Highly Efficient T2 Cobalt Ferrite Nanoparticles Vectorized for Internalization in Cancer Cells
Abstract
:1. Introduction
2. Results and Discussion
2.1. Nanoparticle Physicochemical Properties
2.2. Relaxivity of NP-FA
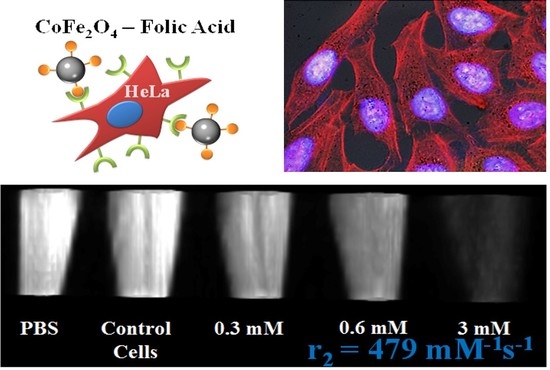
2.3. Cell Viability and Internalization
2.4. Analysis of Cytoskeleton Components
2.5. In Vitro Phantom Image Contrast
3. Materials and Methods
3.1. Synthesis and Functionalization of Cobalt Ferrite Nanoparticles
3.2. Characterization Techniques
3.3. Biological Characterization
3.3.1. Cell Culture
3.3.2. Nanoparticles
3.3.3. Biocompatibility Assay
3.3.4. Internalization
3.3.5. Cytoskeleton Analysis
3.3.6. Confocal Microscopy
3.4. Relaxometric Characterization
3.4.1. Magnetic Relaxivity Measurements
3.4.2. Magnetic Resonance Imaging
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, C.; Zou, B.; Rondinone, A.J.; Zhang, Z.J. Chemical control of superparamagnetic properties of magnesium and cobalt spinel ferrite nanoparticles through atomic level magnetic couplings. J. Am. Chem. Soc. 2000, 122, 6263–6267. [Google Scholar] [CrossRef]
- Gallo-Cordova, Á.; Espinosa, A.; Serrano, A.; Gutiérrez, L.; Menéndez, N.; Del Puerto Morales, M.; Mazario, E. New insights into the structural analysis of maghemite and (MFe2O4, M = Co, Zn) ferrite nanoparticles synthesized by a microwave-assisted polyol process. Mater. Chem. Front. 2020, 4, 3063–3073. [Google Scholar] [CrossRef]
- Stein, C.R.; Bezerra, M.T.S.; Holanda, G.H.A.; André-Filho, J.; Morais, P.C. Structural and magnetic properties of cobalt ferrite nanoparticles synthesized by co-precipitation at increasing temperatures. AIP Adv. 2017, 8, 056303. [Google Scholar] [CrossRef]
- Lasheras, X.; Insausti, M.; Gil de Muro, I.; Garaio, E.; Plazaola, F.; Moros, M.; De Matteis, L.; de la Fuente, J.M.; Lezama, L. Chemical Synthesis and Magnetic Properties of Monodisperse Nickel Ferrite Nanoparticles for Biomedical Applications. J. Phys. Chem. C 2016, 120, 3492–3500. [Google Scholar] [CrossRef] [Green Version]
- Vuong, Q.L.; Berret, J.-F.; Fresnais, J.; Gossuin, Y.; Sandre, O. A Universal Scaling Law to Predict the Efficiency of Magnetic Nanoparticles as MRI T2-Contrast Agents. Adv. Healthc. Mater. 2012, 1, 502–512. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jun, Y.; Huh, Y.-M.; Choi, J.; Lee, J.-H.; Song, H.-T.; Kim, S.; Yoon, S.; Kim, K.-S.; Shin, J.-S.; Suh, J.-S.; et al. Nanoscale Size Effect of Magnetic Nanocrystals and Their Utilization for Cancer Diagnosis via Magnetic Resonance Imaging. J. Am. Chem. Soc. 2005, 127, 5732–5733. [Google Scholar] [CrossRef]
- Lavorato, G.; Alzamora, M.; Contreras, C.; Burlandy, G.; Litterst, F.J.; Baggio-Saitovitch, E. Internal Structure and Magnetic Properties in Cobalt Ferrite Nanoparticles: Influence of the Synthesis Method. Part. Part. Syst. Charact. 2019, 36, 1900061. [Google Scholar] [CrossRef]
- Ali, A.; Zafar, H.; Zia, M.; Ul Haq, I.; Phull, A.R.; Ali, J.S.; Hussain, A. Synthesis, characterization, applications, and challenges of iron oxide nanoparticles. Nanotechnol. Sci. Appl. 2016, 9, 49–67. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tong, S.; Hou, S.; Zheng, Z.; Zhou, J.; Bao, G. Coating Optimization of Superparamagnetic Iron Oxide Nanoparticles for High T2 Relaxivity. Nano Lett. 2010, 10, 4607–4613. [Google Scholar] [CrossRef] [Green Version]
- Pellico, J.; Ruiz-Cabello, J.; Fernández-Barahona, I.; Gutiérrez, L.; Lechuga-Vieco, A.V.; Enríquez, J.A.; Morales, M.P.; Herranz, F. One-Step Fast Synthesis of Nanoparticles for MRI: Coating Chemistry as the Key Variable Determining Positive or Negative Contrast. Langmuir 2017, 33, 10239–10247. [Google Scholar] [CrossRef] [Green Version]
- Alazmi, A.; Singaravelu, V.; Batra, N.M.; Smajic, J.; Alyami, M.; Khashab, N.M.; Costa, P.M.F.J. Cobalt ferrite supported on reduced graphene oxide as a T2 contrast agent for magnetic resonance imaging. RSC Adv. 2019, 9, 6299–6309. [Google Scholar] [CrossRef] [Green Version]
- Geraldes, C.F.G.C.; Laurent, S. Classification and basic properties of contrast agents for magnetic resonance imaging. Contrast Media Mol. Imaging 2009, 4, 1–23. [Google Scholar] [CrossRef]
- Shultz, M.D.; Calvin, S.; Fatouros, P.P.; Morrison, S.A.; Carpenter, E.E. Enhanced ferrite nanoparticles as MRI contrast agents. J. Magn. Magn. Mater. 2007, 311, 464–468. [Google Scholar] [CrossRef]
- Tromsdorf, U.I.; Bigall, N.C.; Kaul, M.G.; Bruns, O.T.; Nikolic, M.S.; Mollwitz, B.; Sperling, R.A.; Reimer, R.; Hohenberg, H.; Parak, W.J.; et al. Size and Surface Effects on the MRI Relaxivity of Manganese Ferrite Nanoparticle Contrast Agents. Nano Lett. 2007, 7, 2422–2427. [Google Scholar] [CrossRef] [PubMed]
- Joshi, H.M.; Lin, Y.P.; Aslam, M.; Prasad, P.V.; Schultz-Sikma, E.A.; Edelman, R.; Meade, T.; Dravid, V.P. Effects of Shape and Size of Cobalt Ferrite Nanostructures on Their MRI Contrast and Thermal Activation. J. Phys. Chem. C 2009, 113, 17761–17767. [Google Scholar] [CrossRef] [Green Version]
- Mazarío, E.; Sánchez-Marcos, J.; Menéndez, N.; Cañete, M.; Mayoral, A.; Rivera-Fernández, S.; De La Fuente, J.M.; Herrasti, P. High specific absorption rate and transverse relaxivity effects in manganese ferrite nanoparticles obtained by an electrochemical route. J. Phys. Chem. C 2015, 119, 6828–6834. [Google Scholar] [CrossRef]
- Lee, J.-H.; Huh, Y.-M.; Jun, Y.; Seo, J.; Jang, J.; Song, H.-T.; Kim, S.; Cho, E.-J.; Yoon, H.-G.; Suh, J.-S.; et al. Artificially engineered magnetic nanoparticles for ultra-sensitive molecular imaging. Nat. Med. 2007, 13, 95–99. [Google Scholar] [CrossRef]
- Kim, D.-H.; Zeng, H.; Ng, T.C.; Brazel, C.S. T1 and T2 relaxivities of succimer-coated MFe23+O4 (M=Mn2+, Fe2+ and Co2+) inverse spinel ferrites for potential use as phase-contrast agents in medical MRI. J. Magn. Magn. Mater. 2009, 321, 3899–3904. [Google Scholar] [CrossRef]
- Albino, M.; Fantechi, E.; Innocenti, C.; López-Ortega, A.; Bonanni, V.; Campo, G.; Pineider, F.; Gurioli, M.; Arosio, P.; Orlando, T.; et al. Role of Zn2+ Substitution on the Magnetic, Hyperthermic, and Relaxometric Properties of Cobalt Ferrite Nanoparticles. J. Phys. Chem. C 2019, 123, 6148–6157. [Google Scholar] [CrossRef] [Green Version]
- Kevadiya, B.D.; Bade, A.N.; Woldstad, C.; Edagwa, B.J.; McMillan, J.M.; Sajja, B.R.; Boska, M.D.; Gendelman, H.E. Development of europium doped core-shell silica cobalt ferrite functionalized nanoparticles for magnetic resonance imaging. Acta Biomater. 2017, 49, 507–520. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yue, H.; Park, J.A.; Ho, S.L.; Ahmad, M.Y.; Cha, H.; Liu, S.; Tegafaw, T.; Marasini, S.; Ghazanfari, A.; Kim, S.; et al. New Class of Efficient T2 Magnetic Resonance Imaging Contrast Agent: Carbon-Coated Paramagnetic Dysprosium Oxide Nanoparticles. Pharmaceuticals 2020, 13, 312. [Google Scholar] [CrossRef] [PubMed]
- Weissleder, R.; Bogdanov, A.; Papisov, M. Drug targeting in magnetic resonance imaging. Magn. Reson. Q. 1992, 8, 55–63. [Google Scholar] [PubMed]
- Bulte, J.W.M.; Kraitchman, D.L. Iron oxide MR contrast agents for molecular and cellular imaging. NMR Biomed. 2004, 17, 484–499. [Google Scholar] [CrossRef] [PubMed]
- Dixit, V.; Van den Bossche, J.; Sherman, D.M.; Thompson, D.H.; Andres, R.P. Synthesis and Grafting of Thioctic Acid—PEG—Folate Conjugates onto Au Nanoparticles for Selective Targeting of Folate Receptor-Positive Tumor Cells. Bioconj. Chem. 2006, 17, 603–609. [Google Scholar] [CrossRef]
- Arbab, A.S.; Wilson, L.B.; Ashari, P.; Jordan, E.K.; Lewis, B.K.; Frank, J.A. A model of lysosomal metabolism of dextran coated superparamagnetic iron oxide (SPIO) nanoparticles: Implications for cellular magnetic resonance imaging. NMR Biomed. 2005, 18, 383–389. [Google Scholar] [CrossRef] [PubMed]
- Laskar, A.; Ghosh, M.; Khattak, S.I.; Li, W.; Yuan, X.-M. Degradation of superparamagnetic iron oxide nanoparticle-induced ferritin by lysosomal cathepsins and related immune response. Nanomedicine 2012, 7, 705–717. [Google Scholar] [CrossRef]
- Falke, S.; Betzel, C. Chapter 6: Dynamic Light Scattering (DLS). In Radiation in Bioanalysis: Spectroscopic Techniques and Theoretical Methods; Pereira, A.S., Tavares, P., Limão-Vieira, P., Eds.; Springer: Cham, Switzerland, 2019; pp. 173–193. ISBN 978-3-030-28247-9. [Google Scholar]
- Kombaiah, K.; Vijaya, J.J.; Kennedy, L.J.; Bououdina, M.; Al Najar, B. Self heating efficiency of CoFe2O4 nanoparticles: A comparative investigation on the conventional and microwave combustion method. J. Alloys Compd. 2018, 735, 1536–1545. [Google Scholar] [CrossRef]
- Phong, P.T.; Phuc, N.X.; Nam, P.H.; Chien, N.V.; Dung, D.D.; Linh, P.H. Size-controlled heating ability of CoFe2O4 nanoparticles for hyperthermia applications. Phys. B Condens. Matter 2018, 531, 30–34. [Google Scholar] [CrossRef]
- Chantrell, R.W.; Walmsley, N.S.; Gore, J.; Maylin, M. Theoretical studies of the field-cooled and zero-field cooled magnetization of interacting fine particles. J. Appl. Phys. 1999, 85, 4340–4342. [Google Scholar] [CrossRef]
- Corot, C.; Robert, P.; Idée, J.-M.; Port, M. Recent advances in iron oxide nanocrystal technology for medical imaging. Adv. Drug Deliv. Rev. 2006, 58, 1471–1504. [Google Scholar] [CrossRef]
- Schultz-Sikma, E.A.; Joshi, H.M.; Ma, Q.; MacRenaris, K.W.; Eckermann, A.L.; Dravid, V.P.; Meade, T.J. Probing the Chemical Stability of Mixed Ferrites: Implications for Magnetic Resonance Contrast Agent Design. Chem. Mater. 2011, 23, 2657–2664. [Google Scholar] [CrossRef] [Green Version]
- Venkatesha, N.; Srivastava, C.; Hegde, V. Synergetic effect of size and morphology of cobalt ferrite nanoparticles on proton relaxivity. IET Nanobiotechnol. 2014, 8, 184–189. [Google Scholar]
- Liu, F.; Laurent, S.; Roch, A.; Vander Elst, L.; Muller, R.N. Size-Controlled Synthesis of CoFe2O4 Nanoparticles Potential Contrast Agent for MRI and Investigation on Their Size-Dependent Magnetic Properties. J. Nanomater. 2013, 2013, 462540. [Google Scholar] [CrossRef] [Green Version]
- Cannas, C.; Musinu, A.; Ardu, A.; Orrù, F.; Peddis, D.; Casu, M.; Sanna, R.; Angius, F.; Diaz, G.; Piccaluga, G. CoFe2O4 and CoFe2O4/SiO2 core/shell nanoparticles: Magnetic and spectroscopic study. Chem. Mater. 2010, 22, 3353–3361. [Google Scholar] [CrossRef]
- Kim, D.-H.; Lee, S.-H.; Kim, K.-N.; Kim, K.-M.; Shim, I.-B.; Lee, Y.-K. Cytotoxicity of ferrite particles by MTT and agar diffusion methods for hyperthermic application. J. Magn. Magn. Mater. 2005, 293, 287–292. [Google Scholar] [CrossRef]
- Mazarío, E.; Herrasti, P.; Morales, M.P.; Menéndez, N. Synthesis and characterization of CoFe2O4 ferrite nanoparticles obtained by an electrochemical method. Nanotechnology 2012, 23, 355708. [Google Scholar] [CrossRef] [PubMed]
- Mazario, E.; Morales, M.P.; Galindo, R.; Herrasti, P.; Menendez, N. Influence of the temperature in the electrochemical synthesis of cobalt ferrites nanoparticles. J. Alloys Compd. 2012, 536, S222–S225. [Google Scholar] [CrossRef]







| Contrast Agents [31] | Material | Coating | D(TEM) nm | r1 | r2 | r2/r1 |
|---|---|---|---|---|---|---|
| This work | CoFe2O4 | Folic acid | 17(4) | 10.3 | 479 | 46 |
| Lee et al. [17] | 2,3-Dimercaptosuccinic acid | 172 | ||||
| Schultz-Sikma et al. [32] | Silica | 7 | 142 | |||
| Joshi et al. [15] | 11-aminoundecanoic acid | 15 | 301 | |||
| Kim et al. [18] | DMSA | 8 | 6 | 392 | 62 | |
| Venkatesha et al. [33] | Chitosan | 6 | 32 | |||
| Liu et al. [34] | Citrate | 37.89 | 27.8 | 75.1 | 2.7 | |
| Sinerem® | γ-Fe2O3 | Dextran | 4–15 | 9.9 | 65 | 7 |
| Resovist® | Carboxydextran | 4–15 | 9.7 | 189 | 19 | |
| VSOP_C184® | Citric acid | 5 | 14 | 33.4 | 2 | |
| Endorem® | Dextran | 4–15 | 10.1 | 120 | 12 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mazarío, E.; Cañete, M.; Herranz, F.; Sánchez-Marcos, J.; de la Fuente, J.M.; Herrasti, P.; Menéndez, N. Highly Efficient T2 Cobalt Ferrite Nanoparticles Vectorized for Internalization in Cancer Cells. Pharmaceuticals 2021, 14, 124. https://doi.org/10.3390/ph14020124
Mazarío E, Cañete M, Herranz F, Sánchez-Marcos J, de la Fuente JM, Herrasti P, Menéndez N. Highly Efficient T2 Cobalt Ferrite Nanoparticles Vectorized for Internalization in Cancer Cells. Pharmaceuticals. 2021; 14(2):124. https://doi.org/10.3390/ph14020124
Chicago/Turabian StyleMazarío, Eva, Magdalena Cañete, Fernando Herranz, Jorge Sánchez-Marcos, Jesús M. de la Fuente, Pilar Herrasti, and Nieves Menéndez. 2021. "Highly Efficient T2 Cobalt Ferrite Nanoparticles Vectorized for Internalization in Cancer Cells" Pharmaceuticals 14, no. 2: 124. https://doi.org/10.3390/ph14020124
APA StyleMazarío, E., Cañete, M., Herranz, F., Sánchez-Marcos, J., de la Fuente, J. M., Herrasti, P., & Menéndez, N. (2021). Highly Efficient T2 Cobalt Ferrite Nanoparticles Vectorized for Internalization in Cancer Cells. Pharmaceuticals, 14(2), 124. https://doi.org/10.3390/ph14020124