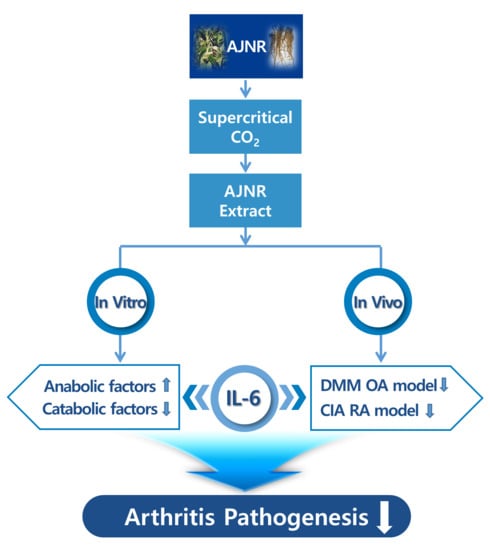
Inhibitory Effects of IL-6-Mediated Matrix Metalloproteinase-3 and -13 by Achyranthes japonica Nakai Root in Osteoarthritis and Rheumatoid Arthritis Mice Models
Abstract
:1. Introduction
2. Results
2.1. Extraction and Identification of AJNR Components
2.2. Effects of AJNR on Cell Viability
2.3. Effects of AJNR on Anabolic or Catabolic Factors in Primary Cultured Articular Chondrocytes
2.4. Effects of AJNR on Chondrocytes Exposed to Pro-Inflammatory Cytokines
2.5. AJNR Attenuated DMM-Induced OA Pathogenesis
2.6. AJNR Attenuated CIA-Induced RA
2.7. AJNR Attenuated CIA-Induced Pannus Formation in Ankle and Knee
2.8. AJNR Inhibited IL-6 Mediated Mmp3 and Mmp13 Expression in DMM-Induced OA Model or CIA-Induced RA Model
3. Discussion
4. Materials and Methods
4.1. Chemicals and Laboratory Ware
4.2. Preparation of AJNR Extracts by Supercritical CO2
4.3. Gas Chromatography-Mass Sepectrometry Analysis
4.4. Primary Culture of Mouse Knee Joint Chondrocytes and Treatment with AJNR
4.5. MTT Cell Viability Assay
4.6. Reverse Transcription-Polymerase Chain Reaction (RT-PCR)
4.7. Animal Model and AJNR Treatment
4.8. Histological Analysis
4.9. Immunohistochemistry
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AJNR | Achyranthes japonica Nakai root |
| OA | osteoarthritis |
| RA | rheumatoid arthritis |
| CO2 | carbon dioxide |
| DMM | destabilization of the medial meniscus |
| CIA | collagenase-induced arthritis |
| Epas1 | endothelial PAS domain protein 1 |
| Esrrg | estrogen-related receptor gamma |
| Nampt | nicotinamide phosphoribosyl-transferase |
| Mtf1 | metal regulatory transcription factor 1 |
| RUNX2 | runt-related transcription factor 2 |
| BATF | basic leucine zipper transcription factor, ATF-like |
| RORα | RAR-related orphan receptor α |
| TLR | toll-like receptor |
| NSAIDs | non-steroidal anti-inflammatory drugs |
| GC-MS | gas chromatography-mass spectrometry |
| Slc39a8 | solute carrier family 39 member 8 |
| Col2a1 | collagen type II alpha 1 chain |
| Sox9 | SRY-box transcription factor 9 |
| Adamts | ADAM metallopeptidase with thrombospondin type 1 motif |
| OARSI | Osteoarthritis Research Society International |
| IP | intraperitoneal |
| H&E | hematoxylin and eosin stain |
| NI | non-immunized |
| DMEM | Dulbecco’s modified Eagle medium |
| MTT | 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide |
| RT-PCR | reverse transcription-polymerase chain reaction |
References
- Sacitharan, P.K. Ageing and Osteoarthritis. Biochem. Cell Biol. Ageing Part II Clin. Sci. 2019, 91, 123–159. [Google Scholar] [CrossRef]
- Yang, S.; Kim, J.; Ryu, J.H.; Oh, H.; Chun, C.H.; Kim, B.J.; Min, B.H.; Chun, J.S. Hypoxia-inducible factor-2alpha is a catabolic regulator of osteoarthritic cartilage destruction. Nat. Med. 2010, 16, 687–693. [Google Scholar] [CrossRef] [PubMed]
- Felson, D.T.; Lawrence, R.C.; Dieppe, P.A.; Hirsch, R.; Helmick, C.G.; Jordan, J.M.; Kington, R.S.; Lane, N.E.; Nevitt, M.C.; Zhang, Y.; et al. Osteoarthritis: New insights. Part 1: The disease and its risk factors. Ann. Intern. Med. 2000, 133, 635–646. [Google Scholar] [CrossRef] [PubMed]
- Dieppe, P.A.; Lohmander, L.S. Pathogenesis and management of pain in osteoarthritis. Lancet 2005, 365, 965–973. [Google Scholar] [CrossRef]
- Aggarwal, B.B.; Gupta, S.C.; Sung, B. Curcumin: An orally bioavailable blocker of TNF and other pro-inflammatory biomarkers. Br. J. Pharm. 2013, 169, 1672–1692. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abramoff, B.; Caldera, F.E. Osteoarthritis: Pathology, Diagnosis, and Treatment Options. Med. Clin. N. Am. 2020, 104, 293–311. [Google Scholar] [CrossRef] [PubMed]
- Scanzello, C.R. Chemokines and inflammation in osteoarthritis: Insights from patients and animal models. J. Orthop. Res. 2017, 35, 735–739. [Google Scholar] [CrossRef] [Green Version]
- Xia, B.; Di, C.; Zhang, J.; Hu, S.; Jin, H.; Tong, P. Osteoarthritis pathogenesis: A review of molecular mechanisms. Calcif. Tissue Int. 2014, 95, 495–505. [Google Scholar] [CrossRef] [Green Version]
- Attur, M.; Al-Mussawir, H.E.; Patel, J.; Kitay, A.; Dave, M.; Palmer, G.; Pillinger, M.H.; Abramson, S.B. Prostaglandin E2 exerts catabolic effects in osteoarthritis cartilage: Evidence for signaling via the EP4 receptor. J. Immunol. 2008, 181, 5082–5088. [Google Scholar] [CrossRef] [Green Version]
- Li, N.; Rivera-Bermudez, M.A.; Zhang, M.; Tejada, J.; Glasson, S.S.; Collins-Racie, L.A.; Lavallie, E.R.; Wang, Y.; Chang, K.C.; Nagpal, S.; et al. LXR modulation blocks prostaglandin E2 production and matrix degradation in cartilage and alleviates pain in a rat osteoarthritis model. Proc. Natl. Acad. Sci. USA 2010, 107, 3734–3739. [Google Scholar] [CrossRef] [Green Version]
- Wang, P.; Guan, P.P.; Guo, C.; Zhu, F.; Konstantopoulos, K.; Wang, Z.Y. Fluid shear stress-induced osteoarthritis: Roles of cyclooxygenase-2 and its metabolic products in inducing the expression of proinflammatory cytokines and matrix metalloproteinases. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2013, 27, 4664–4677. [Google Scholar] [CrossRef] [Green Version]
- Wang, P.; Zhu, F.; Konstantopoulos, K. Prostaglandin E2 induces interleukin-6 expression in human chondrocytes via cAMP/protein kinase A- and phosphatidylinositol 3-kinase-dependent NF-kappaB activation. Am. J. Physio. Cell Physiol. 2010, 298, C1445–C1456. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Son, Y.O.; Kim, H.E.; Choi, W.S.; Chun, C.H.; Chun, J.S. RNA-binding protein ZFP36L1 regulates osteoarthritis by modulating members of the heat shock protein 70 family. Nat. Commun. 2019, 10, 77. [Google Scholar] [CrossRef] [PubMed]
- Son, Y.O.; Park, S.; Kwak, J.S.; Won, Y.; Choi, W.S.; Rhee, J.; Chun, C.H.; Ryu, J.H.; Kim, D.K.; Choi, H.S.; et al. Estrogen-related receptor gamma causes osteoarthritis by upregulating extracellular matrix-degrading enzymes. Nat. Commun. 2017, 8, 2133. [Google Scholar] [CrossRef] [Green Version]
- Yang, S.; Ryu, J.H.; Oh, H.; Jeon, J.; Kwak, J.S.; Kim, J.H.; Kim, H.A.; Chun, C.H.; Chun, J.S. NAMPT (visfatin), a direct target of hypoxia-inducible factor-2alpha, is an essential catabolic regulator of osteoarthritis. Ann. Rheum. Dis. 2015, 74, 595–602. [Google Scholar] [CrossRef] [Green Version]
- Won, Y.; Shin, Y.; Chun, C.H.; Cho, Y.; Ha, C.W.; Kim, J.H.; Chun, J.S. Pleiotropic roles of metallothioneins as regulators of chondrocyte apoptosis and catabolic and anabolic pathways during osteoarthritis pathogenesis. Ann. Rheum. Dis. 2016, 75, 2045–2052. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kamekura, S.; Kawasaki, Y.; Hoshi, K.; Shimoaka, T.; Chikuda, H.; Maruyama, Z.; Komori, T.; Sato, S.; Takeda, S.; Karsenty, G.; et al. Contribution of runt-related transcription factor 2 to the pathogenesis of osteoarthritis in mice after induction of knee joint instability. Arthritis Rheum. 2006, 54, 2462–2470. [Google Scholar] [CrossRef] [PubMed]
- Rhee, J.; Park, S.H.; Kim, S.K.; Kim, J.H.; Ha, C.W.; Chun, C.H.; Chun, J.S. Inhibition of BATF/JUN transcriptional activity protects against osteoarthritic cartilage destruction. Ann. Rheum. Dis. 2017, 76, 427–434. [Google Scholar] [CrossRef] [Green Version]
- Choi, W.S.; Lee, G.; Song, W.H.; Koh, J.T.; Yang, J.; Kwak, J.S.; Kim, H.E.; Kim, S.K.; Son, Y.O.; Nam, H.; et al. The CH25H-CYP7B1-RORalpha axis of cholesterol metabolism regulates osteoarthritis. Nature 2019, 566, 254–258. [Google Scholar] [CrossRef]
- Kim, J.H.; Jeon, J.; Shin, M.; Won, Y.; Lee, M.; Kwak, J.S.; Lee, G.; Rhee, J.; Ryu, J.H.; Chun, C.H.; et al. Regulation of the catabolic cascade in osteoarthritis by the zinc-ZIP8-MTF1 axis. Cell 2014, 156, 730–743. [Google Scholar] [CrossRef] [Green Version]
- Yang, J.I.; Chun, J.S. Upregulated endonuclease Regnase-1 suppresses osteoarthritis by forming a negative feedback loop of catabolic signaling in chondrocytes. Arthritis Res. Ther. 2021, 23, 114. [Google Scholar] [CrossRef]
- Won, Y.; Yang, J.I.; Park, S.; Chun, J.S. Lipopolysaccharide Binding Protein and CD14, Cofactors of Toll-like Receptors, Are Essential for Low-Grade Inflammation-Induced Exacerbation of Cartilage Damage in Mouse Models of Posttraumatic Osteoarthritis. Arthritis Rheumatol. 2021, 73, 1451–1460. [Google Scholar] [CrossRef]
- Choi, W.S.; Yang, J.I.; Kim, W.; Kim, H.E.; Kim, S.K.; Won, Y.; Son, Y.O.; Chun, C.H.; Chun, J.S. Critical role for arginase II in osteoarthritis pathogenesis. Ann. Rheum. Dis. 2019, 78, 421–428. [Google Scholar] [CrossRef] [PubMed]
- Kang, D.; Lee, J.; Wu, C.; Guo, X.; Lee, B.J.; Chun, J.S.; Kim, J.H. The role of selenium metabolism and selenoproteins in cartilage homeostasis and arthropathies. Exp. Mol. Med. 2020, 52, 1198–1208. [Google Scholar] [CrossRef] [PubMed]
- Nelson, A.E. Osteoarthritis year in review 2017: Clinical. Osteoarthr. Cartil. 2018, 26, 319–325. [Google Scholar] [CrossRef] [Green Version]
- Jones, I.A.; Togashi, R.; Wilson, M.L.; Heckmann, N.; Vangsness, C.T., Jr. Intra-articular treatment options for knee osteoarthritis. Nat. Rev. Rheumatol. 2019, 15, 77–90. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Li, J.; Ruan, G.; Wang, G.; Huang, C.; Ding, C. Investigational drugs for the treatment of osteoarthritis, an update on recent developments. Expert Opin. Investig. Drugs 2018, 27, 881–900. [Google Scholar] [CrossRef] [PubMed]
- Padrela, L.; Rodrigues, M.A.; Duarte, A.; Dias, A.M.A.; Braga, M.E.M.; de Sousa, H.C. Supercritical carbon dioxide-based technologies for the production of drug nanoparticles/nanocrystals—A comprehensive review. Adv. Drug Deliv. Rev. 2018, 131, 22–78. [Google Scholar] [CrossRef]
- Deshpande, P.B.; Kumar, G.A.; Kumar, A.R.; Shavi, G.V.; Karthik, A.; Reddy, M.S.; Udupa, N. Supercritical fluid technology: Concepts and pharmaceutical applications. PDA J. Pharm Sci. Technol 2011, 65, 333–344. [Google Scholar] [CrossRef]
- Durante, M.; Lenucci, M.S.; Mita, G. Supercritical carbon dioxide extraction of carotenoids from pumpkin (Cucurbita spp.): A review. Int. J. Mol. Sci. 2014, 15, 6725–6740. [Google Scholar] [CrossRef] [Green Version]
- Arumugham, T.; Rambabu, K.; Hasan, S.W.; Show, P.L.; Rinklebe, J.; Banat, F. Supercritical carbon dioxide extraction of plant phytochemicals for biological and environmental applications—A review. Chemosphere 2021, 271, 129525. [Google Scholar] [CrossRef]
- Wrona, O.; Rafińska, K.; Możeński, C.; Buszewski, B. Supercritical Fluid Extraction of Bioactive Compounds from Plant Materials. J. AOAC Int. 2017, 100, 1624–1635. [Google Scholar] [CrossRef]
- Dragos, D.; Gilca, M.; Gaman, L.; Vlad, A.; Iosif, L.; Stoian, I.; Lupescu, O. Phytomedicine in Joint Disorders. Nutrients 2017, 9, 70. [Google Scholar] [CrossRef]
- Bang, S.Y.; Kim, J.H.; Kim, H.Y.; Lee, Y.J.; Park, S.Y.; Lee, S.J.; Kim, Y. Achyranthes japonica exhibits anti-inflammatory effect via NF-κB suppression and HO-1 induction in macrophages. J. Ethnopharmacol. 2012, 144, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Qian, J.; Dong, H.; Yang, J.; Yu, X.; Chen, J.; Chen, H.; Shi, Q.; Jia, L. The traditional Chinese medicine Achyranthes bidentata and our de novo conception of its metastatic chemoprevention: From phytochemistry to pharmacology. Sci. Rep. 2017, 7, 3888. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.; Lu, X.; Fu, Z.; Zhang, L.; Li, X.; Xu, X.; Ren, Y.; Lu, Y.; Fu, H.; Tian, J. Identification of candidate synovial membrane biomarkers after Achyranthes aspera treatment for rheumatoid arthritis. Biochim. Biophys. Acta 2016, 1864, 308–316. [Google Scholar] [CrossRef] [Green Version]
- Zhai, G. Alteration of Metabolic Pathways in Osteoarthritis. Metabolites 2019, 9, 11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Glasson, S.S.; Chambers, M.G.; Van Den Berg, W.B.; Little, C.B. The OARSI histopathology initiative—Recommendations for histological assessments of osteoarthritis in the mouse. Osteoarthr. Cartil. OARS Osteoarthr. Res. Soc. 2010, 18 (Suppl. 3), S17–S23. [Google Scholar] [CrossRef] [Green Version]
- Goldring, M.B. Update on the biology of the chondrocyte and new approaches to treating cartilage diseases. Best Pract. Res. Clin. Rheumatol. 2006, 20, 1003–1025. [Google Scholar] [CrossRef]
- Dobson, G.P.; Letson, H.L.; Grant, A.; McEwen, P.; Hazratwala, K.; Wilkinson, M.; Morris, J.L. Defining the osteoarthritis patient: Back to the future. Osteoarthr. Cartil. 2018, 26, 1003–1007. [Google Scholar] [CrossRef] [Green Version]
- Csaki, C.; Keshishzadeh, N.; Fischer, K.; Shakibaei, M. Regulation of inflammation signalling by resveratrol in human chondrocytes in vitro. Biochem. Pharm. 2008, 75, 677–687. [Google Scholar] [CrossRef]
- Kim, J.H.; Lee, G.; Won, Y.; Lee, M.; Kwak, J.S.; Chun, C.H.; Chun, J.S. Matrix cross-linking-mediated mechanotransduction promotes posttraumatic osteoarthritis. Proc. Natl. Acad. Sci. USA 2015, 112, 9424–9429. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Zeng, Y. Curcumin reduces inflammation in knee osteoarthritis rats through blocking TLR4 /MyD88/NF-κB signal pathway. Drug Dev. Res. 2019, 80, 353–359. [Google Scholar] [CrossRef]
- Wang, X.Z.; Ding, D.F.; Xue, Y.; Gu, X.F.; Pang, J.; Zhang, M.; Zheng, Y.X.; Cao, Y.L.; Zhan, H.S. Role of TLR4/NF-κB pathway for early change of synovial membrane in knee osteoarthritis rats. Zhongguo Gu Shang 2019, 32, 68–71. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Ahmed, S.; Islam, N.; Goldberg, V.M.; Haqqi, T.M. Epigallocatechin-3-gallate inhibits interleukin-1beta-induced expression of nitric oxide synthase and production of nitric oxide in human chondrocytes: Suppression of nuclear factor kappaB activation by degradation of the inhibitor of nuclear factor kappaB. Arthritis Rheum. 2002, 46, 2079–2086. [Google Scholar] [CrossRef]
- Yang, C.Y.; Chanalaris, A.; Troeberg, L. ADAMTS and ADAM metalloproteinases in osteoarthritis—Looking beyond the ‘usual suspects’. Osteoarthr. Cartil. 2017, 25, 1000–1009. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Behl, T.; Mehta, K.; Sehgal, A.; Singh, S.; Sharma, N.; Ahmadi, A.; Arora, S.; Bungau, S. Exploring the role of polyphenols in rheumatoid arthritis. Crit Rev. Food Sci. Nutr. 2021, 1–22. [Google Scholar] [CrossRef]
- Zhang, Z.J.; Osmałek, T.; Michniak-Kohn, B. Deformable Liposomal Hydrogel for Dermal and Transdermal Delivery of Meloxicam. Int. J. Nanomed. 2020, 15, 9319–9335. [Google Scholar] [CrossRef] [PubMed]
- Nahhas, C.R.; Fuller, B.C.; Hannon, C.P.; Gerlinger, T.L.; Nam, D.; Della Valle, C.J. Which Nonsurgical Treatments Do Patients Believe Are Most Effective for Hip and Knee Arthritis? J. Am. Acad Orthop. Surg. Glob. Res. Rev. 2020, 4, e2000046. [Google Scholar] [CrossRef] [PubMed]
- Schett, G.; Tanaka, Y.; Isaacs, J.D. Why remission is not enough: Underlying disease mechanisms in RA that prevent cure. Nat. Rev. Rheumatol. 2021, 17, 135–144. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.M.; Yun, J.H.; Lee, D.H.; Park, Y.G.; Son, K.H.; Nho, C.W.; Kim, Y.S. Chikusetsusaponin IVa methyl ester induces cell cycle arrest by the inhibition of nuclear translocation of β-catenin in HCT116 cells. Biochem. Biophys. Res. Commun. 2015, 459, 591–596. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Wang, X.; Fang, J.; Chang, Y.; Ning, N.; Guo, H.; Huang, L.; Huang, X. The genus Achyranthes: A review on traditional uses, phytochemistry, and pharmacological activities. J. Ethnopharmacol. 2017, 203, 260–278. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Zhang, Y.; Chen, W.; Liu, C.; Li, X.; Sun, D.; Liu, Z.; Xu, Y.; Mao, X.; Guo, Q.; et al. Achyranthes bidentata extract exerts osteoprotective effects on steroid-induced osteonecrosis of the femoral head in rats by regulating RANKL/RANK/OPG signaling. J. Transl. Med. 2014, 12, 334. [Google Scholar] [CrossRef] [Green Version]
- Ma, D.; Li, Y.; Xiao, W.; Peng, L.; Wang, L.; Liao, Z.; Hu, L. Achyranthes bidentata extract protects chondrocytes functions through suppressing glycolysis and apoptosis via MAPK/AKT signaling axis. Am. J. Transl. Res. 2020, 12, 142–152. [Google Scholar] [PubMed]
- Kim, D.; Lee, D.; Oh, D.; Jeong, H.C.; Lee, S.J.; Sohn, J.; Kim, O.K.; Lee, J. A Mixture Containing Fermented Achyranthes japonica Nakai Ameliorates Osteoarthritis in Knee Joints of Monoiodoacetate-Injected Rats. J. Med. Food 2020, 23, 811–817. [Google Scholar] [CrossRef]
- Lee, S.G.; Lee, E.J.; Park, W.D.; Kim, J.B.; Kim, E.O.; Choi, S.W. Anti-inflammatory and anti-osteoarthritis effects of fermented Achyranthes japonica Nakai. J. Ethnopharmacol. 2012, 142, 634–641. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Wu, G.; Zheng, R. A Systematic Pharmacology and In Vitro Study to Identify the Role of the Active Compounds of Achyranthes bidentata in the Treatment of Osteoarthritis. Med. Sci. Monit. 2020, 26, e925545. [Google Scholar] [CrossRef]
- Kim, J.S.; Lee, S.W.; Kim, S.K.; Na, S.W.; Kim, Y.O. Osteoprotective effects of extract from Achyranthes japonica in ovariectomized rats. J. Exerc. Rehabil. 2014, 10, 372–377. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Melo, M.M.R.; Silvestre, A.J.D.; Silva, C.M. Supercritical fluid extraction of vegetable matrices: Applications, trends and future perspectives of a convincing green technology. J. Supercrit. Fluids 2014, 92, 115–176. [Google Scholar] [CrossRef]
- Brand, D.D.; Latham, K.A.; Rosloniec, E.F. Collagen-induced arthritis. Nat. Protoc. 2007, 2, 1269–1275. [Google Scholar] [CrossRef]
- Zhu, L.; Wei, W.; Zheng, Y.Q.; Jia, X.Y. Effects and mechanisms of total glucosides of paeony on joint damage in rat collagen-induced arthritis. Inflamm. Res. 2005, 54, 211–220. [Google Scholar] [CrossRef]
- Pham, T.; van der Heijde, D.; Altman, R.D.; Anderson, J.J.; Bellamy, N.; Hochberg, M.; Simon, L.; Strand, V.; Woodworth, T.; Dougados, M. OMERACT-OARSI initiative: Osteoarthritis Research Society International set of responder criteria for osteoarthritis clinical trials revisited. Osteoarthr. Cartil. 2004, 12, 389–399. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arden, N.K.; Perry, T.A.; Bannuru, R.R.; Bruyère, O.; Cooper, C.; Haugen, I.K.; Hochberg, M.C.; McAlindon, T.E.; Mobasheri, A.; Reginster, J.Y. Non-surgical management of knee osteoarthritis: Comparison of ESCEO and OARSI 2019 guidelines. Nat. Rev. Rheumatol. 2021, 17, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Gerwin, N.; Bendele, A.M.; Glasson, S.; Carlson, C.S. The OARSI histopathology initiative—recommendations for histological assessments of osteoarthritis in the rat. Osteoarthr. Cartil. 2010, 18, S24–S34. [Google Scholar] [CrossRef] [PubMed] [Green Version]








Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, X.; Kim, D.; Suminda, G.G.D.; Min, Y.; Yang, J.; Kim, M.; Zhao, Y.; Ghosh, M.; Son, Y.-O. Inhibitory Effects of IL-6-Mediated Matrix Metalloproteinase-3 and -13 by Achyranthes japonica Nakai Root in Osteoarthritis and Rheumatoid Arthritis Mice Models. Pharmaceuticals 2021, 14, 776. https://doi.org/10.3390/ph14080776
Zhao X, Kim D, Suminda GGD, Min Y, Yang J, Kim M, Zhao Y, Ghosh M, Son Y-O. Inhibitory Effects of IL-6-Mediated Matrix Metalloproteinase-3 and -13 by Achyranthes japonica Nakai Root in Osteoarthritis and Rheumatoid Arthritis Mice Models. Pharmaceuticals. 2021; 14(8):776. https://doi.org/10.3390/ph14080776
Chicago/Turabian StyleZhao, Xiangyu, Dahye Kim, Godagama Gamaarachchige Dinesh Suminda, Yunhui Min, Jiwon Yang, Mangeun Kim, Yaping Zhao, Mrinmoy Ghosh, and Young-Ok Son. 2021. "Inhibitory Effects of IL-6-Mediated Matrix Metalloproteinase-3 and -13 by Achyranthes japonica Nakai Root in Osteoarthritis and Rheumatoid Arthritis Mice Models" Pharmaceuticals 14, no. 8: 776. https://doi.org/10.3390/ph14080776
APA StyleZhao, X., Kim, D., Suminda, G. G. D., Min, Y., Yang, J., Kim, M., Zhao, Y., Ghosh, M., & Son, Y. -O. (2021). Inhibitory Effects of IL-6-Mediated Matrix Metalloproteinase-3 and -13 by Achyranthes japonica Nakai Root in Osteoarthritis and Rheumatoid Arthritis Mice Models. Pharmaceuticals, 14(8), 776. https://doi.org/10.3390/ph14080776