization of the cannabinoid receptors 1 and 2 in zebrafish larvae using behavioral analysis. Psychopharmacology 2019, 236, 2049–2058. [Google Scholar] [CrossRef] [Green Version]
- Lambert, A.M.; Bonkowsky, J.L.; Masino, M.A. The conserved dopaminergic diencephalospinal tract mediates vertebrate locomotor development in zebrafish larvae. J. Neurosci. 2012, 32, 13488–13500. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Watson, S.; Chambers, D.; Hobbs, C.; Doherty, P.; Graham, A. The endocannabinoid receptor, CB1, is required for normal axonal growth and fasciculation. Mol. Cell Neurosci. 2008, 38, 89–97. [Google Scholar] [CrossRef]
- Falcão, M.A.P.; de Souza, L.S.; Dolabella, S.S.; Guimarães, A.G.; Walker, C.I.B. Zebrafish as an alternative method for determining the embryo toxicity of plant products: A systematic review. Environ. Sci. Pollut. Res. 2018, 25, 35015–35026. [Google Scholar] [CrossRef]
- Hallare, A.; Nagel, K.; Köhler, H.R.; Triebskorn, R. Comparative embryotoxicity and proteotoxicity of three carrier solvents to zebrafish (Danio rerio) embryos. Ecotoxicol. Environ. Saf. 2006, 63, 378–388. [Google Scholar] [CrossRef]
- Li, J.; Zhang, Y.; Liu, K.; He, Q.; Sun, C.; Han, J.; Han, L.; Tian, Q. Xiaoaiping Induces Developmental Toxicity in Zebrafish Embryos Through Activation of ER Stress, Apoptosis and the Wnt Pathway. Front. Pharmacol. 2018, 9, 1250. [Google Scholar] [CrossRef] [Green Version]
- Hernandez, R.E.; Galitan, L.; Cameron, J.; Goodwin, N.; Ramakrishnan, L. Delay of Initial Feeding of Zebrafish Larvae Until 8 Days Postfertilization Has No Impact on Survival or Growth Through the Juvenile Stage. Zebrafish 2018, 15, 515–518. [Google Scholar] [CrossRef] [PubMed]
- Kirla, K.T.; Groh, K.J.; Steuer, A.E.; Poetzsch, M.; Banote, R.K.; Stadnicka-Michalak, J.; Eggen, R.I.L.; Schirmer, K.; Kraemer, T. Zebrafish larvae are insensitive to stimulation by cocaine: Importance of exposure route and toxicokinetics. Toxicol. Sci. 2016, 154, 183–193. [Google Scholar] [CrossRef] [PubMed]
- Brigante, T.A.; Abe, F.R.; Zuardi, A.W.; Hallak, J.E.C.; Crippa, J.A.S.; de Oliveira, D.P. Cannabidiol did not induce teratogenicity or neurotoxicity in exposed zebrafish embryos. Chem. Biol. Interact. 2018, 291, 81–86. [Google Scholar] [CrossRef]
- Hasumi, A.; Maeda, H.; Yoshida, K. Analyzing cannabinoid-induced abnormal behavior in a zebrafish model. PLoS ONE 2020, 15, e0236606. [Google Scholar] [CrossRef] [PubMed]
- Taylor, D.A.; Fennessy, M.R. Biphasic nature of the effects of delta9-tetrahydrocannabinol on body temperature and brain amines of the rat. Eur. J. Pharmacol. 1977, 46, 93–99. [Google Scholar] [CrossRef]
- Migliarini, B.; Carnevali, O. Anandamide modulates growth and lipid metabolism in the zebrafish Danio rerio. Mol. Cell. Endocrinol. 2008, 286, S12–S16. [Google Scholar] [CrossRef]
- Haspula, D.; Clark, M.A. Cannabinoid Receptors: An Update on Cell Signaling, Pathophysiological Roles and Therapeutic Opportunities in Neurological, Cardiovascular, and Inflammatory Diseases. Int. J. Mol. Sci. 2020, 21, 7693. [Google Scholar] [CrossRef]
- Lam, C.S.; Rastegar, S.; Strähle, U. Distribution of cannabinoid receptor 1 in the CNS of zebrafish. Neuroscience 2006, 138, 83–95. [Google Scholar] [CrossRef]
- Fernandez-Ruiz, J.; Hernandez, M.; Ramos, J.A. Cannabinoid-dopamine interaction in the pathophysiology and treatment of CNS disorders. CNS Neurosci. Ther. 2010, 16, 72–91. [Google Scholar] [CrossRef]
- Caprioglio, D.; Allegrone, G.; Pollastro, F.; Vallera, S.; Lopatriello, A.; Colado, J.A.; Munoz, E.; Appendino, G.; Taglialatela-Scafati, O. O-Methyl Phytocannabinoids: Semi-synthesis, Analysis in Cannabis Flowerheads, and Biological Activity. Planta Med. 2019, 85, 981–986. [Google Scholar] [CrossRef]
- Westerfield, M. The Zebrafish Book: A Guide for the Laboratory Use of Zebrafish (Danio rerio), 4th ed; University of Oregon Press: Eugene, OR, USA, 2000. [Google Scholar]
- Xiong, X.; Luo, S.; Wu, B.; Wang, J. Comparative Developmental Toxicity and Stress Protein Responses of Dimethyl Sulfoxide to Rare Minnow and Zebrafish Embryos/Larvae. Zebrafish 2017, 14, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Brogi, L.; Marchese, M.; Cellerino, A.; Licitra, R.; Naef, V.; Mero, S.; Bibbiani, C.; Fronte, B. β-Glucans as Dietary Supplement to Improve Locomotion and Mitochondrial Respiration in a Model of Duchenne Muscular Dystrophy. Nutrients 2021, 13, 1619. [Google Scholar] [CrossRef] [PubMed]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Licitra, R.; Martinelli, M.; Petrocchi Jasinski, L.; Marchese, M.; Kiferle, C.; Fronte, B. In Vivo Evaluation of Cannabis sativa Full Extract on Zebrafish Larvae Development, Locomotion Behavior and Gene Expression. Pharmaceuticals 2021, 14, 1224. https://doi.org/10.3390/ph14121224
Licitra R, Martinelli M, Petrocchi Jasinski L, Marchese M, Kiferle C, Fronte B. In Vivo Evaluation of Cannabis sativa Full Extract on Zebrafish Larvae Development, Locomotion Behavior and Gene Expression. Pharmaceuticals. 2021; 14(12):1224. https://doi.org/10.3390/ph14121224
Chicago/Turabian StyleLicitra, Rosario, Marco Martinelli, Luigi Petrocchi Jasinski, Maria Marchese, Claudia Kiferle, and Baldassare Fronte. 2021. "In Vivo Evaluation of Cannabis sativa Full Extract on Zebrafish Larvae Development, Locomotion Behavior and Gene Expression" Pharmaceuticals 14, no. 12: 1224. https://doi.org/10.3390/ph14121224
APA StyleLicitra, R., Martinelli, M., Petrocchi Jasinski, L., Marchese, M., Kiferle, C., & Fronte, B. (2021). In Vivo Evaluation of Cannabis sativa Full Extract on Zebrafish Larvae Development, Locomotion Behavior and Gene Expression. Pharmaceuticals, 14(12), 1224. https://doi.org/10.3390/ph14121224