Safflower Seed Extract Attenuates the Development of Osteoarthritis by Blocking NF-κB Signaling
Abstract
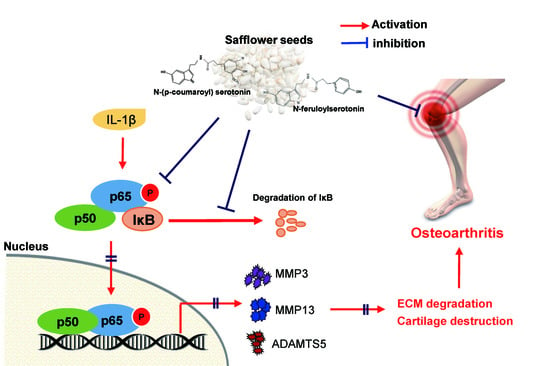
:1. Introduction
2. Results
2.1. Effect of Safflower Seed Extract on Chondrocyte Viability
2.2. Safflower Seed Extract Suppresses IL-1β-Induced Expression of Catabolic Factors in Chondrocytes
2.3. Oral Administration of Safflower Seed Extract Suppresses Cartilage Destruction in the DMM-Induced OA Model
2.4. HPCL Analysis of Safflower Seed Extract
2.5. N-(p-Coumaroyl) Serotonin and N-Feruloyl Serotonin Suppressed the Expression of Catabolic Factors
2.6. Safflower Seed Extract Modulates the NF-KB Signaling Pathway in an In Vitro Model of OA
3. Discussion
4. Materials and Methods
4.1. Reagents and Treatment
4.2. Primary Culture of Articular Chondrocytes
4.3. Cytotoxicity Analysis
4.4. RT-PCR and Quantitative RT-PCR Analyses
4.5. Western Blotting
4.6. IL-1β Assay and Collagenase Activity Assay
4.7. Experimental OA Mouse Model and Oral Gavage
4.8. Safranin O Staining and Immunohistochemical Analysis
4.9. Luciferase Assay
4.10. Statistical Analyses
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Richette, P.; Latourte, A. Osteoarthritis: Value of imaging and biomarkers. La Rev. Du Prat. 2019, 69, 507–509. [Google Scholar] [CrossRef] [Green Version]
- Mehana, E.S.; Khafaga, A.F.; El-Blhei, S.S. The role of matrix metalloproteinases in osteoarthritis pathogenesis: An updated review. Life Sci. 2019, 234, 116786. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Cao, L.; Gao, X.; Chen, Z.; He, Z.; Qian, Y.; Yu, Y.; Wang, G. Ghrelin prevents articular cartilage matrix destruction in human chondrocytes. Biomed. Pharm. 2017, 98, 651–655. [Google Scholar] [CrossRef]
- Zhang, L.; Ma, S.; Su, H.; Cheng, J. Isoliquiritigenin Inhibits IL-1β-Induced Production of Matrix Metalloproteinase in Articular Chondrocytes. Mol. Ther. Methods Clin. Dev. 2018, 9, 153–159. [Google Scholar] [CrossRef] [Green Version]
- Nasi, S.; Ea, H.K.; So, A.; Busso, N. Revisiting the role of interleukin-1 pathway in osteoarthritis: Interleukin-1α and-1β, and NLRP3 inflammasome are not involved in the pathological features of the murine menisectomy model of osteoarthritis. Front. Pharmacol. 2017, 8, 282. [Google Scholar] [CrossRef]
- Choi, M.C.; Jo, J.; Park, J.; Kang, H.K.; Park, Y. NF-κB Signaling Pathways in Osteoarthritic Cartilage Destruction. Cells 2019, 8, 734. [Google Scholar] [CrossRef] [Green Version]
- Mitchell, J.P.; Carmony, R.J. NF-κB and the Transcriptional Control of Inflammation. Int. Rev. Cell Mol. Biol. 2018, 335, 41–84. [Google Scholar] [CrossRef]
- Liang, Y.; Chen, G.; Yang, Y.; Li, Z.; Chen, T.; Sun, W.; Yu, M.; Pan, K.; Guo, W.; Tian, W. Effect of canonical NF-κB signaling pathway on the differentiation of rat dental epithelial stem cells. Stem Cell Res. Ther. 2019, 10, 139. [Google Scholar] [CrossRef]
- Saito, T.; Tanaka, S. Molecular mechanisms underlying osteoarthritis development: Notch and NF-κB. Arthritis Res. Ther. 2017, 15, 94. [Google Scholar] [CrossRef]
- Deng, Y.; Lu, J.; Li, W.; Wu, A.; Zhang, X.; Tong, W.; Ho, K.K.; Qin, L.; Song, H.; Mak, K.K. Reciprocal inhibition of YAP/TAZ and NF-κB regulates osteoarthritic cartilage degradation. Nat. Commun. 2018, 1, 4564. [Google Scholar] [CrossRef] [Green Version]
- Giridharan, S.; Srinivasan, M. Mechanisms of NF-κB p65 and strategies for therapeutic manipulation. J. Inflamm. Res. 2018, 11, 407–419. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mulero, M.C.; Huxford, T.; Ghosh, G. NF-κB, IκB, and IKK: Integral Components of Immune System Signaling. Adv. Exp. Med. Biol. 2019, 1172, 207–226. [Google Scholar] [CrossRef] [PubMed]
- Lepetsos, P.; Papavassiliou, K.; Papavassiliou, A. Redox and NF-κB signaling in osteoarthritis. Free Radic. Biol. Med. 2019, 132, 90–100. [Google Scholar] [CrossRef] [PubMed]
- The Role of Inflammation in the Pathogenesis of Osteoarthritis. Mediat. Inflamm. 2020, 2020, 8293921. [CrossRef] [Green Version]
- Lee, H.; Jang, D.; Jeon, J.; Cho, C.; Choi, S.; Han, S.J.; Oh, E.; Nam, J.; Park, C.H.; Shin, Y.Y.; et al. Seomae mugwort and jaceosidin attenuate osteoarthritic cartilage damage by blocking IκB degradation in mice. J. Cell. Mol. Med. 2020, 24, 8126–8137. [Google Scholar] [CrossRef]
- Henrotin, Y.; Mobasheri, A. Natural products for promoting joint health and managing osteoarthritis. Curr. Rheumatol. Rep. 2018, 20, 72. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.H.; Lee, J.E.; Lee, C.J.; Park, J.S. Natural products as sources of novel drug candidates for the pharmacological management of osteoarthritis: A narrative review. Biomol. Ther. 2019, 27, 503–513. [Google Scholar] [CrossRef] [PubMed]
- Bacchetti, T.; Morresi, C.; Bellachioma, L.; Ferretti, G. Antioxidant and Pro-Oxidant Properties of Carthamus tinctorius, Hydroxy Safflor Yellow A, and Safflor Yellow A. Antioxidants 2020, 9, 119. [Google Scholar] [CrossRef] [Green Version]
- Park, C.H.; Lee, A.Y.; Kim, J.H.; Seong, S.H.; Jang, G.Y.; Cho, E.J.; Choi, J.S.; Kwon, J.; Kim, Y.O.; Lee, S.W.; et al. Protective Effect of Safflower Seed on Cisplatin-Induced Renal Damage in Mice via Oxidative Stress and Apoptosis-Mediated Pathways. Am. J. Chin. Med. 2018, 46, 157–174. [Google Scholar] [CrossRef]
- Orgah, J.O.; He, S.; Wang, Y.; Jiang, M.; Wang, Y.; Orgah, E.A.; Duan, Y.; Zhao, B.; Zhang, B.; Han, J.; et al. Pharmacological potential of the combination of Salvia miltiorrhiza (Danshen) and Carthamus tinctorius (Honghua) for diabetes mellitus and its cardiovascular complications. Pharmacol. Res. 2020, 153, 104654. [Google Scholar] [CrossRef]
- Ibrahim, F.Y.; El-Khateeb, A.Y.; Mohamed, A.H. Rhus and Safflower Extracts as Potential Novel Food Antioxidant, Anticancer, and Antimicrobial Agents Using Nanotechnology. Foods 2019, 8, 139. [Google Scholar] [CrossRef] [Green Version]
- Mohamed, D.A.; Fouda, K.A.; Mohamed, R.S. In vitro Anticancer Activity of Quinoa and Safflower Seeds and Their Preventive Effects on Non-alcoholic Fatty Liver. Pak. J. Biol. Sci. 2019, 22, 383–392. [Google Scholar] [CrossRef] [Green Version]
- Wu, H.; Denna, T.H.; Storkersen, J.N.; Gerrients, V.A. Beyond a neurotransmitter: The role of serotonin in inflammation and immunity. Pharmacol. Res. 2019, 140, 100–114. [Google Scholar] [CrossRef]
- Lazari, D.; Alexiou, G.A.; Markopoulos, G.S.; Vartholomatos, E.; Hodaj, E.; Chousidis, I.; Leonardos, I.; Galani, V.; Kyritsis, A.P.; Vartholomatos, E.; et al. N-(p-coumaroyl) serotonin inhibits glioblastoma cells growth through triggering S-phase arrest and apoptosis. J. Neuro-Oncol. 2017, 132, 373–381. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.L.; Tian, K.; Tang, Z.H.; Chen, X.J.; Bian, Z.X.; Wang, Y.T.; Lu, J.J. Phytochemistry and Pharmacology of Carthamus tinctorius L. Am. J. Chin. Med. 2016, 44, 197–226. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Yue, S.; Yang, Z.; Feng, W.; Meng, X.; Wang, A.; Peng, C.; Wang, C.; Yan, D. Oral hydroxysafflor yellow A reduces obesity in mice by modulating the gut microbiota and serum metabolism. Pharm. Res. 2018, 134, 40–50. [Google Scholar] [CrossRef]
- Park, C.H.; Lee, A.Y.; Kim, J.H.; Seong, S.H.; Cho, E.J.; Choi, J.S.; Kim, M.J.; Yang, S.; Yokozawa, T.; Shin, Y.S. Protective effects of serotonin and its derivatives, N-feruloylserotonin and N-(p-coumaroyl) serotonin, against cisplatin-induced renal damage in mice. Am. J. Chin. Med. 2019, 47, 369–383. [Google Scholar] [CrossRef]
- Chen, D.; Zhao, W.; Wang, T.; Han, L.; Hamilton, J.L.; Im, H.J. Osteoarthritis: Toward a comprehensive understanding of pathological mechanism. Bone Res. 2017, 5, 16044. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Zhou, J.; Wu, J.; Chen, Q.; Du, W.; Fu, F.; Yu, H.; Yao, S.; Jin, H.; Tong, P.; et al. Loganin ameliorates cartilage degeneration and osteoarthritis development in an osteoarthritis mouse model through inhibition of NF-κB activity and pyroptosis in chondrocytes. J. Ethnopharmacol. 2020, 247, 112261. [Google Scholar] [CrossRef] [PubMed]
- Cho, C.; Kang, L.J.; Jang, D.; Jeon, J.; Lee, H.; Choi, S.; Han, S.J.; Oh, E.; Nam, J.; Kim, C.S.; et al. Cirsium japonicum var. maackii and apigenin block Hif-2α-induced osteoarthritic cartilage destruction. J. Cell Mol. Med. 2019, 23, 5369–5379. [Google Scholar] [CrossRef]
- Musumeci, G.; Aiello, F.C.; Szychlinska, M.A.; Di Rosa, M.; Castrogiovanni, P.; Mobasheri, A. Osteoarthritis in the XXIst century: Risk factors and behaviours that influence disease onset and progression. Int. J. Mol. Sci. 2015, 16, 6093–6112. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Kim, J.; Ryu, J.H.; Oh, H.; Chun, C.H.; Kim, B.J.; Min, B.H.; Chun, J.S. Hypoxia-inducible factor-2α is a catabolic regulator of osteoarthritic cartilage destruction. Nat. Med. 2010, 16, 687. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Ouyang, H.; Dass, C.R.; Xu, J. Current research on pharmacologic and regenerative therapies for osteoarthritis. Bone Res. 2016, 4, 15040. [Google Scholar] [CrossRef]
- Daily, J.W.; Yang, M.; Park, S. Efficacy of Turmeric Extracts and Curcumin for Alleviating the Symptoms of Joint Arthritis: A Systematic Review and Meta-Analysis of Randomized Clinical Trials. J. Med. Food 2016, 19, 717–729. [Google Scholar] [CrossRef] [Green Version]
- Park, C.H.; Kim, M.J.; Yang, C.Y.; Yokozawa, T.; Shin, Y.S. Safflower seed extract synergizes the therapeutic effect of cisplatin-induced nephrotoxicity in human colorectal carcinoma RKO cells and RKO-transplanted mice. Drug Discov. Ther. 2019, 13, 328–334. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.H.; He, M.T.; Kim, M.J.; Yang, C.Y.; Shin, Y.S.; Yokozawa, T.; Park, C.H.; Cho, E.J. Saffloer (Carthamus tinctorius L.) seed attenuates memory impairment induced by scopolamine in mice via regulation of cholinergic dysfunction and oxidative stress. Food Funct. 2019, 10, 3650–3659. [Google Scholar] [CrossRef]
- Chakradhar, S.; Perkons, I.; Mišina, I.; Sipeniece, E.; Radziejewska-Kubzdela, E.; Grygier, A.; Rudzińska, M.; Patel, K.S.; Radzimirska-Graczyk, M.; Górnaś, P. Profiling of the bioactive components of safflower seeds and seed oil: Cultivated (Carthamus tinctorius L.) vs. wild (Carthamus oxyacantha M. Bieb.). Eur. Food Res. Technol. 2020, 246, 449–459. [Google Scholar] [CrossRef]
- Loffredo, L.; Perri, L.; Nocella, C.; Violi, F. Antioxidant and antiplatelet activity by polyphenol-rich nutrients: Focus on extra virgin olive oil and cocoa. Br. J. Clin. Pharm. 2017, 83, 96–102. [Google Scholar] [CrossRef] [Green Version]
- Serino, A.; Salazar, G. Protective Role of Polyphenols against Vascular Inflammation, Aging and Cardiovascular Disease. Nutrients 2018, 11, 53. [Google Scholar] [CrossRef] [Green Version]
- Jenei-Lanzl, Z.; Meurer, A.; Zaucke, F. Interleukin-1β signaling in osteoarthritis–chondrocytes in focus. Cell. Signal. 2019, 53, 212–223. [Google Scholar] [CrossRef]
- Lv, M.; Zhou, Y.; Polson, S.W.; Wan, L.Q.; Wang, M.; Han, L.; Wang, L.; Lu, X.L. Identification of Chondrocyte Genes and Signaling Pathways in Response to Acute Joint Inflammation. Sci. Rep. 2019, 9, 93. [Google Scholar] [CrossRef]
- Zhang, Y.; He, F.; Chen, Z.; Su, Q.; Yan, M.; Zhang, Q.; Jun, T.; Qian, L.; Han, Y. Melatonin modulates IL-1β-induced extracellular matrix remodeling in human nucleus pulposus cells and attenuates rat intervertebral disc degeneration and inflammation. Aging 2019, 11, 10499–10512. [Google Scholar] [CrossRef]
- Zeng, L.; Rong, X.F.; Li, R.H.; Wu, X.Y. Icariin inhibits MMP-1, MMP-3 and MMP-13 expression through MAPK pathways in IL-1β-stimulated SW1353 chondrosarcoma cells. Mol. Med. Rep. 2017, 15, 2853–2858. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sirikaew, N.; Chomdej, S.; Tangyuenyong, S.; Tangjitjaroen, W.; Somgird, C.; Thitaram, C.; Ongchai, S. Proinflammatory cytokines and lipopolysaccharides up regulate MMP-3 and MMP-13 production in Asian elephant (Elephas maximus) chondrocytes: Attenuation by anti-arthritic agents. BMC Vet. Res. 2019, 15, 419. [Google Scholar] [CrossRef] [Green Version]
- Nam, D.C.; Kim, B.K.; Lee, H.J.; Shin, H.D.; Lee, C.J.; Hwang, S.C. Effects of prunetin on the proteolytic activity, secretion and gene expression of MMP-3 in vitro and production of MMP-3 in vivo. Korean J. Physiol. Pharm. 2016, 20, 221–228. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahmed, A.S.; Gedin, P.; Hugo, A.; Bakalkin, G.; Kanar, A.; Hart, D.A.; Druid, H.; Svensson, C.; Kosek, E. Activation of NF-κB in synovium versus cartilage from patients with advanced knee osteoarthritis: A potential contributor to inflammatory aspects of disease progression. J. Immunol. 2018, 201, 1918–1927. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.C. NF-κB signaling in inflammation. Signal. Transduct. Target. Ther. 2017, 2, 17023. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mitchell, S.; Vargas, J.; Hoffmann, A. Signaling via the NF-κB system. Wiley Interdiscip. Rev. Syst. Biol. Med. 2016, 8, 227–241. [Google Scholar] [CrossRef] [Green Version]
- Meshram, S.N.; Paul, D.; Manne, R.; Choppara, S.; Sankaran, G.; Agrawal, Y.; Santra, M.K. FBXO32 activates NF-κB through IκBα degradation in inflammatory and genotoxic stress. Int. J. Biochem. Cell Biol. 2017, 92, 134–140. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Yin, Z.; Liu, C.; Liang, H.; Jiang, M.; Tian, J. IL-1β promotes ADAMTS enzyme-mediated aggrecan degradation through NF-κB in human intervertebral disc. J. Orthop. Surg. Res. 2015, 10, 159. [Google Scholar] [CrossRef] [Green Version]
- Chen, W.P.; Hu, Z.N.; Jin, L.B.; Wu, L.D. Licochalcone A Inhibits MMPs and ADAMTSs via the NF-κB and Wnt/β-Catenin Signaling Pathways in Rat Chondrocytes. Cell. Physiol. Biochem. 2017, 43, 937–944. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Glasson, S.S.; Blanchet, T.J.; Morris, E.A. The surgical destabilization of the medial meniscus (DMM) model of osteoarthritis in the 129/SvEv mouse. Osteoarthr. Cart. 2007, 15, 1061–1069. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Samvelyan, H.J.; Hughes, D.; Stevens, C.; Staines, K.A. Models of Osteoarthritis: Relevance and New insight. Calcif. Tissue Int. 2020. [Google Scholar] [CrossRef] [PubMed] [Green Version]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, S.J.; Lim, M.J.; Lee, K.M.; Oh, E.; Shin, Y.S.; Kim, S.; Kim, J.S.; Yun, S.P.; Kang, L.-J. Safflower Seed Extract Attenuates the Development of Osteoarthritis by Blocking NF-κB Signaling. Pharmaceuticals 2021, 14, 258. https://doi.org/10.3390/ph14030258
Han SJ, Lim MJ, Lee KM, Oh E, Shin YS, Kim S, Kim JS, Yun SP, Kang L-J. Safflower Seed Extract Attenuates the Development of Osteoarthritis by Blocking NF-κB Signaling. Pharmaceuticals. 2021; 14(3):258. https://doi.org/10.3390/ph14030258
Chicago/Turabian StyleHan, Seong Jae, Min Ju Lim, Kwang Min Lee, Eunjeong Oh, Yu Su Shin, Seokho Kim, Joong Sun Kim, Seung Pil Yun, and Li-Jung Kang. 2021. "Safflower Seed Extract Attenuates the Development of Osteoarthritis by Blocking NF-κB Signaling" Pharmaceuticals 14, no. 3: 258. https://doi.org/10.3390/ph14030258
APA StyleHan, S. J., Lim, M. J., Lee, K. M., Oh, E., Shin, Y. S., Kim, S., Kim, J. S., Yun, S. P., & Kang, L. -J. (2021). Safflower Seed Extract Attenuates the Development of Osteoarthritis by Blocking NF-κB Signaling. Pharmaceuticals, 14(3), 258. https://doi.org/10.3390/ph14030258