Association between Statin Use and Gastric Cancer: A Nested Case-Control Study Using a National Health Screening Cohort in Korea
Abstract
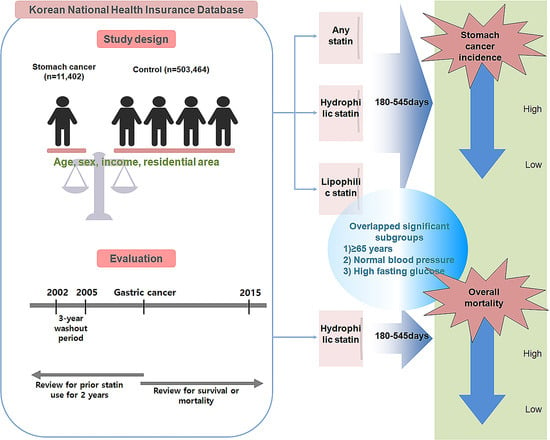
:1. Introduction
2. Results
2.1. Baseline Characteristics of the Study Participants
2.2. Odds Ratios of the Incidence of Gastric Cancer for the Duration of Use and Types of Statins
2.3. Odds Ratios of Mortality in Gastric Cancer Patients for the Duration of Use and Types of Statins
3. Discussion
4. Materials and Methods
4.1. Ethical Approval and Study Population
4.2. Exposure (Statin)
4.3. Outcome (Gastric Cancer)
4.4. Covariates
4.5. Statistical Analyses
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jung, K.W.; Won, Y.J.; Hong, S.; Kong, H.J.; Im, J.S.; Seo, H.G. Prediction of Cancer Incidence and Mortality in Korea, 2021. Cancer Res. Treat. 2021, 53, 316–322. [Google Scholar] [CrossRef] [PubMed]
- Hundahl, S.A.; Phillips, J.L.; Menck, H.R. The National Cancer Data Base Report on poor survival of U.S. gastric carcinoma patients treated with gastrectomy: Fifth Edition American Joint Committee on Cancer staging, proximal disease, and the “different disease” hypothesis. Cancer 2000, 88, 921–932. [Google Scholar] [CrossRef]
- Jun, J.K.; Choi, K.S.; Lee, H.Y.; Suh, M.; Park, B.; Song, S.H.; Jung, K.W.; Lee, C.W.; Choi, I.J.; Park, E.C.; et al. Effectiveness of the Korean National Cancer Screening Program in Reducing Gastric Cancer Mortality. Gastroenterology 2017, 152, 1319–1328.e7. [Google Scholar] [CrossRef]
- Iannelli, F.; Lombardi, R.; Milone, M.R.; Pucci, B.; De Rienzo, S.; Budillon, A.; Bruzzese, F. Targeting Mevalonate Pathway in Cancer Treatment: Repurposing of Statins. Recent Pat. Anticancer Drug Discov. 2018, 13, 184–200. [Google Scholar] [CrossRef]
- Ortiz, N.; Delgado-Carazo, J.C.; Diaz, C. Importance of Mevalonate Pathway Lipids on the Growth and Survival of Primary and Metastatic Gastric Carcinoma Cells. Clin. Exp. Gastroenterol. 2021, 14, 217–228. [Google Scholar] [CrossRef]
- Liu, Q.; Xia, H.; Zhou, S.; Tang, Q.; Zhou, J.; Ren, M.; Bi, F. Simvastatin Inhibits the Malignant Behaviors of Gastric Cancer Cells by Simultaneously Suppressing YAP and β-Catenin Signaling. OncoTargets Ther. 2020, 13, 2057–2066. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nielsen, S.F.; Nordestgaard, B.G.; Bojesen, S.E. Statin use and reduced cancer-related mortality. N. Engl. J. Med. 2012, 367, 1792–1802. [Google Scholar] [CrossRef] [Green Version]
- Ford, I.; Murray, H.; Packard, C.J.; Shepherd, J.; Macfarlane, P.W.; Cobbe, S.M.; West of Scotland Coronary Prevention Study Group. Long-term follow-up of the West of Scotland Coronary Prevention Study. N. Engl. J. Med. 2007, 357, 1477–1486. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, M.R.; Mascitelli, L.; Pezzetta, F. Do statins prevent or promote cancer? Curr. Oncol. 2008, 15, 76–77. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bates, G.J.; Fox, S.B.; Han, C.; Leek, R.D.; Garcia, J.F.; Harris, A.L.; Banham, A.H. Quantification of regulatory T cells enables the identification of high-risk breast cancer patients and those at risk of late relapse. J. Clin. Oncol. 2006, 24, 5373–5380. [Google Scholar] [CrossRef] [PubMed]
- Vogel, T.J.; Goodman, M.T.; Li, A.J.; Jeon, C.Y. Statin treatment is associated with survival in a nationally representative population of elderly women with epithelial ovarian cancer. Gynecol. Oncol. 2017, 146, 340–345. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.D.; Zeng, K.; Xue, F.Q.; Chen, J.H.; Chen, Y.Q. Statins are associated with reduced risk of gastric cancer: A meta-analysis. Eur. J. Clin. Pharmacol. 2013, 69, 1855–1860. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Yi, Z.; Guan, X.; Zeng, Y.X.; Ma, F. The relationship between statins and breast cancer prognosis varies by statin type and exposure time: A meta-analysis. Breast Cancer Res. Treat. 2017, 164, 1–11. [Google Scholar] [CrossRef]
- Yang, H.C.; Islam, M.M.; Nguyen, P.A.A.; Wang, C.H.; Poly, T.N.; Huang, C.W.; Li, Y.J. Development of a Web-Based System for Exploring Cancer Risk With Long-term Use of Drugs: Logistic Regression Approach. JMIR Public Health Surveill. 2021, 7, e21401. [Google Scholar] [CrossRef]
- Yang, P.R.; Tsai, Y.Y.; Chen, K.J.; Yang, Y.H.; Shih, W.T. Statin Use Improves Overall Survival of Patients with Gastric Cancer after Surgery and Adjuvant Chemotherapy in Taiwan: A Nationwide Matched Cohort Study. Cancers 2020, 12, 2055. [Google Scholar] [CrossRef] [PubMed]
- You, H.S.; You, N.; Lee, J.W.; Lim, H.J.; Kim, J.; Kang, H.T. Inverse Association between Statin Use and Stomach Cancer Incidence in Individuals with Hypercholesterolemia, from the 2002–2015 NHIS-HEALS Data. Int. J. Environ. Res. Public Health 2020, 17, 1054. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, J.; Lee, S.H.; Hur, K.Y.; Woo, S.Y.; Kim, S.W.; Kang, W.K. Statins and the risk of gastric cancer in diabetes patients. BMC Cancer 2012, 12, 596. [Google Scholar] [CrossRef] [Green Version]
- Lim, T.; Lee, I.; Kim, J.; Kang, W.K. Synergistic Effect of Simvastatin plus Radiation in Gastric Cancer and Colorectal Cancer: Implications of BIRC5 and Connective Tissue Growth Factor. Int. J. Radiat. Oncol. Biol. Phys. 2015, 93, 316–325. [Google Scholar] [CrossRef]
- Goulitquer, S.; Croyal, M.; Lalande, J.; Royer, A.L.; Guitton, Y.; Arzur, D.; Durand, S.; Le Jossic-Corcos, C.; Bouchereau, A.; Potin, P.; et al. Consequences of blunting the mevalonate pathway in cancer identified by a pluri-omics approach. Cell Death Dis. 2018, 9, 745. [Google Scholar] [CrossRef]
- Chushi, L.; Wei, W.; Kangkang, X.; Yongzeng, F.; Ning, X.; Xiaolei, C. HMGCR is up-regulated in gastric cancer and promotes the growth and migration of the cancer cells. Gene 2016, 587, 42–47. [Google Scholar] [CrossRef]
- Follet, J.; Corcos, L.; Baffet, G.; Ezan, F.; Morel, F.; Simon, B.; Le Jossic-Corcos, C. The association of statins and taxanes: An efficient combination trigger of cancer cell apoptosis. Br. J. Cancer 2012, 106, 685–692. [Google Scholar] [CrossRef] [Green Version]
- Cheung, K.S.; Chan, E.W.; Wong, A.Y.S.; Chen, L.; Seto, W.K.; Wong, I.C.K.; Leung, W.K. Statins Were Associated with a Reduced Gastric Cancer Risk in Patients with Eradicated Helicobacter Pylori Infection: A Territory-Wide Propensity Score Matched Study. Cancer Epidemiol. Biomark. Prev. 2020, 29, 493–499. [Google Scholar] [CrossRef]
- Ma, Z.; Wang, W.; Jin, G.; Chu, P.; Li, H. Effect of statins on gastric cancer incidence: A meta-analysis of case control studies. J. Cancer Res. Ther. 2014, 10, 859–865. [Google Scholar] [CrossRef]
- Cho, M.H.; Yoo, T.G.; Jeong, S.M.; Shin, D.W. Association of Aspirin, Metformin, and Statin Use with Gastric Cancer Incidence and Mortality: A Nationwide Cohort Study. Cancer Prev. Res. 2021, 14, 95–104. [Google Scholar] [CrossRef] [PubMed]
- Graaf, M.R.; Beiderbeck, A.B.; Egberts, A.C.; Richel, D.J.; Guchelaar, H.J. The risk of cancer in users of statins. J. Clin. Oncol. 2004, 22, 2388–2394. [Google Scholar] [CrossRef] [PubMed]
- Shimoyama, S. Statins and gastric cancer risk. Hepatogastroenterology 2011, 58, 1057–1061. [Google Scholar] [PubMed]
- Bujanda, L.; Rodriguez-Gonzalez, A.; Sarasqueta, C.; Eizaguirre, E.; Hijona, E.; Marin, J.J.; Perugorria, M.J.; Banales, J.M.; Cosme, A. Effect of pravastatin on the survival of patients with advanced gastric cancer. Oncotarget 2016, 7, 4379–4384. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Konings, I.R.; van der Gaast, A.; van der Wijk, L.J.; de Jongh, F.E.; Eskens, F.A.; Sleijfer, S. The addition of pravastatin to chemotherapy in advanced gastric carcinoma: A randomised phase II trial. Eur. J. Cancer 2010, 46, 3200–3204. [Google Scholar] [CrossRef] [PubMed]
- Chiu, H.F.; Ho, S.C.; Chang, C.C.; Wu, T.N.; Yang, C.Y. Statins are associated with a reduced risk of gastric cancer: A population-based case-control study. Am. J. Gastroenterol. 2011, 106, 2098–2103. [Google Scholar] [CrossRef] [PubMed]
- Spence, A.D.; Busby, J.; Hughes, C.M.; Johnston, B.T.; Coleman, H.G.; Cardwell, C.R. Statin use and survival in patients with gastric cancer in two independent population-based cohorts. Pharmacoepidemiol. Drug Saf. 2019, 28, 460–470. [Google Scholar] [CrossRef]
- Karp, I.; Behlouli, H.; Lelorier, J.; Pilote, L. Statins and cancer risk. Am. J. Med. 2008, 121, 302–309. [Google Scholar] [CrossRef]
- Chung, H.; Kim, H.J.; Jung, H.C.; Lee, S.K.; Kim, S.G. Statins and metachronous recurrence after endoscopic resection of early gastric cancer: A nationwide Korean cohort study. Gastric Cancer 2020, 23, 659–666. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.Y.; Min, C.; Oh, D.J.; Choi, H.G. Tobacco Smoking and Alcohol Consumption Are Related to Benign Parotid Tumor: A Nested Case-Control Study Using a National Health Screening Cohort. Clin. Exp. Otorhinolaryngol. 2019, 12, 412–419. [Google Scholar] [CrossRef] [Green Version]
- Song, J.W.; Chung, K.C. Observational studies: Cohort and case-control studies. Plast. Reconstr. Surg. 2010, 126, 2234–2242. [Google Scholar] [CrossRef] [Green Version]
- Orkaby, A.R.; Driver, J.A.; Ho, Y.L.; Lu, B.; Costa, L.; Honerlaw, J.; Galloway, A.; Vassy, J.L.; Forman, D.E.; Gaziano, J.M.; et al. Association of Statin Use With All-Cause and Cardiovascular Mortality in US Veterans 75 Years and Older. JAMA 2020, 324, 68–78. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.Y.; Min, C.; Oh, D.J.; Choi, H.G. Bidirectional Association Between GERD and Asthma: Two Longitudinal Follow-Up Studies Using a National Sample Cohort. J. Allergy Clin. Immunol. Pract. 2020, 8, 1005–1013.e9. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.Y.; Oh, D.J.; Park, B.; Choi, H.G. Bell’s palsy and obesity, alcohol consumption and smoking: A nested case-control study using a national health screening cohort. Sci. Rep. 2020, 10, 4248. [Google Scholar] [CrossRef]
- Austin, P.C. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples. Stat. Med. 2009, 28, 3083–3107. [Google Scholar] [CrossRef] [Green Version]
- Li, F.; Thomas, L.E.; Li, F. Addressing Extreme Propensity Scores via the Overlap Weights. Am. J. Epidemiol. 2019, 188, 250–257. [Google Scholar] [CrossRef] [Green Version]
- Thomas, L.E.; Li, F.; Pencina, M.J. Overlap Weighting: A Propensity Score Method That Mimics Attributes of a Randomized Clinical Trial. JAMA 2020, 323, 2417–2418. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Morgan, K.L.; Zaslavsky, A.M. Balancing covariates via propensity score weighting. J. Am. Stat. Assoc. 2017, 113, 390–400. [Google Scholar] [CrossRef] [Green Version]





| Characteristics | Before Overlap Weighting Adjustment | After Overlap Weighting Adjustment | ||||
|---|---|---|---|---|---|---|
| Gastric Cancer | Control | Standardized Difference | Gastric Cancer | Control | Standardized Difference | |
| Age (%) | 0.00 | 0.00 | ||||
| 40–44 | 0.51 | 0.51 | 0.47 | 0.47 | ||
| 45–49 | 4.54 | 4.54 | 4.27 | 4.27 | ||
| 50–54 | 11.51 | 11.51 | 11.43 | 11.43 | ||
| 55–59 | 13.46 | 13.46 | 13.47 | 13.47 | ||
| 60–64 | 17.22 | 17.22 | 17.20 | 17.20 | ||
| 65–69 | 17.47 | 17.47 | 17.46 | 17.46 | ||
| 70–74 | 17.63 | 17.63 | 17.71 | 17.71 | ||
| 75–79 | 10.9 | 10.9 | 11.03 | 11.03 | ||
| 80–84 | 5.46 | 5.46 | 5.64 | 5.64 | ||
| 85+ | 1.31 | 1.31 | 1.32 | 1.32 | ||
| Sex (%) | 0.00 | 0.00 | ||||
| Male | 73.55 | 73.55 | 73.18 | 73.17 | ||
| Female | 26.45 | 26.45 | 26.82 | 26.83 | ||
| Income (%) | 0.00 | 0.00 | ||||
| 1 (lowest) | 15.69 | 15.69 | 15.60 | 15.60 | ||
| 2 | 12.33 | 12.33 | 12.32 | 12.32 | ||
| 3 | 16.03 | 16.03 | 15.80 | 15.80 | ||
| 4 | 21.46 | 21.46 | 21.64 | 21.64 | ||
| 5 (highest) | 34.5 | 34.5 | 34.64 | 34.64 | ||
| Region of residence (%) | 0.00 | 0.00 | ||||
| Urban | 41.08 | 41.08 | 40.99 | 40.99 | ||
| Rural | 58.92 | 58.92 | 59.01 | 59.01 | ||
| Obesity † (%) | 0.07 | 0.00 | ||||
| Underweight | 3.78 | 2.76 | 3.54 | 3.54 | ||
| Normal | 36.83 | 35.41 | 36.44 | 36.44 | ||
| Overweight | 25.97 | 27.57 | 26.35 | 26.35 | ||
| Obese I | 34.05 | 31.50 | 31.16 | 31.16 | ||
| Obese II | 2.36 | 2.77 | 2.51 | 2.51 | ||
| Smoking status (%) | 0.09 | 0.00 | ||||
| Nonsmoker | 56.8 | 61.1 | 57.57 | 57.57 | ||
| Past smoker | 19.52 | 17.58 | 19.48 | 19.48 | ||
| Current smoker | 23.69 | 21.33 | 22.95 | 22.95 | ||
| Alcohol consumption (%) | 0.09 | 0.00 | ||||
| <1 time a week | 55.61 | 60.22 | 56.09 | 56.09 | ||
| ≥1 time a week | 44.39 | 39.78 | 43.91 | 43.91 | 0.00 | |
| SBP (Mean, SD) | 128.43 (16.58) | 128.58 (16.93) | 0.01 | 128.50 (13.85) | 128.51 (7.21) | 0.00 |
| DBP (Mean, SD) | 78.74 (10.60) | 78.96 (10.60) | 0.02 | 78.78 (8.85) | 78.78 (4.48) | 0.00 |
| Total cholesterol (Mean, SD) | 193.15 (39.30) | 195.84 (38.06) | 0.07 | 193.90 (32.67) | 193.90 (16.01) | 0.00 |
| Fasting blood glucose (Mean, SD) | 104.32 (32.77) | 102.73 (30.42) | 0.05 | 103.98 (26.75) | 103.98 (13.44) | 0.00 |
| CCI score (Mean, SD) | 2.19 (2.63) | 0.71 (1.37) | 0.71 | 1.42 (1.68) | 1.42 (0.87) | 0.00 |
| Dyslipidemia history (%) | 37.33 | 42.07 | 0.10 | 39.51 | 39.51 | 0.00 |
| Characteristics | Gastric Cancer | Control | Odds Ratios for Gastric Cancer (95% Confidence Interval) | |||
|---|---|---|---|---|---|---|
| % | % | Crude | p-Value | Overlap Weighted Model † | p-Value | |
| Any statin | ||||||
| <180 days | 87.7 | 86.8 | 1 | 1 | ||
| 180–545 days | 5.7 | 6.3 | 0.89 (0.80–0.98) | 0.021 * | 0.88 (0.81–0.86) | 0.002 * |
| >545 days | 6.6 | 6.9 | 0.94 (0.86–1.04) | 0.214 | 0.96 (0.89–1.04) | 0.292 |
| Hydrophilic statins | ||||||
| <180 days | 97.8 | 97.7 | 1 | 1 | ||
| 180–545 days | 1.1 | 1.3 | 0.85 (0.68–0.95) | 0.149 | 0.78 (0.66–0.92) | 0.004 * |
| >545 days | 1.1 | 1.0 | 1.11 (0.89–1.39) | 0.368 | 1.03 (0.86–1.23) | 0.733 |
| Lipophilic statins | ||||||
| <180 days | 89.7 | 88.8 | 1 | 1 | ||
| 180–545 days | 5.3 | 5.7 | 0.91 (0.82–1.01) | 0.074 | 0.91 (0.84–0.99) | 0.039 * |
| >545 days | 5.0 | 5.5 | 0.91 (0.82–1.01) | 0.085 | 0.96 (0.88–1.04) | 0.327 |
| Characteristics | Before Overlap Weighting Adjustment | After Overlap Weighting Adjustment | ||||
|---|---|---|---|---|---|---|
| Deceased pts | Survived pts | Standardized Difference | Deceased pts | Survived pts | Standardized Difference | |
| Age (%) | 0.56 | 0.00 | ||||
| 40–44 | 0.52 | 0.51 | 0.47 | 0.47 | ||
| 45–49 | 3.94 | 4.75 | 3.82 | 3.82 | ||
| 50–54 | 7.19 | 13.05 | 7.36 | 7.36 | ||
| 55–59 | 8.32 | 15.29 | 9.00 | 9.00 | ||
| 60–64 | 11.95 | 19.09 | 13.05 | 13.05 | ||
| 65–69 | 16.46 | 17.83 | 17.52 | 17.52 | ||
| 70–74 | 19.84 | 16.84 | 20.68 | 20.68 | ||
| 75–79 | 17.63 | 8.51 | 16.75 | 16.75 | ||
| 80–84 | 11.04 | 3.47 | 9.24 | 9.24 | ||
| 85+ | 3.12 | 0.66 | 2.11 | 2.11 | ||
| Sex (%) | 0.08 | 0.00 | ||||
| Male | 76.27 | 72.58 | 74.75 | 74.75 | ||
| Female | 23.73 | 27.42 | 25.25 | 25.25 | ||
| Income (%) | 0.14 | 0.00 | ||||
| 1 (lowest) | 19.53 | 14.32 | 18.50 | 18.50 | ||
| 2 | 12.69 | 12.21 | 13.00 | 13.0 | ||
| 3 | 17.58 | 15.47 | 16.87 | 16.87 | ||
| 4 | 19.45 | 22.18 | 20.74 | 20.74 | ||
| 5 (highest) | 30.75 | 35.83 | 30.89 | 30.89 | ||
| Residence (%) | 0.08 | 0.00 | ||||
| Urban | 38.03 | 42.16 | 38.31 | 38.31 | ||
| Rural | 61.97 | 57.84 | 61.69 | 61.69 | ||
| Obesity † (%) | 0.22 | 0.00 | ||||
| Underweight | 7.28 | 2.54 | 6.12 | 6.12 | ||
| Normal | 40.45 | 35.54 | 40.98 | 40.98 | ||
| Overweight | 24.25 | 26.58 | 24.22 | 24.22 | ||
| Obese I | 26.5 | 32.67 | 26.86 | 26.87 | ||
| Obese II | 1.52 | 2.67 | 1.82 | 1.82 | ||
| Smoking (%) | 0.05 | 0.00 | ||||
| Non | 58.68 | 56.13 | 58.33 | 58.32 | ||
| Past | 14.85 | 21.17 | 17.39 | 17.39 | ||
| Current | 26.46 | 22.7 | 24.28 | 24.28 | ||
| Alcohol (%) | 0.27 | 0.00 | ||||
| <1 time/week | 65.31 | 52.17 | 61.99 | 61.98 | ||
| ≥1 time/week | 34.69 | 47.83 | 38.01 | 38.02 | 0.00 | |
| SBP (Mean, SD) | 130.35 (18.07) | 127.75 (15.96) | 0.15 | 129.85 (11.75) | 129.85 (6.57) | 0.00 |
| DBP (Mean, SD) | 79.27 (11.28) | 78.55 (10.34) | 0.07 | 78.95 (7.29) | 78.95 (4.08) | 0.00 |
| TCL (Mean, SD) | 190.08 (41.15) | 194.25 (38.57) | 0.10 | 190.50 (26.82) | 190.50 (15.06) | 0.00 |
| FBGL (Mean, SD) | 106.25 (37.71) | 103.64 (30.80) | 0.07 | 106.29 (24.08) | 106.29 (15.77) | 0.00 |
| CCI score (Mean, SD) | 4.60 (2.82) | 1.34 (1.94) | 1.35 | 3.17 (1.74) | 3.17 (1.09) | 0.00 |
| Dyslipidemia (%) | 21.7 | 42.89 | 0.47 | 28.42 | 28.42 | 0.00 |
| Treatment (%) | 0.77 | 0.00 | ||||
| Only surgery | 59.64 | 90.65 | 74.58 | 74.58 | ||
| Surgery + RT/CT | 40.36 | 9.35 | 25.42 | 25.42 | ||
| Characteristics | Deceased | Survived | Odds Ratios for Mortality (95% Confidence Interval) | |||
|---|---|---|---|---|---|---|
| % | % | Crude | p-Value | Overlap Weighted Model † | p-Value | |
| Any statin | ||||||
| <180 days | 91.1 | 86.5 | 1 | 1 | ||
| 180–545 days | 4.7 | 6.0 | 0.74 (0.60–0.93) | 0.008 * | 1.15 (0.94–1.40) | 0.164 |
| >545 days | 4.2 | 7.4 | 0.53 (0.42–0.67) | <0.001 * | 0.85 (0.69–1.04) | 0.106 |
| Hydrophilic statins | ||||||
| <180 days | 98.5 | 97.5 | 1 | 1 | ||
| 180–545 days | 0.6 | 1.3 | 0.47 (0.27–0.83) | 0.009 * | 0.47 (0.29–0.77) | 0.003 * |
| >545 days | 0.9 | 1.2 | 0.75 (0.46–1.22) | 0.243 | 1.17 (0.75–1.81) | 0.495 |
| Lipophilic statins | ||||||
| <180 days | 92.5 | 88.7 | 1 | 1 | ||
| 180–545 days | 4.4 | 5.6 | 0.76 (0.61–0.95) | 0.016 * | 1.21 (0.99–1.49) | 0.064 |
| >545 days | 3.0 | 5.7 | 0.51 (0.39–0.66) | <0.001 * | 0.82 (0.65–1.03) | 0.086 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kwon, M.J.; Kang, H.S.; Kim, J.-H.; Kim, J.H.; Kim, S.H.; Kim, N.Y.; Nam, E.S.; Min, K.-W.; Choi, H.G. Association between Statin Use and Gastric Cancer: A Nested Case-Control Study Using a National Health Screening Cohort in Korea. Pharmaceuticals 2021, 14, 1283. https://doi.org/10.3390/ph14121283
Kwon MJ, Kang HS, Kim J-H, Kim JH, Kim SH, Kim NY, Nam ES, Min K-W, Choi HG. Association between Statin Use and Gastric Cancer: A Nested Case-Control Study Using a National Health Screening Cohort in Korea. Pharmaceuticals. 2021; 14(12):1283. https://doi.org/10.3390/ph14121283
Chicago/Turabian StyleKwon, Mi Jung, Ho Suk Kang, Joo-Hee Kim, Ji Hee Kim, Se Hoon Kim, Nan Young Kim, Eun Sook Nam, Kyueng-Whan Min, and Hyo Geun Choi. 2021. "Association between Statin Use and Gastric Cancer: A Nested Case-Control Study Using a National Health Screening Cohort in Korea" Pharmaceuticals 14, no. 12: 1283. https://doi.org/10.3390/ph14121283
APA StyleKwon, M. J., Kang, H. S., Kim, J. -H., Kim, J. H., Kim, S. H., Kim, N. Y., Nam, E. S., Min, K. -W., & Choi, H. G. (2021). Association between Statin Use and Gastric Cancer: A Nested Case-Control Study Using a National Health Screening Cohort in Korea. Pharmaceuticals, 14(12), 1283. https://doi.org/10.3390/ph14121283