Methyl Jasmonate and Methyl-β-Cyclodextrin Individually Boost Triterpenoid Biosynthesis in Chlamydomonas Reinhardtii UVM4
Abstract
:1. Introduction
2. Results
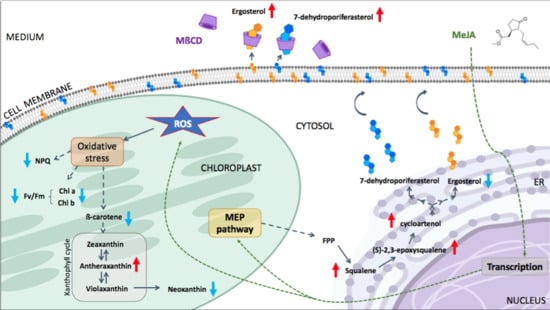
2.1. Methyl Jasmonate Triggers an Oxidative Stress in C. reinhardtii
2.2. MeJA and MβCD Have No Synergistic Effects on Triterpenoids Abundance
2.3. Cyclodextrin Sequesters Sterols to the Growth Media
3. Discussion
4. Materials and Methods
4.1. Strains, Culture Conditions and Treatments
4.2. Photosynthetic Activity and NPQ
4.3. ROS Detection
4.4. Viability Analysis
4.5. Metabolites Extraction and Quantification
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hill, R.A.; Connolly, J.D. Triterpenoids. Nat. Prod. Rep. 2020, 37, 962–998. [Google Scholar] [CrossRef] [Green Version]
- Thimmappa, R.; Geisler, K.; Louveau, T.; O’Maille, P.; Osbourn, A. Triterpene Biosynthesis in Plants. Annu. Rev. Plant Biol. 2014, 65, 225–257. [Google Scholar] [CrossRef]
- Kumar, V.; Kumar, C.S.; Hari, G.; Venugopal, N.K.; Vijendra, P.D. Homology modeling and docking studies on oxidosqualene cyclases associated with primary and secondary metabolism of Centella asiatica. SpringerPlus 2013, 2, 189. [Google Scholar] [CrossRef] [Green Version]
- Moses, T.; Pollier, J.; Thevelein, J.M.; Goossens, A. Bioengineering of plant (tri)terpenoids: From metabolic engineering of plants to synthetic biology in vivo and in vitro. N. Phytol. 2013, 200, 27–43. [Google Scholar] [CrossRef] [PubMed]
- Genser, B.; Silbernagel, G.; De Backer, G.; Bruckert, E.; Carmena, R.; Chapman, M.J.; Deanfield, J.; Descamps, O.S.; Rietzschel, E.R.; Dias, K.C.; et al. Plant sterols and cardiovascular disease: A systematic review and meta-analysis†. Eur. Hear. J. 2012, 33, 444–451. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jäpelt, R.B.; Jakobsen, J. Vitamin D in plants: A review of occurrence, analysis, and biosynthesis. Front. Plant Sci. 2013, 4, 136. [Google Scholar] [CrossRef] [Green Version]
- Pisha, E.; Chai, H.; Lee, I.-S.; Chagwedera, T.E.; Farnsworth, N.R.; Cordell, G.A.; Beecher, C.W.; Fong, H.H.; Kinghorn, A.D.; Brown, D.M.; et al. Discovery of betulinic acid as a selective inhibitor of human melanoma that functions by induction of apoptosis. Nat. Med. 1995, 1, 1046–1051. [Google Scholar] [CrossRef]
- Soica, C.; Danciu, C.; Savoiu-Balint, G.; Borcan, F.; Ambrus, R.; Zupkó, I.; Bojin, F.; Coricovac, D.; Ciurlea, S.; Avram, S.; et al. Betulinic Acid in Complex with a Gamma-Cyclodextrin Derivative Decreases Proliferation and in Vivo Tumor Development of Non-Metastatic and Metastatic B164A5 Cells. Int. J. Mol. Sci. 2014, 15, 8235–8255. [Google Scholar] [CrossRef] [Green Version]
- Spanova, M.; Daum, G. Squalene—Biochemistry, molecular biology, process biotechnology, and applications. Eur. J. Lipid Sci. Technol. 2011, 113, 1299–1320. [Google Scholar] [CrossRef]
- D’Adamo, S.; Di Visconte, G.S.; Lowe, G.; Szaub-Newton, J.; Beacham, T.; Landels, A.; Allen, M.J.; Spicer, A.; Matthijs, M. Engineering the unicellular alga Phaeodactylum tricornutum for high-value plant triterpenoid production. Plant Biotechnol. J. 2018, 17, 75–87. [Google Scholar] [CrossRef] [Green Version]
- Moses, T.; Pollier, J.; Almagro, L.; Buyst, D.; Van Montagu, M.; Pedreño, M.A.; Martins, J.C.; Thevelein, J.H.; Goossens, A. Combinatorial biosynthesis of sapogenins and saponins in Saccharomyces cerevisiae using a C-16α hydroxylase from Bupleurum falcatum. Proc. Natl. Acad. Sci. USA 2014, 111, 1634–1639. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fabris, M.; Abbriano, R.M.; Pernice, M.; Sutherland, D.L.; Commault, A.S.; Hall, C.C.; Labeeuw, L.; McCauley, J.I.; Kuzhiuparambil, U.; Ray, P.; et al. Emerging Technologies in Algal Biotechnology: Toward the Establishment of a Sustainable, Algae-Based Bioeconomy. Front. Plant Sci. 2020, 11, 279. [Google Scholar] [CrossRef] [Green Version]
- Neupert, J.; Karcher, D.; Bock, R. Generation of Chlamydomonas strains that efficiently express nuclear transgenes. Plant J. 2009, 57, 1140–1150. [Google Scholar] [CrossRef]
- Lauersen, K.J.; Baier, T.; Wichmann, J.; Wördenweber, R.; Mussgnug, J.H.; Hübner, W.; Huser, T.; Kruse, O. Efficient phototrophic production of a high-value sesquiterpenoid from the eukaryotic microalga Chlamydomonas reinhardtii. Metab. Eng. 2016, 38, 331–343. [Google Scholar] [CrossRef] [PubMed]
- Commault, A.S.; Fabris, M.; Kuzhiumparambil, U.; Adriaans, J.; Pernice, M.; Ralph, P.J. Methyl jasmonate treatment affects the regulation of the 2-C-methyl-D-erythritol 4-phosphate pathway and early steps of the triterpenoid biosynthesis in Chlamydomonas reinhardtii. Algal Res. 2019, 39, 101462. [Google Scholar] [CrossRef]
- Ho, T.-T.; Murthy, H.N.; Park, S.-Y. Methyl Jasmonate Induced Oxidative Stress and Accumulation of Secondary Metabolites in Plant Cell and Organ Cultures. Int. J. Mol. Sci. 2020, 21, 716. [Google Scholar] [CrossRef] [Green Version]
- Sabater-Jara, A.B.; Onrubia, M.; Moyano, E.; Bonfill, M.; Palazón, J.; Pedreño, M.A.; Cusidó, R.M. Synergistic effect of cyclodextrins and methyl jasmonate on taxane production in Taxus x media cell cultures. Plant Biotechnol. J. 2014, 12, 1075–1084. [Google Scholar] [CrossRef] [PubMed]
- Lijavetzky, D.; Almagro, L.; Belchí-Navarro, S.; Martínez-Zapater, J.M.; Bru, R.; A Pedreño, M. Synergistic effect of methyljasmonate and cyclodextrin on stilbene biosynthesis pathway gene expression and resveratrol production in Monastrell grapevine cell cultures. BMC Res. Notes 2008, 1, 132. [Google Scholar] [CrossRef] [Green Version]
- Zhou, P.; Yang, J.; Zhu, J.; He, S.; Zhang, W.; Yu, R.; Zi, J.; Xuesong, H.; Huang, X. Effects of β-cyclodextrin and methyl jasmonate on the production of vindoline, catharanthine, and ajmalicine in Catharanthus roseus cambial meristematic cell cultures. Appl. Microbiol. Biotechnol. 2015, 99, 7035–7045. [Google Scholar] [CrossRef]
- Almagro, L.; Gutierrez, J.; Pedreño, M.A.; Sottomayor, M. Synergistic and additive influence of cyclodextrins and methyl jasmonate on the expression of the terpenoid indole alkaloid pathway genes and metabolites in Catharanthus roseus cell cultures. Plant Cell Tissue Organ Cult. 2014, 119, 543–551. [Google Scholar] [CrossRef]
- Sharma, K.; Zafar, R. Optimization of methyl jasmonate and β-cyclodextrin for enhanced production of taraxerol and taraxasterol in (Taraxacum officinale Weber) cultures. Plant Physiol. Biochem. 2016, 103, 24–30. [Google Scholar] [CrossRef]
- Zhang, L.; Xing, D. Methyl Jasmonate Induces Production of Reactive Oxygen Species and Alterations in Mitochondrial Dynamics that Precede Photosynthetic Dysfunction and Subsequent Cell Death. Plant Cell Physiol. 2008, 49, 1092–1111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perreca, E.; Rohwer, J.; González-Cabanelas, D.; Loreto, F.; Schmidt, A.; Gershenzon, J.; Wright, L.P. Effect of Drought on the Methylerythritol 4-Phosphate (MEP) Pathway in the Isoprene Emitting Conifer Picea glauca. Front. Plant Sci. 2020, 11, 546295. [Google Scholar] [CrossRef] [PubMed]
- Jung, S. Effect of chlorophyll reduction in Arabidopsis thaliana by methyl jasmonate or norflurazon on antioxidant systems. Plant Physiol. Biochem. 2004, 42, 225–231. [Google Scholar] [CrossRef] [PubMed]
- Kovač, M.; Ravnikar, M. The effect of jasmonic acid on the photosynthetic pigments of potato plants grown in vitro. Plant Sci. 1994, 103, 11–17. [Google Scholar] [CrossRef]
- Demmig-Adams, B.; Gilmore, A.M.; Adams, W.W., 3rd. Carotenoids 3: In vivo function of carotenoids in higher plants. FASEB J. 1996, 10, 403–412. [Google Scholar] [CrossRef]
- Jahns, P.; Latowski, D.; Strzalka, K. Mechanism and regulation of the violaxanthin cycle: The role of antenna proteins and membrane lipids. Biochim. Biophys. Acta 2009, 1787, 3–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peers, G.; Truong, T.B.; Ostendorf, E.; E Busch, A.; Elrad, D.; Grossman, A.R.; Hippler, M.; Niyogi, K.K. An ancient light-harvesting protein is critical for the regulation of algal photosynthesis. Nat. Cell Biol. 2009, 462, 518–521. [Google Scholar] [CrossRef]
- Girolomoni, L.; Cazzaniga, S.; Pinnola, A.; Perozeni, F.; Ballottari, M.; Bassi, R. LHCSR3 is a nonphotochemical quencher of both photosystems inChlamydomonas reinhardtii. Proc. Natl. Acad. Sci. USA 2019, 116, 4212–4217. [Google Scholar] [CrossRef] [Green Version]
- Pinnola, A. The rise and fall of Light-Harvesting Complex Stress-Related proteins as photoprotection agents during evolution. J. Exp. Bot. 2019, 70, 5527–5535. [Google Scholar] [CrossRef]
- Vidal-Meireles, A.; Tóth, D.; Kovács, L.; Neupert, J.; Tóth, S.Z. Ascorbate Deficiency Does Not Limit Nonphotochemical Quenching in Chlamydomonas reinhardtii. Plant Physiol. 2020, 182, 597–611. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Z.; Peers, G.; Dent, R.M.; Bai, Y.; Yang, S.Y.; Apel, W.; Leonelli, L.; Niyogi, K.K. Evolution of an atypical de-epoxidase for photoprotection in the green lineage. Nat. Plants 2016, 2, 16140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Briceño, Z.; Almagro, L.; Sabater-Jara, A.B.; Calderón, A.A.; A Pedreño, M.; Ferrer, M.A. Enhancement of phytosterols, taraxasterol and induction of extracellular pathogenesis-related proteins in cell cultures of Solanum lycopersicum cv Micro-Tom elicited with cyclodextrins and methyl jasmonate. J. Plant Physiol. 2012, 169, 1050–1058. [Google Scholar] [CrossRef] [PubMed]
- Oliva, E.; Mathiron, D.; Bertaut, E.; Landy, D.; Cailleu, D.; Pilard, S.; Clement, C.; Courot, E.; Bonnet, V.; Djedaïni-Pilard, F. Physico-chemical studies of resveratrol, methyl-jasmonate and cyclodextrin interactions: An approach to resveratrol bioproduction optimization. RSC Adv. 2018, 8, 1528–1538. [Google Scholar] [CrossRef] [Green Version]
- Cui, Y.; Li, X.; Yu, M.; Li, R.; Fan, L.; Zhu, Y.; Lin, J. Sterols regulate endocytic pathways during flg22-induced defense responses in Arabidopsis. Development 2018, 145, dev165688. [Google Scholar] [CrossRef] [Green Version]
- Fabris, M.; Matthijs, M.; Carbonelle, S.; Moses, T.; Pollier, J.; Dasseville, R.; Baart, G.J.E.; Vyverman, W.; Goossens, A. Tracking the sterol biosynthesis pathway of the diatomPhaeodactylum tricornutum. N. Phytol. 2014, 204, 521–535. [Google Scholar] [CrossRef]
- Jaramillo-Madrid, A.C.; Ashworth, J.; Fabris, M.; Ralph, P.J. Phytosterol biosynthesis and production by diatoms (Bacillariophyceae). Phytochemistry 2019, 163, 46–57. [Google Scholar] [CrossRef]
- Miras-Moreno, B.; Almagro, L.; Pedreño, M. Ángeles; Sabater-Jara, A.B. Effect of terbinafine on the biosynthetic pathway of isoprenoid compounds in carrot suspension cultured cells. Plant Cell Rep. 2018, 37, 1011–1019. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Commault, A.S.; Kuzhiumparambil, U.; Herdean, A.; Fabris, M.; Jaramillo-Madrid, A.C.; Abbriano, R.M.; Ralph, P.J.; Pernice, M. Methyl Jasmonate and Methyl-β-Cyclodextrin Individually Boost Triterpenoid Biosynthesis in Chlamydomonas Reinhardtii UVM4. Pharmaceuticals 2021, 14, 125. https://doi.org/10.3390/ph14020125
Commault AS, Kuzhiumparambil U, Herdean A, Fabris M, Jaramillo-Madrid AC, Abbriano RM, Ralph PJ, Pernice M. Methyl Jasmonate and Methyl-β-Cyclodextrin Individually Boost Triterpenoid Biosynthesis in Chlamydomonas Reinhardtii UVM4. Pharmaceuticals. 2021; 14(2):125. https://doi.org/10.3390/ph14020125
Chicago/Turabian StyleCommault, Audrey S., Unnikrishnan Kuzhiumparambil, Andrei Herdean, Michele Fabris, Ana Cristina Jaramillo-Madrid, Raffaela M. Abbriano, Peter J. Ralph, and Mathieu Pernice. 2021. "Methyl Jasmonate and Methyl-β-Cyclodextrin Individually Boost Triterpenoid Biosynthesis in Chlamydomonas Reinhardtii UVM4" Pharmaceuticals 14, no. 2: 125. https://doi.org/10.3390/ph14020125
APA StyleCommault, A. S., Kuzhiumparambil, U., Herdean, A., Fabris, M., Jaramillo-Madrid, A. C., Abbriano, R. M., Ralph, P. J., & Pernice, M. (2021). Methyl Jasmonate and Methyl-β-Cyclodextrin Individually Boost Triterpenoid Biosynthesis in Chlamydomonas Reinhardtii UVM4. Pharmaceuticals, 14(2), 125. https://doi.org/10.3390/ph14020125