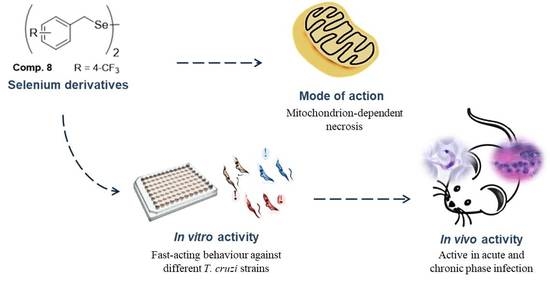
Library of Selenocyanate and Diselenide Derivatives as In Vivo Antichagasic Compounds Targeting Trypanosoma cruzi Mitochondrion
Abstract
:1. Introduction
2. Results and Discussion
2.1. In Vitro Biological Evaluation
2.2. In Vivo Biological Evaluation
2.3. Mode of Action Studies
3. Materials and Methods
3.1. Chemistry
3.2. In Vitro Activity Assays
3.2.1. Screening against Extracellular Epimastigotes
3.2.2. Cytotoxicity Test
3.2.3. Screening against Intracellular Amastigotes and Infected Cells
3.2.4. Screening against Bloodstream Trypomastigotes
3.3. In Vivo Activity Assays on BALB/c Mice
3.3.1. Ethics Statement
3.3.2. Infection and Treatment
3.3.3. Screening Assays on Mice
3.4. Mode of Action Studies
3.4.1. H Nuclear Magnetic Resonance (NMR) Analysis of Excreted Metabolites
3.4.2. Flow Cytometry Analysis of Mitochondrial Membrane Potential and Nucleic Acid Levels
3.4.3. SOD Enzymatic Inhibition Analysis
3.5. Statistical Analyses
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bern, C. Chagas’ disease. N. Engl. J. Med. 2015, 373, 456–466. [Google Scholar] [CrossRef]
- Hashimoto, K.; Yoshioka, K. Review: Surveillance of Chagas Disease. Adv. Parasitol. 2012, 79, 375–428. [Google Scholar]
- Moncayo, A.; Silveira, A.C. Current Epidemiological Trends of Chagas Disease in Latin America and Future Challenges: Epidemiology, Surveillance, And Health Policies, 2nd ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2017; ISBN 9780128010297. [Google Scholar]
- Hernández, C.; Vera, M.J.; Cucunubá, Z.; Flórez, C.; Cantillo, O.; Buitrago, L.S.; González, M.S.; Ardila, S.; Dueñas, L.Z.; Tovar, R.; et al. High-Resolution Molecular Typing of Trypanosoma cruzi in 2 Large Outbreaks of Acute Chagas Disease in Colombia. J. Infect. Dis. 2016, 214, 1252–1255. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kessler, R.L.; Contreras, V.T.; Marliére, N.P.; Aparecida Guarneri, A.; Villamizar Silva, L.H.; Mazzarotto, G.A.C.A.; Batista, M.; Soccol, V.T.; Krieger, M.A.; Probst, C.M. Recently differentiated epimastigotes from Trypanosoma cruzi are infective to the mammalian host. Mol. Microbiol. 2017, 104, 712–736. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tyler, K.M.; Engman, D.M. The life cycle of Trypanosoma cruzi revisited. Int. J. Parasitol. 2001, 31, 472–481. [Google Scholar] [CrossRef]
- Tarleton, R.L. CD8+ T Cells in Trypanosoma cruzi Infection. Semin. Immunopathol. 2015, 37, 233–238. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cardillo, F.; De Pinho, R.T.; Antas, P.R.Z.; Mengel, J. Immunity and immune modulation in Trypanosoma cruzi infection. Pathog. Dis. 2015, 73, ftv082. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cunha-Neto, E.; Chevillard, C. Chagas Disease Cardiomyopathy: Immunopathology and Genetics. Mediators Inflamm. 2014, 2014, 683230. [Google Scholar] [CrossRef] [Green Version]
- Ribeiro, A.L.; Nunes, M.P.; Teixeira, M.M.; Rocha, M.O.C. Diagnosis and management of Chagas disease and cardiomyopathy. Nat. Rev. Cardiol. 2012, 9, 576–589. [Google Scholar] [CrossRef] [PubMed]
- Morillo, C.A.; Waskin, H.; Sosa-Estani, S.; del Carmen Bangher, M.; Cuneo, C.; Milesi, R.; Mallagray, M.; Apt, W.; Beloscar, J.; Gascon, J.; et al. Benznidazole and Posaconazole in Eliminating Parasites in Asymptomatic T. Cruzi Carriers: The STOP-CHAGAS Trial. J. Am. Coll. Cardiol. 2017, 69, 939–947. [Google Scholar] [CrossRef]
- Cruz-Chan, J.V.; Villanueva-Lizama, L.E.; Versteeg, L.; Damania, A.; Villar, M.J.; González-López, C.; Keegan, B.; Pollet, J.; Gusovsky, F.; Hotez, P.J.; et al. Vaccine-linked chemotherapy induces IL-17 production and reduces cardiac pathology during acute Trypanosoma cruzi infection. Sci. Rep. 2021, 11, 3222. [Google Scholar] [CrossRef]
- Quijano-Hernandez, I.; Dumonteil, E. Advances and challenges towards a vaccine against Chagas disease. Hum. Vaccines 2011, 7, 1184–1191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garg, N.; Bhatia, V. Current status and future prospects for a vaccine against American trypanosomiasis. Expert Rev. Vaccines 2005, 4, 867–880. [Google Scholar] [CrossRef]
- Martín-Escolano, J.; Medina-Carmona, E.; Martín-Escolano, R. Chagas disease: Current view of an ancient and global chemotherapy challenge. ACS Infect. Dis. 2020, 6, 2830–2843. [Google Scholar] [CrossRef] [PubMed]
- Gaspar, L.; Moraes, C.B.; Freitas-Junior, L.; Ferrari, S.; Costantino, L.; Costi, M.; Coron, R.; Smith, T.; Siqueira-Neto, J.; McKerrow, J.; et al. Current and Future Chemotherapy for Chagas Disease. Curr. Med. Chem. 2015, 22, 4293–4312. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Molina, I.; Goḿez, I.; Prat, J.; Salvador, F.; Treviño, B.; Sulleiro, E.; Serre, N.; Pou, D.; Roure, S.; Cabezos, J.; et al. Randomized trial of posaconazole and benznidazole for chronic Chagas’ disease. N. Engl. J. Med. 2014, 370, 1899–1908. [Google Scholar] [CrossRef]
- Morillo, C.A.; Marin-Neto, J.A.; Avezum, A.; Sosa-Estani, S.; Rassi, A.; Rosas, F.; Villena, E.; Quiroz, R.; Bonilla, R.; Britto, C.; et al. Randomized trial of benznidazole for chronic chagas’ cardiomyopathy. N. Engl. J. Med. 2015, 373, 1295–1306. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aldasoro, E.; Posada, E.; Requena-Méndez, A.; Calvo-Cano, A.; Serret, N.; Casellas, A.; Sanz, S.; Soy, D.; Pinazo, J.; Gascon, J. What to expect and when: Benznidazole toxicity in chronic Chagas’ disease treatment. J. Antimicrob. Chemother. 2018, 73, 1060–1067. [Google Scholar] [CrossRef] [Green Version]
- Wilkinson, S.R.; Taylor, M.C.; Horn, D.; Kelly, J.M.; Cheeseman, I. A mechanism for cross-resistance to nifurtimox and benznidazole in trypanosomes. Proc. Natl. Acad. Sci. USA 2008, 105, 5022–5027. [Google Scholar] [CrossRef] [Green Version]
- Mejia, A.M.; Hall, B.S.; Taylor, M.C.; Gómez-Palacio, A.; Wilkinson, S.R.; Triana-Chávez, O.; Kelly, J.M. Benznidazole-resistance in trypanosoma cruzi is a readily acquired trait that can arise independently in a single population. J. Infect. Dis. 2012, 206, 220–228. [Google Scholar] [CrossRef] [Green Version]
- Villarreal, D.; Barnabé, C.; Sereno, D.; Tibayrenc, M. Lack of correlation between in vitro susceptibility to Benznidazole and phylogenetic diversity of Trypanosoma cruzi, the agent of Chagas disease. Exp. Parasitol. 2004, 108, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Baquedano, Y.; Moreno, E.; Espuelas, S.; Nguewa, P.; Font, M.; Gutierrez, K.J.; Jiménez-Ruiz, A.; Palop, J.A.; Sanmartín, C. Novel hybrid selenosulfonamides as potent antileishmanial agents. Eur. J. Med. Chem. 2014, 74, 116–123. [Google Scholar] [CrossRef] [PubMed]
- Baquedano, Y.; Alcolea, V.; Toro, M.Á.; Gutiérrez, K.J.; Nguewa, P.; Font, M.; Moreno, E.; Espuelas, S.; Jiménez-Ruiz, A.; Palop, J.A.; et al. Novel heteroaryl selenocyanates and diselenides as potent antileishmanial agents. Antimicrob. Agents Chemother. 2016, 60, 3802–3812. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martín-Montes, Á.; Plano, D.; Martín-Escolano, R.; Alcolea, V.; Díaz, M.; Pérez-Silanes, S.; Espuelas, S.; Moreno, E.; Marín, C.; Gutiérrez-Sánchez, R.; et al. Library of seleno-compounds as novel agents against Leishmania species. Antimicrob. Agents Chemother. 2017, 61, e02546-16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scarim, C.B.; Chelucci, R.C.; dos Santos, J.L.; Chin, C.M. The use of Sulfonamide Derivatives in the Treatment of Trypanosomatid Parasites including Trypanosoma cruzi, Trypanosoma brucei, and Leishmania ssp. Med. Chem. 2019, 16, 24–38. [Google Scholar] [CrossRef] [PubMed]
- Alcolea, V.; Pérez-Silanes, S. Selenium as an interesting option for the treatment of Chagas disease: A review. Eur. J. Med. Chem. 2020, 206, 112673. [Google Scholar] [CrossRef]
- Stolwijk, J.M.; Garje, R.; Sieren, J.C.; Buettner, G.R.; Zakharia, Y. Understanding the redox biology of selenium in the search of targeted cancer therapies. Antioxidants 2020, 9, 420. [Google Scholar] [CrossRef] [PubMed]
- Iman, M.; Kaboutaraki, H.; Jafari, R.; Hosseini, S.; Moghimi, A.; Khamesipour, A.; Harchegani, A.; Davood, A. Molecular Dynamics Simulation and Docking Studies of Selenocyanate Derivatives as Anti-Leishmanial Agents. Comb. Chem. High. Throughput Screen. 2016, 19, 847–854. [Google Scholar] [CrossRef] [PubMed]
- Romanha, A.J.; De Castro, S.L.; De Nazaré, M.; Soeiro, C.; Lannes-vieira, J.; Ribeiro, I.; Talvani, A.; Bourdin, B.; Blum, B.; Olivieri, B.; et al. In vitro and in vivo experimental models for drug screening and development for Chagas disease. Mem. Inst. Oswaldo Cruz 2010, 105, 233–238. [Google Scholar] [CrossRef]
- Nwaka, S.; Besson, D.; Ramirez, B.; Maes, L.; Matheeussen, A.; Bickle, Q.; Mansour, N.R.; Yousif, F.; Townson, S.; Gokool, S.; et al. Integrated dataset of screening hits against multiple neglected disease pathogens. PLoS Negl. Trop. Dis. 2011, 5. [Google Scholar] [CrossRef] [Green Version]
- Don, R.; Ioset, J.R. Screening strategies to identify new chemical diversity for drug development to treat kinetoplastid infections. Parasitology 2014, 141, 140–146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Katsuno, K.; Burrows, J.N.; Duncan, K.; Van Huijsduijnen, R.H.; Kaneko, T.; Kita, K.; Mowbray, C.E.; Schmatz, D.; Warner, P.; Slingsby, B.T. Hit and lead criteria in drug discovery for infectious diseases of the developing world. Nat. Rev. Drug Discov. 2015, 14, 751–758. [Google Scholar] [CrossRef] [PubMed]
- Chatelain, E. Chagas disease drug discovery: Toward a new era. J. Biomol. Screen. 2015, 20, 22–35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Rycker, M.; O’Neill, S.; Joshi, D.; Campbell, L.; Gray, D.W.; Fairlamb, A.H. A Static-Cidal Assay for Trypanosoma brucei to Aid Hit Prioritisation for Progression into Drug Discovery Programmes. PLoS Negl. Trop. Dis. 2012, 6, e1932. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chatelain, E.; Konar, N. Translational challenges of animal models in chagas disease drug development: A review. Drug Des. Dev. Ther. 2015, 9, 4807–4823. [Google Scholar] [CrossRef] [Green Version]
- DNDi Target Product Profile for Chagas Disease. Available online: https://www.dndi.org/diseases-projects/chagas/chagas-target-product-profile/ (accessed on 10 January 2021).
- Francisco, A.F.; Jayawardhana, S.; Lewis, M.D.; White, K.L.; Shackleford, D.M.; Chen, G.; Saunders, J.; Osuna-Cabello, M.; Read, K.D.; Charman, S.A.; et al. Nitroheterocyclic drugs cure experimental Trypanosoma cruzi infections more effectively in the chronic stage than in the acute stage. Sci. Rep. 2016, 6, 35351. [Google Scholar] [CrossRef] [Green Version]
- Santos, D.M.; Martins, T.A.F.; Caldas, I.S.; Diniz, L.F.; Machado-Coelho, G.L.L.; Carneiro, C.M.; de P. Oliveira, R.; Talvani, A.; Lana, M.; Bahia, M.T. Benznidazole alters the pattern of Cyclophosphamide-induced reactivation in experimental Trypanosoma cruzi-dependent lineage infection. Acta Trop. 2010, 113, 134–138. [Google Scholar] [CrossRef]
- Martín-Escolano, R.; Cebrián, R.; Maqueda, M.; Romero, D.; Rosales, M.J.; Sánchez-Moreno, M.; Marín, C. Assessing the effectiveness of AS-48 in experimental mice models of Chagas’ disease. J. Antimicrob. Chemother. 2020, 75, 1537–1545. [Google Scholar] [CrossRef]
- Bustamante, J.M.; Craft, J.M.; Crowe, B.D.; Ketchie, S.A.; Tarleton, R.L. New, combined, and reduced dosing treatment protocols cure trypanosoma cruzi infection in mice. J. Infect. Dis. 2014, 209, 150–162. [Google Scholar] [CrossRef]
- Martín-Escolano, R.; Molina-Carreño, D.; Delgado-Pinar, E.; Martin-Montes, Á.; Clares, M.P.; Medina-Carmona, E.; Pitarch-Jarque, J.; Martín-Escolano, J.; Rosales, M.J.; García-España, E.; et al. New polyamine drugs as more effective antichagas agents than benznidazole in both the acute and chronic phases. Eur. J. Med. Chem. 2019, 164, 27–46. [Google Scholar] [CrossRef]
- Kayama, H.; Takeda, K. The Innate Immune Response to Trypanosoma Cruzi Infection. Microbes Infect. 2010, 12, 511–517. [Google Scholar] [CrossRef] [PubMed]
- Bringaud, F.; Rivière, L.; Coustou, V. Energy metabolism of trypanosomatids: Adaptation to available carbon sources. Mol. Biochem. Parasitol. 2006, 149, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Mauregi, D.A.; Cannata, J.J.B.; Cazzulo, J.-J. Glucose Metabolism in Trypanosoma Cruzi. Essays Biochem. 2011, 51, 15–30. [Google Scholar]
- De Deken, R.H. The Crabtree effect: A regulatory system in yeast. J. Gen. Microbiol. 1966, 44, 149–156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kirkinezos, I.G.; Moraes, C.T. Reactive oxygen species and mitochondrial diseases. Semin. Cell Dev. Biol. 2001, 12, 449–457. [Google Scholar] [CrossRef]
- Abengózar, M.Á.; Cebrián, R.; Saugar, J.M.; Gárate, T.; Valdivia, E.; Martínez-Bueno, M.; Maqueda, M.; Rivas, L. Enterocin AS-48 as evidence for the use of bacteriocins as new leishmanicidal agents. Antimicrob. Agents Chemother. 2017, 61, e02288-16. [Google Scholar] [CrossRef] [Green Version]
- Hall, B.S.; Wilkinson, S.R. Activation of benznidazole by trypanosomal type I nitroreductases results in glyoxal formation. Antimicrob. Agents Chemother. 2012, 56, 115–123. [Google Scholar] [CrossRef] [Green Version]
- Lee, W.K.; Thévenod, F. A role for mitochondrial aquaporins in cellular life-and-death decisions? Am. J. Physiol. Cell Physiol. 2006, 291, C195–C202. [Google Scholar] [CrossRef]
- Verma, N.K.; Singh, G.; Dey, C.S. Miltefosine induces apoptosis in arsenite-resistant Leishmania donovani promastigotes through mitochondrial dysfunction. Exp. Parasitol. 2007, 116, 1–13. [Google Scholar] [CrossRef]
- Michels, P.A.M.; Bringaud, F.; Herman, M.; Hannaert, V. Metabolic functions of glycosomes in trypanosomatids. Biochim. Biophys. Acta Mol. Cell Res. 2006, 1763, 1463–1477. [Google Scholar] [CrossRef]
- Beltran-Hortelano, I.; Perez-Silanes, S.; Galiano, S. Trypanothione Reductase and Superoxide Dismutase as Current Drug Targets for Trypanosoma cruzi: An Overview of Compounds with Activity against Chagas Disease. Curr. Med. Chem. 2017, 24, 1066–1138. [Google Scholar] [CrossRef]
- Maes, L.; Vanden Berghe, D.; Germonprez, N.; Quirijnen, L.; Cos, P.; De Kimpe, N.; Van Puyvelde, L. In Vitro and In Vivo Activities of a Triterpenoid Saponin Extract (PX-6518) from the Plant Maesa balansae against Visceral Leishmania Species. Antimicrob. Agents Chemother. 2004, 48, 130–136. [Google Scholar] [CrossRef] [Green Version]
- Kendall, G.; Wilderspin, A.F.; Ashall, F.; Miles, M.A.; Kelly, J.M. Trypanosoma cruzi glycosomal glyceraldehyde-3-phosphate dehydrogenase does not conform to the ‘hotspot’ topogenic signal model. EMBO J. 1990, 9, 2751–2758. [Google Scholar] [CrossRef] [PubMed]
- Martín-Escolano, R.; Moreno-Viguri, E.; Santivanez-Veliz, M.; Martin-Montes, A.; Medina-Carmona, E.; Paucar, R.; Marín, C.; Azqueta, A.; Cirauqui, N.; Pey, A.L.; et al. Second Generation of Mannich Base-Type Derivatives with in Vivo Activity against Trypanosoma cruzi. J. Med. Chem. 2018, 61, 5643–5663. [Google Scholar] [CrossRef] [PubMed]
- Pless-Petig, G.; Metzenmacher, M.; Türk, T.R.; Rauen, U. Aggravation of cold-induced injury in Vero-B4 cells by RPMI 1640 medium—Identification of the responsible medium components. BMC Biotechnol. 2012, 12, 73. [Google Scholar] [CrossRef] [Green Version]
- Francisco, A.F.; Lewis, M.D.; Jayawardhana, S.; Taylor, M.C.; Chatelain, E.; Kelly, J.M. Limited ability of posaconazole to cure both acute and chronic Trypanosoma cruzi infections revealed by highly sensitive in vivo imaging. Antimicrob. Agents Chemother. 2015, 59, 4653–4661. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ye, X.; Ding, J.; Zhou, X.; Chen, G.; Liu, S.F. Divergent roles of endothelial NF-κB in multiple organ injury and bacterial clearance in mouse models of sepsis. J. Exp. Med. 2008, 205, 1303–1315. [Google Scholar] [CrossRef] [Green Version]
- Paucar, R.; Martín-Escolano, R.; Moreno-Viguri, E.; Azqueta, A.; Cirauqui, N.; Marín, C.; Sánchez-Moreno, M.; Pérez-Silanes, S. Rational modification of Mannich base-type derivatives as novel antichagasic compounds: Synthesis, in vitro and in vivo evaluation. Bioorg. Med. Chem. 2019, 27, 3902–3917. [Google Scholar] [CrossRef]
- Fernandez-Becerra, C.; Sanchez-Moreno, M.; Osuna, A.; Opperdoes, F.R. Comparative Aspects of Energy Metabolism in Plant Trypanosomatids. J. Eukaryot. Microbiol. 1997, 44, 523–529. [Google Scholar] [CrossRef]
- Martín-Escolano, R.; Aguilera-Venegas, B.; Marín, C.; Martín-Montes, Á.; Martín-Escolano, J.; Medina-Carmona, E.; Arán, V.J.; Sánchez-Moreno, M. Synthesis and Biological in vitro and in vivo Evaluation of 2-(5-Nitroindazol-1-yl)ethylamines and Related Compounds as Potential Therapeutic Alternatives for Chagas Disease. ChemMedChem 2018, 13, 2104–2118. [Google Scholar] [CrossRef]
- Sandes, J.M.; Fontes, A.; Regis-da-Silva, C.G.; Brelaz De Castro, M.C.A.; Lima-Junior, C.G.; Silva, F.P.L.; Vasconcellos, M.L.A.A.; Figueiredo, R.C.B.Q. Trypanosoma cruzi Cell Death Induced by the Morita-Baylis-Hillman Adduct 3-Hydroxy-2-Methylene-3-(4-Nitrophenylpropanenitrile). PLoS ONE 2014, 9, e93936. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- López-Céspedes, Á.; Villagrán, E.; Briceño Álvarez, K.; De Diego, J.A.; Hernández-Montiel, H.L.; Saldaña, C.; Snchez-Moreno, M.; Marín, C. Trypanosoma cruzi: Seroprevalence Detection in Suburban Population of Santiago de Querétaro (Mexico). Sci. World J. 2012, 2012, 914129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beyer, W.F.; Fridovich, I. Assaying for superoxide dismutase activity: Some large consequences of minor changes in conditions. Anal. Biochem. 1987, 161, 559–566. [Google Scholar] [CrossRef]





| Comp | Activity IC50 (µM) a | Toxicity IC50 (µm) b Vero Cell | Selectivity Index c | ||||
|---|---|---|---|---|---|---|---|
| Epimast. | Amast. | Trypomast. | Epimast. | Amast. | Trypomast. | ||
| BZN | 16.9 ± 1.8 | 8.3 ± 0.7 | 12.4 ± 1.1 | 80.4 ± 7.1 | 5 | 10 | 7 |
| 8 | 1.9 ± 0.1 | 0.8 ± 0.2 | 1.5 ± 0.3 | 44.3 ± 2.7 | 23 (5) | 55 (6) | 30 (4) |
| 10 | 1.8 ± 0.2 | 1.2 ± 0.2 | 1.7 ± 0.3 | 134.6 ± 6.3 | 75 (15) | 112 (11) | 79 (11) |
| 11 | 3.0 ± 0.4 | 3.1 ± 0.1 | 4.5 ± 0.6 | 61.3 ± 3.7 | 20 (4) | 20 (2) | 14 (2) |
| 15 | 0.9 ± 0.1 | 0.5 ± 0.0 | 1.3 ± 0.3 | 17.9 ± 1.0 | 20 (4) | 36 (4) | 14 (2) |
| 20 | 1.3 ± 0.2 | 1.0 ± 0.1 | 0.9 ± 0.0 | 16.4 ± 0.5 | 13 (3) | 16 (2) | 18 (3) |
| 21 | 1.0 ± 0.2 | 0.8 ± 0.1 | 0.9 ±0.1 | 21.7 ± 1.8 | 22 (4) | 27 (3) | 24 (3) |
| 43 | 5.1 ± 0.6 | 4.2 ± 0.7 | 3.7 ± 0.2 | 66.9 ± 6.2 | 13 (3) | 16 (2) | 18 (3) |
| 46 | 2.7 ± 0.8 | 2.3 ± 0.1 | 2.0 ± 0.3 | 48.6 ± 3.5 | 18 (4) | 21 (2) | 24 (3) |
| 47 | 3.6 ± 0.3 | 3.0 ± 0.4 | 3.8 ± 0.4 | 44.7 ± 3.0 | 12 (2) | 15 (2) | 12 (2) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martín-Escolano, R.; Molina-Carreño, D.; Plano, D.; Espuelas, S.; Rosales, M.J.; Moreno, E.; Aydillo, C.; Sanmartín, C.; Sánchez-Moreno, M.; Marín, C. Library of Selenocyanate and Diselenide Derivatives as In Vivo Antichagasic Compounds Targeting Trypanosoma cruzi Mitochondrion. Pharmaceuticals 2021, 14, 419. https://doi.org/10.3390/ph14050419
Martín-Escolano R, Molina-Carreño D, Plano D, Espuelas S, Rosales MJ, Moreno E, Aydillo C, Sanmartín C, Sánchez-Moreno M, Marín C. Library of Selenocyanate and Diselenide Derivatives as In Vivo Antichagasic Compounds Targeting Trypanosoma cruzi Mitochondrion. Pharmaceuticals. 2021; 14(5):419. https://doi.org/10.3390/ph14050419
Chicago/Turabian StyleMartín-Escolano, Rubén, Daniel Molina-Carreño, Daniel Plano, Socorro Espuelas, María J. Rosales, Esther Moreno, Carlos Aydillo, Carmen Sanmartín, Manuel Sánchez-Moreno, and Clotilde Marín. 2021. "Library of Selenocyanate and Diselenide Derivatives as In Vivo Antichagasic Compounds Targeting Trypanosoma cruzi Mitochondrion" Pharmaceuticals 14, no. 5: 419. https://doi.org/10.3390/ph14050419
APA StyleMartín-Escolano, R., Molina-Carreño, D., Plano, D., Espuelas, S., Rosales, M. J., Moreno, E., Aydillo, C., Sanmartín, C., Sánchez-Moreno, M., & Marín, C. (2021). Library of Selenocyanate and Diselenide Derivatives as In Vivo Antichagasic Compounds Targeting Trypanosoma cruzi Mitochondrion. Pharmaceuticals, 14(5), 419. https://doi.org/10.3390/ph14050419