Action of Carvacrol on Parascaris sp. and Antagonistic Effect on Nicotinic Acetylcholine Receptors
Abstract
:1. Introduction
2. Results
2.1. Acetylcholine-Induced Contraction of Parascaris sp. Body Muscle Flap Preparation
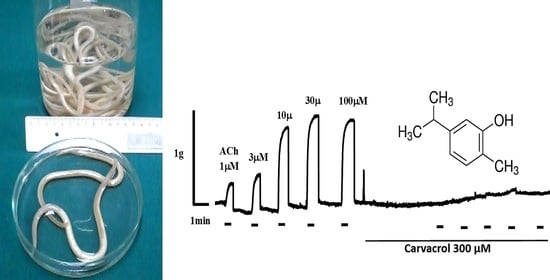
2.2. Carvacrol Abolishes Acetylcholine-Induced Contractions of Parascaris sp. Muscle Strips
2.3. Effect of Carvacrol on the Parascaris sp. Morantel-AChR Expressed in Xenopus oocytes
2.4. Effect of Carvacrol on Parascaris sp. and Ascaris suum Nicotine-Sensitive AChRs Expressed in Xenopus oocytes
3. Discussion
4. Materials and Methods
4.1. Parascaris sp. Muscle Flap Contraction
4.2. Two-Electrode Voltage-Clamp Electrophysiology in Xenopus laevis oocytes
4.3. Drugs
4.4. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nielsen, M.K. Universal challenges for parasite control: A perspective from equine parasitology. Trends Parasitol. 2015, 31, 282–284. [Google Scholar] [CrossRef]
- Reinemeyer, C.R.; Nielsen, M.K. Parasitism and colic. Vet. Clin. N. Am. Equine Pract. 2009, 25, 233–245. [Google Scholar] [CrossRef]
- Salle, G.; Guillot, J.; Tapprest, J.; Foucher, N.; Sevin, C.; Laugier, C. Compilation of 29 years of postmortem examinations identifies major shifts in equine parasite prevalence from 2000 onwards. Int. J. Parasitol. 2020, 50, 125–132. [Google Scholar] [CrossRef]
- Gokbulut, C.; McKellar, Q.A. Anthelmintic drugs used in equine species. Vet. Parasitol. 2018, 261, 27–52. [Google Scholar] [CrossRef]
- von Samson-Himmelstjerna, G. Anthelmintic resistance in equine parasites-detection, potential clinical relevance and implications for control. Vet. Parasitol. 2012, 185, 2–8. [Google Scholar] [CrossRef]
- Matthews, J.B. Anthelmintic resistance in equine nematodes. Int. J. Parasitol. Drugs Drug Resist. 2014, 4, 310–315. [Google Scholar] [CrossRef] [Green Version]
- Nielsen, M.K.; Reinemeyer, C.R.; Donecker, J.M.; Leathwick, D.M.; Marchiondo, A.A.; Kaplan, R.M. Anthelmintic resistance in equine parasites--current evidence and knowledge gaps. Vet. Parasitol. 2014, 204, 55–63. [Google Scholar] [CrossRef]
- Peregrine, A.S.; Molento, M.B.; Kaplan, R.M.; Nielsen, M.K. Anthelmintic resistance in important parasites of horses: Does it really matter? Vet. Parasitol. 2014, 201, 1–8. [Google Scholar] [CrossRef]
- Martin, F.; Hoglund, J.; Bergstrom, T.F.; Karlsson Lindsjo, O.; Tyden, E. Resistance to pyrantel embonate and efficacy of fenbendazole in Parascaris univalens on Swedish stud farms. Vet. Parasitol. 2018, 264, 69–73. [Google Scholar] [CrossRef]
- Raza, A.; Qamar, A.G.; Hayat, K.; Ashraf, S.; Williams, A.R. Anthelmintic resistance and novel control options in equine gastrointestinal nematodes. Parasitology 2019, 146, 425–437. [Google Scholar] [CrossRef]
- Anthony, J.P.; Fyfe, L.; Smith, H. Plant active components-a resource for antiparasitic agents? Trends Parasitol. 2005, 21, 462–468. [Google Scholar] [CrossRef]
- Kaplan, R.M.; Storey, B.E.; Vidyashankar, A.N.; Bissinger, B.W.; Mitchell, S.M.; Howell, S.B.; Mason, M.E.; Lee, M.D.; Pedroso, A.A.; Akashe, A.; et al. Antiparasitic efficacy of a novel plant-based functional food using an Ascaris suum model in pigs. Acta Trop. 2014, 139, 15–22. [Google Scholar] [CrossRef]
- Saha, S.; Lachance, S. Effect of essential oils on cattle gastrointestinal nematodes assessed by egg hatch, larval migration and mortality testing. J. Helminthol. 2019, 94, e111. [Google Scholar] [CrossRef]
- Lopez, V.; Cascella, M.; Benelli, G.; Maggi, F.; Gomez-Rincon, C. Green drugs in the fight against Anisakis simplex-larvicidal activity and acetylcholinesterase inhibition of Origanum compactum essential oil. Parasitl. Res. 2018, 117, 861–867. [Google Scholar] [CrossRef] [Green Version]
- Baser, K.H. Biological and pharmacological activities of carvacrol and carvacrol bearing essential oils. Curr. Pharm. Des. 2008, 14, 3106–3119. [Google Scholar] [CrossRef]
- Friedman, M. Chemistry and multibeneficial bioactivities of carvacrol (4-isopropyl-2-methylphenol), a component of essential oils produced by aromatic plants and spices. J. Agric. Food Chem. 2014, 62, 7652–7670. [Google Scholar] [CrossRef]
- Sakkas, H.; Papadopoulou, C. Antimicrobial Activity of Basil, Oregano, and Thyme Essential Oils. J. Microbiol. Biotechnol. 2017, 27, 429–438. [Google Scholar] [CrossRef] [Green Version]
- Lei, J.; Leser, M.; Enan, E. Nematicidal activity of two monoterpenoids and SER-2 tyramine receptor of Caenorhabditis elegans. Biochem. Pharmacol. 2010, 79, 1062–1071. [Google Scholar] [CrossRef]
- Andre, W.P.; Ribeiro, W.L.; Cavalcante, G.S.; dos Santos, J.M.; Macedo, I.T.; de Paula, H.C.; de Freitas, R.M.; de Morais, S.M.; de Melo, J.V.; Bevilaqua, C.M. Comparative efficacy and toxic effects of carvacryl acetate and carvacrol on sheep gastrointestinal nematodes and mice. Vet. Parasitol. 2016, 218, 52–58. [Google Scholar] [CrossRef] [Green Version]
- Katiki, L.M.; Barbieri, A.M.E.; Araujo, R.C.; Verissimo, C.J.; Louvandini, H.; Ferreira, J.F.S. Synergistic interaction of ten essential oils against Haemonchus contortus in vitro. Vet. Parasitol. 2017, 243, 47–51. [Google Scholar] [CrossRef]
- Hierro, I.; Valero, A.; Perez, P.; Gonzalez, P.; Cabo, M.M.; Montilla, M.P.; Navarro, M.C. Action of different monoterpenic compounds against Anisakis simplex s.l. L3 larvae. Phytomedicine 2004, 11, 77–82. [Google Scholar] [CrossRef]
- Andres, M.F.; Gonzalez-Coloma, A.; Sanz, J.; Burillo, J.; Sainz, P. Nematicidal activity of essential oils: A review. Phytochem. Rev. 2012, 11, 371–390. [Google Scholar] [CrossRef] [Green Version]
- Abdel-Rahman, F.H.; Alaniz, N.M.; Saleh, M.A. Nematicidal activity of terpenoids. J. Environ. Sci. Health B 2013, 48, 16–22. [Google Scholar] [CrossRef]
- Marjanovic, S.D.; Bogunovic, D.; Milovanovic, M.; Marinkovic, D.; Zdravkovic, N.; Magas, V.; Trailovic, M.S. Antihelminic Activity of Carvacrol, Thymol, Cinnamaldehyde and P-Cymen against the Free-Living Nematode Caenorhabditis Elegans and Rat Pinworm Syphacia Muris. Acta Vet. Beogr. 2018, 68, 445–456. [Google Scholar] [CrossRef] [Green Version]
- Hernando, G.; Turani, O.; Bouzat, C. Caenorhabditis elegans muscle Cys-loop receptors as novel targets of terpenoids with potential anthelmintic activity. PLoS Negl. Trop. Dis. 2019, 13, e0007895. [Google Scholar] [CrossRef]
- Trailovic, S.M.; Marjanovic, D.S.; Nedeljkovic Trailovic, J.; Robertson, A.P.; Martin, R.J. Interaction of carvacrol with the Ascaris suum nicotinic acetylcholine receptors and gamma-aminobutyric acid receptors, potential mechanism of antinematodal action. Parasitol. Res. 2015, 114, 3059–3068. [Google Scholar] [CrossRef] [Green Version]
- Choudhary, S.; Marjianovic, D.S.; Wong, C.R.; Zhang, X.; Abongwa, M.; Coats, J.R.; Trailovic, S.M.; Martin, R.J.; Robertson, A.P. Menthol acts as a positive allosteric modulator on nematode levamisole sensitive nicotinic acetylcholine receptors. Int. J. Parasitol. Drugs Drug Resist. 2019, 9, 44–53. [Google Scholar] [CrossRef]
- Marjanovic, D.S.; Zdravkovic, N.; Milovanovic, M.; Trailovic, J.N.; Robertson, A.P.; Todorovic, Z.; Trailovic, S.M. Carvacrol acts as a potent selective antagonist of different types of nicotinic acetylcholine receptors and enhances the effect of monepantel in the parasitic nematode Ascaris suum. Vet. Parasitol. 2020, 278, 109031. [Google Scholar] [CrossRef]
- Courtot, E.; Charvet, C.L.; Beech, R.N.; Harmache, A.; Wolstenholme, A.J.; Holden-Dye, L.; O’Connor, V.; Peineau, N.; Woods, D.J.; Neveu, C. Functional Characterization of a Novel Class of Morantel-Sensitive Acetylcholine Receptors in Nematodes. PLoS Pathog. 2015, 11, e1005267. [Google Scholar] [CrossRef] [Green Version]
- Blanchard, A.; Guegnard, F.; Charvet, C.L.; Crisford, A.; Courtot, E.; Sauve, C.; Harmache, A.; Duguet, T.; O’Connor, V.; Castagnone-Sereno, P.; et al. Deciphering the molecular determinants of cholinergic anthelmintic sensitivity in nematodes: When novel functional validation approaches highlight major differences between the model Caenorhabditis elegans and parasitic species. PLoS Pathog. 2018, 14, e1006996. [Google Scholar] [CrossRef]
- Beech, R.N.; Neveu, C. The evolution of pentameric ligand-gated ion-channels and the changing family of anthelmintic drug targets. Parasitology 2015, 142, 303–317. [Google Scholar] [CrossRef]
- Richmond, J.E.; Jorgensen, E.M. One GABA and two acetylcholine receptors function at the C. elegans neuromuscular junction. Nat. Neurosci. 1999, 2, 791–797. [Google Scholar] [CrossRef]
- Raymond, V.; Mongan, N.P.; Sattelle, D.B. Anthelmintic actions on homomer-forming nicotinic acetylcholine receptor subunits: Chicken alpha7 and ACR-16 from the nematode Caenorhabditis elegans. Neuroscience 2000, 101, 785–791. [Google Scholar] [CrossRef]
- Boulin, T.; Fauvin, A.; Charvet, C.L.; Cortet, J.; Cabaret, J.; Bessereau, J.L.; Neveu, C. Functional reconstitution of Haemonchus contortus acetylcholine receptors in Xenopus oocytes provides mechanistic insights into levamisole resistance. Br. J. Pharmacol. 2011, 164, 1421–1432. [Google Scholar] [CrossRef] [Green Version]
- Abongwa, M.; Buxton, S.K.; Courtot, E.; Charvet, C.L.; Neveu, C.; McCoy, C.J.; Verma, S.; Robertson, A.P.; Martin, R.J. Pharmacological profile of Ascaris suum ACR-16, a new homomeric nicotinic acetylcholine receptor widely distributed in Ascaris tissues. Br. J. Pharmacol. 2016, 173, 2463–2477. [Google Scholar] [CrossRef] [Green Version]
- Charvet, C.L.; Guegnard, F.; Courtot, E.; Cortet, J.; Neveu, C. Nicotine-sensitive acetylcholine receptors are relevant pharmacological targets for the control of multidrug resistant parasitic nematodes. Int. J. Parasitol. Drugs Drug Resist. 2018, 8, 540–549. [Google Scholar] [CrossRef]
- Trailovic, S.M.; Zurovac, Z.; Gruborovic, S.; Marjanovic, D.S.; Nedeljkovic-Trailovic, J. Presynaptic and postsynaptic regulation of muscle contractions in the ascarid nematode Ascaris suum: A target for drug action. J. Helminthol. 2016, 90, 698–705. [Google Scholar] [CrossRef]
- Zheng, F.; Robertson, A.P.; Abongwa, M.; Yu, E.W.; Martin, R.J. The Ascaris suum nicotinic receptor, ACR-16, as a drug target: Four novel negative allosteric modulators from virtual screening. Int. J. Parasitol. Drugs Drug Resist. 2016, 6, 60–73. [Google Scholar] [CrossRef] [Green Version]
- Robertson, A.P.; Clark, C.L.; Burns, T.A.; Thompson, D.P.; Geary, T.G.; Trailovic, S.M.; Martin, R.J. Paraherquamide and 2-deoxy-paraherquamide distinguish cholinergic receptor subtypes in Ascaris muscle. J. Pharmacol. Exp. Ther. 2002, 302, 853–860. [Google Scholar] [CrossRef] [Green Version]
- Sangster, N.C.; Davis, C.W.; Collins, G.H. Effects of cholinergic drugs on longitudinal contraction in levamisole-susceptible and -resistant Haemonchus contortus. Int. J. Parasitol. 1991, 21, 689–695. [Google Scholar] [CrossRef]
- Kopp, S.R.; Coleman, G.T.; McCarthy, J.S.; Kotze, A.C. Phenotypic characterization of two Ancylostoma caninum isolates with different susceptibilities to the anthelmintic pyrantel. Antimicrob. Agents Chemother. 2008, 52, 3980–3986. [Google Scholar] [CrossRef] [Green Version]
- Martin, R.J.; Robertson, A.P.; Buxton, S.K.; Beech, R.N.; Charvet, C.L.; Neveu, C. Levamisole receptors: A second awakening. Trends Parasitol. 2012, 28, 289–296. [Google Scholar] [CrossRef] [Green Version]
- Robertson, A.P.; Bjorn, H.E.; Martin, R.J. Resistance to levamisole resolved at the single-channel level. FASEB J. 1999, 13, 749–760. [Google Scholar] [CrossRef]
- Qian, H.; Martin, R.J.; Robertson, A.P. Pharmacology of N-, L-, and B-subtypes of nematode nAChR resolved at the single-channel level in Ascaris suum. FASEB J. 2006, 20, 2606–2608. [Google Scholar] [CrossRef] [Green Version]
- Qian, H.; Robertson, A.P.; Powell-Coffman, J.A.; Martin, R.J. Levamisole resistance resolved at the single-channel level in Caenorhabditis elegans. FASEB J. 2008, 22, 3247–3254. [Google Scholar] [CrossRef] [Green Version]
- Garcia-Bustos, J.F.; Sleebs, B.E.; Gasser, R.B. An appraisal of natural products active against parasitic nematodes of animals. Parasit. Vectors 2019, 12, 306. [Google Scholar] [CrossRef] [Green Version]
- Miro, M.V.; CR, E.S.; Viviani, P.; Luque, S.; Lloberas, M.; Costa-Junior, L.M.; Lanusse, C.; Virkel, G.; Lifschitz, A. Combination of bioactive phytochemicals and synthetic anthelmintics: In vivo and in vitro assessment of the albendazole-thymol association. Vet. Parasitol. 2020, 281, 109121. [Google Scholar] [CrossRef]
- Minsakorn, S.; Watthanadirek, A.; Poolsawat, N.; Puttarak, P.; Chawengkirttikul, R.; Anuracpreeda, P. The anthelmintic potentials of medicinal plant extracts and an isolated compound (rutin, C27H30O16) from Terminalia catappa L. against Gastrothylax crumenifer. Vet. Parasitol. 2021, 291, 109385. [Google Scholar] [CrossRef]
- Silva, C.R.; Lifschitz, A.L.; Macedo, S.R.D.; Campos, N.; Viana-Filho, M.; Alcantara, A.C.S.; Araujo, J.G.; Alencar, L.M.R.; Costa-Junior, L.M. Combination of synthetic anthelmintics and monoterpenes: Assessment of efficacy, and ultrastructural and biophysical properties of Haemonchus contortus using atomic force microscopy. Vet. Parasitol. 2021, 290, 109345. [Google Scholar] [CrossRef]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trailovic, S.M.; Rajkovic, M.; Marjanovic, D.S.; Neveu, C.; Charvet, C.L. Action of Carvacrol on Parascaris sp. and Antagonistic Effect on Nicotinic Acetylcholine Receptors. Pharmaceuticals 2021, 14, 505. https://doi.org/10.3390/ph14060505
Trailovic SM, Rajkovic M, Marjanovic DS, Neveu C, Charvet CL. Action of Carvacrol on Parascaris sp. and Antagonistic Effect on Nicotinic Acetylcholine Receptors. Pharmaceuticals. 2021; 14(6):505. https://doi.org/10.3390/ph14060505
Chicago/Turabian StyleTrailovic, Sasa M., Milan Rajkovic, Djordje S. Marjanovic, Cédric Neveu, and Claude L. Charvet. 2021. "Action of Carvacrol on Parascaris sp. and Antagonistic Effect on Nicotinic Acetylcholine Receptors" Pharmaceuticals 14, no. 6: 505. https://doi.org/10.3390/ph14060505
APA StyleTrailovic, S. M., Rajkovic, M., Marjanovic, D. S., Neveu, C., & Charvet, C. L. (2021). Action of Carvacrol on Parascaris sp. and Antagonistic Effect on Nicotinic Acetylcholine Receptors. Pharmaceuticals, 14(6), 505. https://doi.org/10.3390/ph14060505