Pharyngeal Pumping and Tissue-Specific Transgenic P-Glycoprotein Expression Influence Macrocyclic Lactone Susceptibility in Caenorhabditis elegans
Abstract
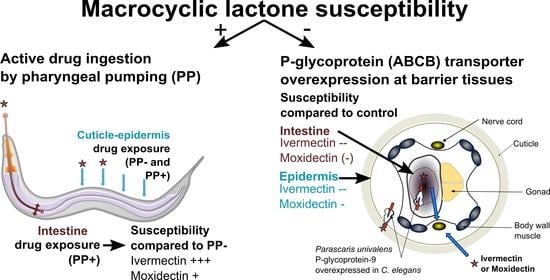
:1. Introduction
2. Results
2.1. Tissue-Specific Expression Patterns of Pun-PGP-9 in a Cel-pgp-9 Mutant Strain
2.2. Motility Assays in Transgenic and Wildtype Caenorhabditis elegans
2.2.1. The Effect of Active Drug Ingestion on Ivermectin and Moxidectin Susceptibility
2.2.2. Cel-pgp-9 Loss-of-Function Strain Susceptibility Phenotype in Adult Stage
2.2.3. The Effect of Epidermal Pun-PGP-9 Expression on Ivermectin and Moxidectin Susceptibility
2.2.4. The Effect of Intestinal Pun-PGP-9 Expression on Ivermectin and Moxidectin Susceptibility
2.2.5. A Comparison of Moxidectin and Ivermectin Susceptibility in Transgenic and Wildtype Strains
3. Discussion
3.1. The Role of Pun-PGP-9 in Ivermectin and Moxidectin Susceptibility
3.2. The Intestinal and Transcuticlular-Epidermal Uptake of Ivermectin and Moxidectin
3.3. Experimental Limitations and Relevance for Parasitic Nematodes
4. Conclusions
5. Materials and Methods
5.1. Plasmids and Plasmid Construction
5.2. Generation of Caenorhabditis elegans Strains and Maintenance
5.3. Verification of Pun-PGP-9 Expression by RT-PCR and Immunofluorescence
5.4. Trashing Assay
5.5. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shoop, W.L.; Mrozik, H.; Fisher, M.H. Structure and activity of avermectins and milbemycins in animal health. Vet. Parasitol. 1995, 59, 139–156. [Google Scholar] [CrossRef]
- Kaplan, R.M. Biology, epidemiology, diagnosis, and management of anthelmintic resistance in gastrointestinal nematodes of livestock. Vet. Clin. N. Am. Small Anim. Pract. 2020, 36, 17–30. [Google Scholar] [CrossRef]
- Nielsen, M.K.; Reinemeyer, C.R.; Donecker, J.M.; Leathwick, D.M.; Marchiondo, A.A.; Kaplan, R.M. Anthelmintic resistance in equine parasites—Current evidence and knowledge gaps. Vet. Parasitol. 2014, 204, 55–63. [Google Scholar] [CrossRef]
- Wolstenholme, A.J.; Evans, C.C.; Jimenez, P.D.; Moorhead, A.R. The emergence of macrocyclic lactone resistance in the canine heartworm, Dirofilaria immitis. Parasitology 2015, 142, 1249–1259. [Google Scholar] [CrossRef]
- Jimenez Castro, P.D.; Howell, S.B.; Schaefer, J.J.; Avramenko, R.W.; Gilleard, J.S.; Kaplan, R.M. Multiple drug resistance in the canine hookworm Ancylostoma caninum: An emerging threat? Parasit. Vectors 2019, 12, 576. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Osei-Atweneboana, M.Y.; Awadzi, K.; Attah, S.K.; Boakye, D.A.; Gyapong, J.O.; Prichard, R.K. Phenotypic evidence of emerging ivermectin resistance in Onchocerca volvulus. PLoS Negl. Trop. Dis. 2011, 5, e998. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hotez, P.J.; Brindley, P.J.; Bethony, J.M.; King, C.H.; Pearce, E.J.; Jacobson, J. Helminth infections: The great neglected tropical diseases. J. Clin. Investig. 2008, 118, 1311–1321. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nielsen, M.K. Universal challenges for parasite control: A perspective from equine parasitology. Trends Parasitol. 2015, 31, 282–284. [Google Scholar] [CrossRef] [PubMed]
- Wolstenholme, A.J.; Rogers, A.T. Glutamate-gated chloride channels and the mode of action of the avermectin/milbemycin anthelmintics. Parasitology 2005, 131, S85–S95. [Google Scholar] [CrossRef] [PubMed]
- Dent, J.A.; Smith, M.M.; Vassilatis, D.K.; Avery, L. The genetics of ivermectin resistance in Caenorhabditis elegans. Proc. Natl. Acad. Sci. USA 2000, 97, 2674–2679. [Google Scholar] [CrossRef] [Green Version]
- Janssen, I.J.; Krücken, J.; Demeler, J.; Basiaga, M.; Kornas, S.; von Samson-Himmelstjerna, G. Genetic variants and increased expression of Parascaris equorum P-glycoprotein-11 in populations with decreased ivermectin susceptibility. PLoS ONE 2013, 8, e61635. [Google Scholar] [CrossRef] [PubMed]
- Bourguinat, C.; Keller, K.; Blagburn, B.; Schenker, R.; Geary, T.G.; Prichard, R.K. Correlation between loss of efficacy of macrocyclic lactone heartworm anthelmintics and P-glycoprotein genotype. Vet. Parasitol. 2011, 176, 374–381. [Google Scholar] [CrossRef] [PubMed]
- Janssen, I.J.; Krücken, J.; Demeler, J.; von Samson-Himmelstjerna, G. Caenorhabditis elegans: Modest increase of susceptibility to ivermectin in individual P-glycoprotein loss-of-function strains. Exp. Parasitol. 2013, 134, 171–177. [Google Scholar] [CrossRef] [PubMed]
- Ardelli, B.F.; Prichard, R.K. Inhibition of P-glycoprotein enhances sensitivity of Caenorhabditis elegans to ivermectin. Vet. Parasitol. 2013, 191, 264–275. [Google Scholar] [CrossRef]
- Aller, S.G.; Yu, J.; Ward, A.; Weng, Y.; Chittaboina, S.; Zhuo, R.; Harrell, P.M.; Trinh, Y.T.; Zhang, Q.; Urbatsch, I.L.; et al. Structure of P-glycoprotein reveals a molecular basis for poly-specific drug binding. Science 2009, 323, 1718–1722. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reyes-Guerrero, D.E.; Cedillo-Borda, M.; Alonso-Morales, R.; Alonso-Díaz, M.; Olmedo-Juárez, A.; Mendoza-de-Gives, P.; López-Arellano, M.E. Comparative study of transcription profiles of the P-glycoprotein transporters of two Haemonchus contortus isolates: Susceptible and resistant to ivermectin. Mol. Biochem. Parasitol. 2020. [Google Scholar] [CrossRef]
- Khan, S.; Nisar, A.; Yuan, J.; Luo, X.; Dou, X.; Liu, F.; Zhao, X.; Li, J.; Ahmad, H.; Mehmood, S.A.; et al. A whole genome re-sequencing based GWA analysis reveals candidate genes associated with ivermectin resistance in Haemonchus contortus. Genes 2020, 11, 367. [Google Scholar] [CrossRef] [Green Version]
- Choi, Y.J.; Bisset, S.A.; Doyle, S.R.; Hallsworth-Pepin, K.; Martin, J.; Grant, W.N.; Mitreva, M. Genomic introgression mapping of field-derived multiple-anthelmintic resistance in Teladorsagia circumcincta. PLoS Genet. 2017, 13, e1006857. [Google Scholar] [CrossRef]
- Peachey, L.E.; Pinchbeck, G.L.; Matthews, J.B.; Burden, F.A.; Lespine, A.; von Samson-Himmelstjerna, G.; Krücken, J.; Hodgkinson, J.E. P-glycoproteins play a role in ivermectin resistance in cyathostomins. Int. J. Parasitol. Drugs Drug Resist. 2017, 7, 388–398. [Google Scholar] [CrossRef]
- Coghlan, A.; Tyagi, R.; Cotton, J.A.; Holroyd, N.; Rosa, B.A.; Tsai, I.J.; Laetsch, D.R.; Beech, R.N.; Day, T.A.; Hallsworth-Pepin, K.; et al. Comparative genomics of the major parasitic worms. Nat. Genet. 2019, 51, 163–174. [Google Scholar] [CrossRef] [Green Version]
- Sheps, J.A.; Ralph, S.; Zhao, Z.; Baillie, D.L.; Ling, V. The ABC transporter gene family of Caenorhabditis elegans has implications for the evolutionary dynamics of multidrug resistance in eukaryotes. Genome Biol. 2004, 5, R15. [Google Scholar] [CrossRef] [Green Version]
- Laing, R.; Kikuchi, T.; Martinelli, A.; Tsai, I.J.; Beech, R.N.; Redman, E.; Holroyd, N.; Bartley, D.J.; Beasley, H.; Britton, C.; et al. The genome and transcriptome of Haemonchus contortus, a key model parasite for drug and vaccine discovery. Genome Biol. 2013, 14, R88. [Google Scholar] [CrossRef] [Green Version]
- Gerhard, A.P.; Krücken, J.; Heitlinger, E.; Janssen, I.J.I.; Basiaga, M.; Kornaś, S.; Beier, C.; Nielsen, M.K.; Davis, R.E.; Wang, J.; et al. The P-glycoprotein repertoire of the equine parasitic nematode Parascaris univalens. Sci. Rep. 2020, 10, 13586. [Google Scholar] [CrossRef] [PubMed]
- Kaschny, M.; Demeler, J.; Janssen, I.J.; Kuzmina, T.A.; Besognet, B.; Kanellos, T.; Kerboeuf, D.; von Samson-Himmelstjerna, G.; Krücken, J. Macrocyclic lactones differ in interaction with recombinant P-glycoprotein 9 of the parasitic nematode Cylicocylus elongatus and ketoconazole in a yeast growth assay. PLoS Pathog. 2015, 11, e1004781. [Google Scholar] [CrossRef] [Green Version]
- Turnbull, F.; Jonsson, N.N.; Kenyon, F.; Skuce, P.J.; Bisset, S.A. P-glycoprotein-9 and macrocyclic lactone resistance status in selected strains of the ovine gastrointestinal nematode, Teladorsagia circumcincta. Int. J. Parasitol. Drugs Drug Resist. 2018, 8, 70–80. [Google Scholar] [CrossRef]
- Kellerová, P.; Matoušková, P.; Lamka, J.; Vokřál, I.; Szotáková, B.; Zajíčková, M.; Pasák, M.; Skálová, L. Ivermectin-induced changes in the expression of cytochromes P450 and efflux transporters in Haemonchus contortus female and male adults. Vet. Parasitol. 2019. [Google Scholar] [CrossRef]
- Jesudoss Chelladurai, J.; Brewer, M.T. Detection and quantification of Parascaris P-glycoprotein drug transporter expression with a novel mRNA hybridization technique. Vet. Parasitol. 2019, 267, 75–83. [Google Scholar] [CrossRef]
- Zhao, Z.; Sheps, J.A.; Ling, V.; Fang, L.L.; Baillie, D.L. Expression analysis of ABC transporters reveals differential functions of tandemly duplicated genes in Caenorhabditis elegans. J. Mol. Biol. 2004, 344, 409–417. [Google Scholar] [CrossRef] [PubMed]
- Godoy, P.; Lian, J.; Beech, R.N.; Prichard, R.K. Haemonchus contortus P-glycoprotein-2: In situ localisation and characterisation of macrocyclic lactone transport. Int. J. Parasitol. 2015, 45, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Godoy, P.; Che, H.; Beech, R.N.; Prichard, R.K. Characterization of Haemonchus contortus P-glycoprotein-16 and its interaction with the macrocyclic lactone anthelmintics. Mol. Biochem. Parasitol. 2015, 204, 11–15. [Google Scholar] [CrossRef]
- Ballent, M.; Lifschitz, A.; Virkel, G.; Sallovitz, J.; Lanusse, C. Modulation of the P-glycoprotein-mediated intestinal secretion of ivermectin: In vitro and in vivo assessments. Drug Metab. Dispos. 2006, 34, 457–463. [Google Scholar] [CrossRef] [PubMed]
- Merola, V.M.; Eubig, P.A. Toxicology of avermectins and milbemycins (macrocylic lactones) and the role of P-glycoprotein in dogs and cats. Vet. Clin. N. Am. Small Anim. Pract. 2012, 42, 313–333. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kiki-Mvouaka, S.; Menez, C.; Borin, C.; Lyazrhi, F.; Foucaud-Vignault, M.; Dupuy, J.; Collet, X.; Alvinerie, M.; Lespine, A. Role of P-glycoprotein in the disposition of macrocyclic lactones: A comparison between ivermectin, eprinomectin, and moxidectin in mice. Drug Metab. Dispos. 2010, 38, 573–580. [Google Scholar] [CrossRef] [PubMed]
- Avery, L.; Shtonda, B.B. Food transport in the C. elegans pharynx. J. Exp. Biol. 2003, 206, 2441–2457. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, B.-M.; Avery, L. Serotonin activates overall feeding by activating two separate neural pathways in Caenorhabditis elegans. J. Neurosci. 2012, 32, 1920–1931. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Y.; Falck, J.R.; Rothe, M.; Schunck, W.H.; Menzel, R. Role of CYP eicosanoids in the regulation of pharyngeal pumping and food uptake in Caenorhabditis elegans. J. Lipid Res. 2015, 56, 2110–2123. [Google Scholar] [CrossRef] [Green Version]
- Dalliere, N.; Bhatla, N.; Luedtke, Z.; Ma, D.K.; Woolman, J.; Walker, R.J.; Holden-Dye, L.; O’Connor, V. Multiple excitatory and inhibitory neural signals converge to fine-tune Caenorhabditis elegans feeding to food availability. FASEB J. 2016, 30, 836–848. [Google Scholar] [CrossRef] [Green Version]
- Kennedy, B.P.; Aamodt, E.J.; Allen, F.L.; Chung, M.A.; Heschl, M.F.; McGhee, J.D. The gut esterase gene (ges-1) from the nematodes Caenorhabditis elegans and Caenorhabditis briggsae. J. Exp. Biol. 1993, 229, 890–908. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Kirch, S.; Ambros, V. The Caenorhabditis elegans heterochronic gene pathway controls stage-specific transcription of collagen genes. Development 1995, 121, 2471–2478. [Google Scholar]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Janssen, I.J.; Krücken, J.; Demeler, J.; von Samson-Himmelstjerna, G. Transgenically expressed Parascaris P-glycoprotein-11 can modulate ivermectin susceptibility in Caenorhabditis elegans. Int. J. Parasitol. Drugs Drug Resist. 2015, 5, 44–47. [Google Scholar] [CrossRef] [Green Version]
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020. [Google Scholar]
- Mani, T.; Bourguinat, C.; Keller, K.; Ashraf, S.; Blagburn, B.; Prichard, R.K. Interaction of macrocyclic lactones with a Dirofilaria immitis P-glycoprotein. Int. J. Parasitol. 2016, 46, 631–640. [Google Scholar] [CrossRef]
- Godoy, P.; Che, H.; Beech, R.N.; Prichard, R.K. Characterisation of P-glycoprotein-9.1 in Haemonchus contortus. Parasit. Vectors 2016, 9, 52. [Google Scholar] [CrossRef] [Green Version]
- Prichard, R.K.; Geary, T.G. Perspectives on the utility of moxidectin for the control of parasitic nematodes in the face of developing anthelmintic resistance. Int. J. Parasitol. Drugs Drug Resist. 2019, 10, 69–83. [Google Scholar] [CrossRef]
- Cooper, L.G.; Caffe, G.; Cerutti, J.; Nielsen, M.K.; Anziani, O.S. Reduced efficacy of ivermectin and moxidectin against Parascaris spp. in foals from Argentina. Vet. Parasitol. Reg. Stud. Rep. 2020, 20, 100388. [Google Scholar] [CrossRef]
- Hunt-Newbury, R.; Viveiros, R.; Johnsen, R.; Mah, A.; Anastas, D.; Fang, L.; Halfnight, E.; Lee, D.; Lin, J.; Lorch, A.; et al. High-throughput in vivo analysis of gene expression in Caenorhabditis elegans. PLoS Biol. 2007, 5, e237. [Google Scholar] [CrossRef] [PubMed]
- Thompson, D.; Ho, N.; Sims, S.; Geary, T. Mechanistic approaches to quantitate anthelmintic absorption by gastrointestinal nematodes. Parasitol. Today 1993, 9, 31–35. [Google Scholar] [CrossRef]
- Ho, N.F.H.; Geary, T.G.; Raub, T.J.; Barsuhn, C.L.; Thompson, D.P. Biophysical transport properties of the cuticle of Ascaris suum. Mol. Biochem. Parasitol. 1990, 41, 153–165. [Google Scholar] [CrossRef]
- Weeks, J.C.; Robinson, K.J.; Lockery, S.R.; Roberts, W.M. Anthelmintic drug actions in resistant and susceptible C. elegans revealed by electrophysiological recordings in a multichannel microfluidic device. Int. J. Parasitol. Drugs Drug Resist. 2018, 8, 607–628. [Google Scholar] [CrossRef] [PubMed]
- O’Lone, R.B.; Campbell, W.C. Effect of refrigeration on the antinematodal efficacy of ivermectin. J. Parasitol. 2001, 87, 452–454. [Google Scholar] [CrossRef]
- Ho, N.F.; Geary, T.G.; Barsuhn, C.L.; Sims, S.M.; Thompson, D.P. Mechanistic studies in the transcuticular delivery of antiparasitic drugs. II: Ex vivo/in vitro correlation of solute transport by Ascaris suum. Mol. Biochem. Parasitol. 1992, 52, 1–13. [Google Scholar] [CrossRef]
- Prichard, R.; Ménez, C.; Lespine, A. Moxidectin and the avermectins: Consanguinity but not identity. Int. J. Parasitol. Drugs Drug Resist. 2012, 2, 134–153. [Google Scholar] [CrossRef] [PubMed]
- Craven, J.; Bjorn, H.; Hennesy, D.R.; Friis, C. The effects of body composition on the pharmacokinetics of subcutaneously injected ivermectin and moxidectin in pigs. J. Vet. Pharmacol. Ther. 2002, 25, 227–232. [Google Scholar] [CrossRef]
- Mullaney, B.C.; Ashrafi, K.C. C. elegans fat storage and metabolic regulation. Biochim. Biophys. Acta 2009, 1791, 474–478. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Castelletto, M.L.; Gang, S.S.; Hallem, E.A. Recent advances in functional genomics for parasitic nematodes of mammals. J. Exp. Biol. 2020, 223, jeb206482. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wolstenholme, A.J. Glutamate-gated Chloride Channels. J. Biol. Chem. 2012, 287, 40232–40238. [Google Scholar] [CrossRef] [Green Version]
- Hu, Y.; Ellis, B.L.; Yiu, Y.Y.; Miller, M.M.; Urban, J.F.; Shi, L.Z.; Aroian, R.V. An extensive comparison of the effect of anthelmintic classes on diverse nematodes. PLoS ONE 2013, 8, e70702. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Demeler, J.; Gill, J.H.; von Samson-Himmelstjerna, G.; Sangster, N.C. The in vitro assay profile of macrocyclic lactone resistance in three species of sheep trichostrongyloids. Int. J. Parasitol. Drugs Drug Resist. 2013, 3, 109–118. [Google Scholar] [CrossRef] [Green Version]
- George, M.M.; Lopez-Soberal, L.; Storey, B.E.; Howell, S.B.; Kaplan, R.M. Motility in the L3 stage is a poor phenotype for detecting and measuring resistance to avermectin/milbemycin drugs in gastrointestinal nematodes of livestock. Int. J. Parasitol. Drugs Drug Resist. 2017, 8, 22–30. [Google Scholar] [CrossRef] [PubMed]
- Lloberas, M.; Alvarez, L.; Entrocasso, C.; Virkel, G.; Ballent, M.; Mate, L.; Lanusse, C.; Lifschitz, A. Comparative tissue pharmacokinetics and efficacy of moxidectin, abamectin and ivermectin in lambs infected with resistant nematodes: Impact of drug treatments on parasite P-glycoprotein expression. Int. J. Parasitol. Drugs Drug Resist. 2013, 3, 20–27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leathwick, D.M.; Miller, C.M.; Waghorn, T.S.; Schwendel, H.; Lifschitz, A. Route of administration influences the concentration of ivermectin reaching nematode parasites in the gastrointestinal tract of cattle. Int. J. Parasitol. Drugs Drug Resist. 2020, 14, 152–158. [Google Scholar] [CrossRef]
- Lloberas, M.; Alvarez, L.; Entrocasso, C.; Virkel, G.; Lanusse, C.; Lifschitz, A. Measurement of ivermectin concentrations in target worms and host gastrointestinal tissues: Influence of the route of administration on the activity against resistant Haemonchus contortus in lambs. Exp. Parasitol. 2012, 131, 304–309. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, L.; Suarez, G.; Ceballos, L.; Moreno, L.; Canton, C.; Lifschitz, A. Integrated assessment of ivermectin pharmacokinetics, efficacy against resistant Haemonchus contortus and P-glycoprotein expression in lambs treated at three different dosage levels. Vet. Parasitol. 2015, 210. [Google Scholar] [CrossRef]
- Rieckher, M.; Kyparissidis-Kokkinidis, I.; Zacharopoulos, A.; Kourmoulakis, G.; Tavernarakis, N.; Ripoll, J.; Zacharakis, G. A Customized Light Sheet Microscope to Measure Spatio-Temporal Protein Dynamics in Small Model Organisms. PLoS ONE 2015, 10, e0127869. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stiernagle, T. Maintenance of C. elegans. WormBook 2006. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Evans, T.C. (Ed.) Transformation and microinjection. In WormBook; The C. elegans Research Community, WormBook: Pasadena, CA, USA, 2006. [Google Scholar] [CrossRef] [Green Version]
- Duerr, J.S. Immunohistochemistry. WormBook 2006. [Google Scholar] [CrossRef] [PubMed] [Green Version]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gerhard, A.P.; Krücken, J.; Neveu, C.; Charvet, C.L.; Harmache, A.; von Samson-Himmelstjerna, G. Pharyngeal Pumping and Tissue-Specific Transgenic P-Glycoprotein Expression Influence Macrocyclic Lactone Susceptibility in Caenorhabditis elegans. Pharmaceuticals 2021, 14, 153. https://doi.org/10.3390/ph14020153
Gerhard AP, Krücken J, Neveu C, Charvet CL, Harmache A, von Samson-Himmelstjerna G. Pharyngeal Pumping and Tissue-Specific Transgenic P-Glycoprotein Expression Influence Macrocyclic Lactone Susceptibility in Caenorhabditis elegans. Pharmaceuticals. 2021; 14(2):153. https://doi.org/10.3390/ph14020153
Chicago/Turabian StyleGerhard, Alexander P., Jürgen Krücken, Cedric Neveu, Claude L. Charvet, Abdallah Harmache, and Georg von Samson-Himmelstjerna. 2021. "Pharyngeal Pumping and Tissue-Specific Transgenic P-Glycoprotein Expression Influence Macrocyclic Lactone Susceptibility in Caenorhabditis elegans" Pharmaceuticals 14, no. 2: 153. https://doi.org/10.3390/ph14020153
APA StyleGerhard, A. P., Krücken, J., Neveu, C., Charvet, C. L., Harmache, A., & von Samson-Himmelstjerna, G. (2021). Pharyngeal Pumping and Tissue-Specific Transgenic P-Glycoprotein Expression Influence Macrocyclic Lactone Susceptibility in Caenorhabditis elegans. Pharmaceuticals, 14(2), 153. https://doi.org/10.3390/ph14020153