Mechanistic Insight into the Effects of Curcumin on Neuroinflammation-Driven Chronic Pain
Abstract
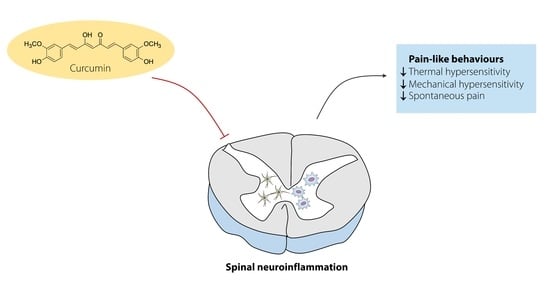
:1. Introduction
2. Overview of Curcumin
3. Role of Glia in Neuroinflammation-Mediated Chronic Pain
4. In Vitro Effects of Curcumin on Neuroinflammation
5. In Vivo Effects of Curcumin on Neuroinflammation-Driven Chronic Pain
| No | Cell | Exposure | Concentration (µM) | Effects | Reference |
|---|---|---|---|---|---|
| 1 | Rat primary microglia and BV-2 microglial cells | 50 µg/mL gangliosides, 100 ng/mL LPS, and 10 U/mL IFNγ | 5–10 | ↓ COX-2 and iNOS | (H. Y. Kim et al., 2003) [65] |
| ↓ JAK-STAT signaling pathway | |||||
| 2 | BV-2 microglial cells | 0.2 ng/mL LPS | 2–16 | ↓ COX-2 production | Kang et al., 2004) [59] |
| ↓ NF-κβ signaling and AP-1 binding activity | |||||
| 3 | BV-2 microglial cells | 0.5 μg/mL LPS | 10–20 | ↓ NO, iNOS, COX-2, PGE2, TNF-α, IL1-β, and IL-6 | (C.-Y. Jin et al., 2007) [58] |
| ↓ NF-κβ signaling pathway | |||||
| 4 | BV-2 microglial cells | 100 ng/mL LPS | 20 | ↑ the expression of IL-4 and PPAR-α | (Karlstetter et al., 2011) [62] |
| ↓ TLR2 and PGE2 | |||||
| ↓ the expression of C3, NOS2, STAT1, MCP-1 | |||||
| 5 | N9 microglial cells | 1 µg/mL HIV-1 gp120 | 15 | ↓ ROS, TNF-α and MCP-1 productions | (Guo et al., 2013) [63] |
| 6 | Primary cells containing neurons and microglia | 1 μg/mL LPS | 2 | ↓ TLR4, MyD88, CD11b, IL-1β, IL-6, and RANTES | (H. Zhu et al., 2014) [64] |
| ↓ NF-κβ signaling pathway | |||||
| 7 | Primary microglial cells | 1 μg/mL LPS | 10–25 | ↓ IL1-β, TNF-α, MCP-1, MIP-1α | (J.-J. Chen et al., 2015) [17] |
| 8 | BV-2 microglial cells | 1 μg/mL LPS | 10–50 | ↓ NO, iNOS, TNF-α, IL-1β, and IL-6 | (Cianciulli et al., 2016) [60] |
| ↓ PI3K/Akt and NF-κβ signaling pathways | |||||
| 9 | BV-2 microglial cells | 0.1 μg/mL Pam3CSK4 | 10–20 | ↓ NO, TNF-α, NOS2, COX-2, PGE2 | M. Jin et al., 2018) [66] |
| ↑ HO-1 and Nrf2 expression | |||||
| ↓ NF-κβ and MAPK signaling pathways | |||||
| 10 | BV-2 microglial cells | 5 μg/mL LTA | 10–20 | ↓ TNF-α, NO, NOS2,COX-2, and PGE2 | (Y. Yu et al., 2018) [67] |
| ↑ HO-1 and Nrf2 expression | |||||
| ↓ MAPK signaling pathways | |||||
| 11 | BV-2 microglial cells | 1 μg/mL LPS | 10–50 | ↑ anti-inflammatory cytokines (IL-4 and IL-10) and ↑ SOCS-1 | (Porro et al., 2019) [61] |
| ↓ JAK-STAT signaling pathway |
| No | Cell | Exposure | Concentration (µM) | Effects | Reference |
|---|---|---|---|---|---|
| 1 | C6 astroglial cells | 6 pg/mL LPS 100 U/mL of IFNγ | - | ↓ NO production | (Soliman and Mazzio 1998) [72] |
| 2 | Primary astrocytes | 5 μg/mL LPS | 10 | ↓ MIP-2 expression | (M. Tomita et al., 2005) [68] |
| 3 | Primary astrocytes | 200 µM H2O2 | 10 | ↓ Intracellular ROS and ↑ activation of Nrf2 signaling pathways | (Jiang et al., 2011) [74] |
| 4 | C6 astroglial cells | 1 μg/mL LPS | 10–25 | ↓ MCP-1 and JNK pathway | (Z.-J. Zhang et al., 2012) [100] |
| 5 | U-373 MG cells | 0.5 μg/mL LPS | 5 | ↓ the expression of IL-6, MMP9 enzyme activity, MCP-1 | (Seyedzadeh et al., 2014) [70] |
| 6 | Primary astrocytes | 1 μg/mL LPS | 10–25 | ↓ IL1-β, TNF-α, MCP-1, MIP-1α | (J.-J. Chen et al., 2015) [17] |
| 7 | Primary glial cells containing microglia, oligodendrocytes, and astrocytes | 5 mM fructose | 0.5–2 | ↓ the expression of fractalkine and its receptor, CX3CR1 | (M. X. Xu et al., 2016) [75] |
| ↓ the GFAP, the marker of astrocyte activation | |||||
| 8 | Primary astrocytes | 800 μM MPP+ | 8 | ↓ the release of TNF-α and IL-6 | (S. Yu et al., 2016) [76] |
| ↑ the expression of IL-10 | |||||
| ↓ the expression of TLR4 and inhibited NF-κβ pathway | |||||
| 9 | Primary astrocytes | 20 ng/mL TNF-α + 10 ng/mL IL-1β | 1 | ↓ GFAP, MCP-1, RANTES, CXCL10 | (Yuan et al., 2017) [71] |
| ↓ NF-κβ signaling pathway | |||||
| 10 | Primary astrocytes | 10 ng/mL IL-1β | 10 | ↓ the expression of COX-2 and IL-6 | (Drion et al., 2018) [69] |
| ↓ the activation of the MAPK pathways through p44 and p42 | |||||
| ↓ the phosphorylation of ERK1/2 | |||||
| 11 | Human Astrocytes-spinal cord | 50 µM H2O2 | 1 | ↓ Intracellular ROS, GFAP, prdx6, TNF-α | (Daverey and Agrawal 2018) [73] |
| ↓ NF-κβ pathway and ↑ Nrf2 expression |
| No | Animal | Animal Model of Pain | Dose (mg/kg/d) | Duration (Days) | Effects on Inflammatory Mediators in The Spinal Cord | Pain-Like Behaviors | References | ||
|---|---|---|---|---|---|---|---|---|---|
| Pain Hypersensitivity | Spontaneous Pain | ||||||||
| Thermal | Mechanical | ||||||||
| 1 | Sprague–Dawley rats | SIDN | 60 mg/kg/d (p.o) | 25 | ↓ TNF-α and TNFR1 | ✔ | ✔ | N/A | (Y. Li et al., 2013) [23] |
| 2 | HbSS-BERK sickle mice | Sickle cell disease | 15 mg/kg/d (p.o) | 28 | ↓ the expression of Iba1 and GFAP, Substance P, and ROS | ✔ | ✔ | N/A | (Valverde et al., 2016) [18] |
| 3 | Sprague–Dawley rats | OIPN | 12.5–50 mg/kg/d (p.o) | 28 | ↑ SOD, GSH-Px, CAT, and ↓ MDA | ✔ | ✔ | N/A | (X. Zhang et al., 2020) [24] |
| ↓ TNF-α, IL-1β, IL-6, and NF-κβ activity | |||||||||
| 4 | ICR mice | CCI | 25–200 mg/kg/d (p.o) | 14 | TNF-α and IL-6 | ✔ | ✔ | N/A | (Limcharoen et al., 2021) [83] |
| 5 | Sprague–Dawley rats | SCI | 40 mg/kg/d (i.p) | 7 | ↓ GFAP expression | N/A | N/A | N/A | (M. S. Lin et al., 2011) [85] |
| 6 | Sprague–Dawley rats | SCI | 40 mg/kg (i.p) | 1 | ↓ RANTES and iNOS | N/A | N/A | N/A | (M.-S. Lin et al., 2011) [86] |
| 7 | Sprague–Dawley rats | CCI | 100 mg/kg/(i.p) | 14 | ↓ CX3CR1 | ✔ | ✔ | N/A | Zheng et al., 2011) [91] |
| 8 | Sprague–Dawley rats | CCI | 50 mg/kg/d (i.p) | 7 and 14 | ↓ GFAP and phospho-ERK | ✔ | ✔ | N/A | (F. T. Ji et al., 2013) [82] |
| 9 | Sprague–Dawley rats | CCI | 40 and 60 mg/kg/d (i.p) | 7 | ↓ BDNF and COX-2, and P300/CBP HAT activity | ✔ | ✔ | N/A | (X. Zhu et al., 2014) [21] |
| 10 | Sprague–Dawley rats | CCI | 100 mg/kg/d (i.p) | 14 | ↓ NF-κβ p65 and CX3CR1 | ✔ | ✔ | N/A | (Cao et al., 2014) [81] |
| 11 | Sprague–Dawley rats | SCI | 100 mg/kg (i.p) | 1 | ↓ TLR4, TNF-α, IL-1β, IL-6 and NF-κβ activity | N/A | N/A | ✔ | (Ni et al., 2015) [20] |
| 12 | Charles-Foster strain rats | CFA | 100 mg/kg/d (i.p) | 2 | No effect on the cytokines | ✔ | N/A | N/A | (Singh and Vinayak 2015) [84] |
| 13 | BALB/c mice | SNI | 60–120 mg/kg/0.5 d (i.p) | 7 | ↓ GFAP, IL-1β, NALP1 inflammasome and JAK2-STAT3 signaling | ✔ | ✔ | N/A | (S. Liu et al., 2016) [22] |
| 14 | Sprague–Dawley rats | Brachial Plexus Avulsion | 60 mg/kg/d (i.p) | 28 | ↓ GFAP, TNF-α, IL-6, c-FOS, and NGF | ✔ | ✔ | N/A | (Xie et al., 2019) [19] |
| 15 | Sprague–Dawley rats | CFA | 0.1 mg and 1 mg (i.t) | 3 | ↓ IL-1β, TNF-α, MCP-1 and MIP-1α | ✔ | ✔ | N/A | (J.-J. Chen et al., 2015) [17] |
| 16 | ICR mice | CCI | CurDDG, 25–200 mg/kg/d (p.o) | 14 | ↓ TNF-α and IL-6 | ✔ | ✔ | N/A | (Limcharoen et al., 2021) [83] |
| 17 | ICR mice | CCI | CurDG, 25–200 mg/kg/d (p.o) | 14 | ↓ TNF-α and IL-6 | ✔ | ✔ | N/A | (Limcharoen et al., 2020) [101] |
| 18 | CD-1 mice | CCI | PLGA-CUR, 5–25 µg/mouse. (i.t) | 1 | ↓ TNF-α, IL-β, IL-6 and BDNF | ✔ | ✔ | N/A | (Pieretti et al., 2017) [102] |
| 19 | ICR mice | OIH | PLGA-CUR 20 mg/kg, (p.o) | 1 | ↓ spinal CaMKIIα | ✔ | ✔ | N/A | (Hu et al., 2016) [103] |
6. The Activation of Glial Cells in Humans with Chronic Pain and the Potential Use of Curcumin
7. Mechanism of Action of Curcumin on Neuroinflammation-Driven Chronic Pain
7.1. MAPK Pathway
7.2. NF-κβ Pathway
7.3. JAK-STAT Pathway
8. Role of Curcumin on Epigenetic Modulation and Inflammasome on Neuroinflammation-Driven Chronic Pain
9. Physical and Pharmacokinetic Properties of Curcumin
9.1. Solubility and Stability
9.2. Absorption
9.3. Metabolism and Elimination
| Model | Dose/Concentration | Main Finding | Ref |
|---|---|---|---|
| Absorption | |||
| Caco-2 permeability assay | 20 μg/mL | Permeation rate 7.1 × 10−6 cm/s | (Yu and Huang, 2011) [168] |
| Caco-2 permeability assay | 20 μg/mL | Permeation rate 8.4 × 10−6 cm/s | (Yu and Huang, 2012) [167] |
| Reverted rat gut sacs | 100 μg/mL | Amount of curcumin in the serosal fluid of the jejunum > duodenum and ileum | (Y.-M. Tsai et al., 2012) [178] |
| comparable amount of curcumin in sac tissue of duodenum, jejunum, and ileum | |||
| Caco-2 permeability assay | 30 μg/mL | Permeability coefficient 5.14 × 10−8 cm/s | (J. Wang et al., 2015) [166] |
| In vivo biodistribtion in rats | 70 mg/kg | Curcumin was absorbed through intestinal segments, including the duodenum, jejunum, and ileum | |
| In situ single-pass intestinal perfusion test (SPIP) | 5 µg/mL curcumin | Permeability coefficient of curcumin in duodenum > jejunum and ileum | (H. Ji et al., 2016) [164] |
| Rat intestinal perfusion study | 40 μg/mL curcumin | Absorption rate and effective permeability of curcumin in the duodenum > jejunum and ileum | (Tian, Asghar, Wu, Chen et al., 2017) [165] |
| Distribution | |||
| Male albino rats | 400 mg curcumin | Portal blood, stomach, intestine, liver, and kidney | (Ravindranath and Chandrasekhara, 1980) [179] |
| Female BALB/c mice | 100 mg/kg body weight, i.p | Plasma, liver, kidneys, spleen, brain, and intestines 1 h after i.p. administration | (Pan, Huang, and Lin 1999) [171] |
| Male Wistar albino rats | 340 mg/kg | Intestinal mucosa, liver, kidney, and heart | (Marczylo, Steward, and Gescher 2009) [174] |
| Male ICR mice | 20 and 400 mg/kg. p.o. | Plasma and central nervous system (brain and spinal cord) | (Szymusiak et al., 2016) [87] |
| Metabolism (metabolites) | |||
| In vitro: hepatocytes or liver microsomes | 0.1–5 μg/mL | 60%–90% of curcumin was metabolized within 30 min | (Wahlström and Blennow 1978) [176] |
| In vitro hepatocytes | 100 μM | Hexahydrocurcumin and hexahydrocurcuminol | (Ireson et al., 2001) [172] |
| Male Sprague–Dawley rats | 0.6–12 mg, i.v | glucuronides of tetrahydrocurcumin and Hexahydrocurcumin, dihydroferulic acid, and ferulic acid | (Holder, Plummer, and Ryan 1978) [173] |
| Female BALB/c mice | 100 mg/kg body weight, i.p | Curcumin-glucuronoside, dihydrocurcumin-glucuronoside, tetrahydrocurcumin-glucuronoside, and tetrahydrocurcumin | (Pan, Huang, and Lin 1999) [171] |
| Female F344 rats | 500 mg/kg, p.o and 40 mg/kg, i.v. | Major: curcumin glucuronide and curcumin sulfate minor: hexahydrocurcumin, hexahydrocurcuminol, hexahydrocurcumin glucuronide | (Ireson et al., 2001) [172] |
| Male Wistar albino rats | 340 mg/kg, p.o. | Phenolic glucuronides and alcoholic glucuronides (plasma and urine) | (Marczylo, Steward, and Gescher 2009) [174] |
| Human | Curcuminoids, 3.6 g/d for 29 days, p.o | Curcumin glucuronide and curcumin sulfate (plasma and urine) | (R.A. Sharma et al., 2004) [177] |
| Elimination | |||
| Sprague–Dawley rats | 1 g/kg | 75% of curcumin excreted in the feces, the undetectable amount in urine | (Wahlström and Blennow 1978) [176] |
| Sprague–Dawley rats | 0.6 mg/dose | -[3H]-curcumin metabolites—89.4% (feces) 6.3% (urine) | (Holder, Plummer, and Ryan 1978) [173] |
| High extent of biliary excretion of curcumin | |||
| Male Wistar albino rats | 340 mg/kg, p.o. | 2.0 ng/mL of curcumin (urine) | (Marczylo, Steward, and Gescher 2009) [174] |
| Human | Curcumuminoids, 3.6 g/d for 29 days, p.o | Curcumin and its metabolites (urine and feces) | (R.A. Sharma et al., 2004) [177] |
9.4. Bioavailability and BBB Penetration
| No | Species | Route | Dose (mg/kg) | Pharmacokinetic Parameters | Ref | |||
|---|---|---|---|---|---|---|---|---|
| Plasma Concentration (ng/mL) | tmax (min) | t 1/2 (min) | F (%) | |||||
| Mice | ||||||||
| 1 | Male ICR mice | p.o | 20 | 0.60 ± 0.44 | 15 | N/A | N/A | (Szymusiak et al., 2016) [87] |
| 400 | 79.82 ± 49.00 | 3 | ||||||
| 2 | Male C57BL/6 mice | p.o | 25 | 14 ± 3 | 120 | N/A | N/A | (C. Wang et al., 2015) [197] |
| 3 | BALB/C mice | p.o | 150 | 800 ± 200 | 60 ± 34.2 | N/A | N/A | (S. Kumar et al., 2016) [198] |
| 4 | Female BALB/c mice | p.o | 1000 | 220 | N/A | N/A | (Pan, Huang, and Lin 1999) [171] | |
| i.p. | 100 | 2250 | ||||||
| Rats | ||||||||
| 1 | Male Sprague–Dawley rats | p.o | 20 | 82 ± 8 | 30 | N/A | N/A | (C. Liu et al., 2018) [199] |
| 2 | Male Sprague–Dawley rats | p.o | 50 | 290 ± 110 | 60 | N/A | N/A | (Baek and Cho 2017) [200] |
| 3 | Male Sprague–Dawley rats | p.o | 50 | 22 ± 6 | N/A | N/A | 2.60 ± 1.03 | (Tian, Asghar, Wu, Kambere Amerigos et al., 2017) [180] |
| 4 | Male Sprague–Dawley rats | p.o | 50 | 5.08 ± 1.18 | 60 | 541.11 ± 395.78 | N/A | (Yutong Wang et al., 2017) [201] |
| 5 | Sprague–Dawley rats | p.o | 50 | 109.84 ± 85.89 | 69 ± 44.4 | N/A | N/A | (J. Wang, Ma, and Tu 2015) [166] |
| 6 | Male Wistar rats | p.o | 50 | 2120 ± 340 | 34.8 ± 12 | 570 ± 12 | N/A | (H. Ji et al., 2016) [164] |
| 7 | Male Wistar rats | p.o | 50 | 87.06 ± 24.02 | 39.6 ± 3.6 | 29.4 ± 4.8 | N/A | (Wan et al., 2012) [202] |
| 8 | Albino Wistar rats | p.o | 50 | 9.58 ± 0.4 | 30 ± 0.0 | 75 ± 2.25 | N/A | (Chaurasia et al., 2015) [203] |
| 9 | Male Wistar rats | p.o | 50 | 4.07 ± 0.56 | 30 | 66.54 ± 7.44 | N/A | (Khalil et al., 2013) [204] |
| 10 | Male Sprague–Dawley rats | p.o | 100 | 470 ± 180 | 60 | N/A | N/A | (Peng et al., 2018a) [205] |
| 11 | Male Sprague–Dawley rats | p.o | 100 | 1550 ± 210 | 102 ± 16 | 74.2 ± 5.9 | 4.73 | (X. Xie et al., 2011) [181] |
| 12 | Male Sprague–Dawley rats | p.o | 100 | 21.6 ± 3.6 | 60 | 246 ± 18 | N/A | (Shukla et al., 2017) [206] |
| 13 | Male Sprague–Dawley rats | p.o | 100 | 35 ± 8.0 | 80 ± 10 | 207 ± 94 | 0.9 | (Onoue et al., 2010) [182] |
| 14 | Wistar rats | p.o | 100 | 126 ± 13.56 | 60 | 70.2 ± 2.4 | N/A | (A. Kumar et al., 2016) [207] |
| 15 | Male Sprague–Dawley rats | p.o | 150 | 1480 ± 30 | 15 ± 0.00 | 304.2 ± 24.6 | N/A | (Q. Zhang et al., 2018) [208] |
| 16 | Male Sprague–Dawley rats | p.o | 250 | 90.3 ± 15.5 | 30 | N/A | N/A | (Shaikh et al., 2009) [209] |
| 17 | Male Sprague–Dawley rats | p.o | 250 | 32.29 ± 14.93 | 34.8 ± 12 | 28.2 ± 8.4 | N/A | (Joshi et al., 2013) [210] |
| 18 | Male Sprague–Dawley rats | p.o | 500 | 60 ± 10 | 41.7 ± 5.4 | 44.5 ± 7.5 | 1% | (Yang et al., 2007) [183] |
| 19 | Male Wistar rats | p.o | 500 | 3.2 ± 1.4 | 30 | N/A | N/A | (Teixeira et al., 2016) [211] |
| 20 | Sprague–Dawley rats | p.o | 1000 | 950 ± 120 | 84 ± 33 | 184.8 ± 75.6 | N/A | (Hu et al., 2015) [212] |
| 21 | Male Sprague–Dawley rats | p.o | 1000 | 28 ± 10 | 45 | N/A | N/A | (Y.-M. Tsai et al., 2012) [178] |
| 22 | Male Sprague–Dawley rats | p.o | 1000 | 15 ± 12 | 50 ± 32 | 95 ± 35 | N/A | (Chang et al., 2013) [213] |
| 23 | Male Sprague–Dawley rats | p.o | 1000 | 22 ± 2 | N/A | N/A | 0.21 | (Y.-M. Tsai et al., 2011) [184] |
| 24 | Wistar Albino Rats | p.o | 1000 | 258.64 | 103.2 | 76.8 | N/A | (Gupta and Dixit 2011) [214] |
| 25 | Sprague–Dawley rats | p.o | 1000 | 830 ± 830 | 180.0 ± 60.0 | 61.24 ± 15.17 | N/A | (Hu et al., 2012) [215] |
| 26 | Male Sprague–Dawley rats | i.v. | 10 | 360 ± 50 | N/A | 28.1 ± 5.6 | N/A | (Yang et al., 2007) [183] |
| 27 | Male Sprague–Dawley rats | i.v. | 10 | 8820 ± 110 | 3 | N/A | N/A | (X. Xie et al., 2011) [181] |
| 28 | Male Sprague–Dawley rats | i.v. | 10 | 4200 ± 1800 | N/A | N/A | N/A | (Y.-M. Tsai et al., 2011) [184] |
| Species | Route | Form of Curcumin | Dose (Cur/Cur equivalent) | Plasma Conc. (ng/mL) | ||||
| 1 | Human | p.o | Curcumin | 4000–8000 mg/day (3 months) | 187.9–652 | (A.L. Cheng et al., 2001) [185] | ||
| 2 | Human | p.o | Curcuminoid capsules (8.2% curcumin) | 36 and 180 mg curcumin/day (29 days) | Undetectable | (R A Sharma et al., 2001) [186] | ||
| 3 | Human | p.o | Curcuminoid capsules (90% of curcumin) | 3.6 g/day | 4.1 ± 0.2 | (Sharma, R.A et al., 2004) [177] | ||
| 4 | Healthy human | p.o | Standardized curcuminoid mixture | 1295 mg | 9.0 ± 2.8 | (Cuomo et al., 2011) [187] | ||
| p.o | Lecithin formulation of standardized curcuminoid mixture (Meriva) | 297 mg | 50.3 ± 12.7 | |||||
| 5 | Healthy human | p.o | Curcumin | 30 mg | 1.8 ± 2.8 | (Sasaki et al., 2011) [188] | ||
| p.o | Curcumin colloidal nanoparticles (Theracurmin™) | 30 mg | 29.5 ± 12.9 | |||||
| 6 | Healthy human | p.o | Curcumin | 410 mg | 2.6 ± 4.9 | (Schiborr et al., 2014) [189] | ||
| p.o | Liquid micelles of curcumin (NovaSol®) | 410 mg | 1189.1 ± 518.7 | |||||
| p.o | micronized curcumin | 410 mg | 15.3 ± 8.9 | |||||
| No. | Species | Route | Dose | Concentration | Ref | ||
|---|---|---|---|---|---|---|---|
| Brain (ng/g) | Spinal Cord (ng/g) | Plasma (ng/mL) | |||||
| 1 | NMRI mice | p.o | 50 mg/kg | Undetectable | N/A | N/A | (Schiborr et al., 2010) [190] |
| C57BL/6 mice | i.p | 100 mg/kg | 4160–5010 | ||||
| 2 | Wistar rats | p.o | 200 mg/kg | 1.40 ± 0.80 | N/A | N/A | (IM, K.; Maliakel et al., 2015) [216] |
| 3 | Male ICR mice | p.o | 400 mg/kg | 30.32 ± 3.10 | 129.16 ± 63.12 | 79.82 ± 49.00 | (Szymusiak et al., 2016) [87] |
| 20 mg/kg | 2.03 ± 0.69 | 23.49 ± 11.57 | 0.60 ± 0.44 | ||||
| 4 | Male C57BL/6 mice | p.o | 50 mg/kg/d for 2 days | 41.1 ± 6.7 | N/A | 8.2 ± 1.8 | (Sorrenti et al., 2018) [192] |
| Male C57BL/6 mice-induced by LPS | p.o. | 50 mg/kg/d for 2 days | 108.3 ± 25.8 | N/A | 4.8 ± 0.9 | ||
| 5 | Female BALB/c mice | i.p. | 100 mg/kg | 410 ± 10 | N/A | N/A | (Pan, Huang, and Lin 1999) [171] |
| 6 | Kunming male mice | i.v. | 10 mg/kg | 25.7 | N/A | N/A | (Sun et al., 2010) [217] |
| 7 | C57BL/6 mice | i.v. | 5 mg/dose | 2–5 | N/A | 2–5 | (Dende et al., 2017) [218] |
9.5. Drug-Interactions
10. Strategies to Improve the Efficacy of Curcumin on Neuroinflammation-Driven Chronic Pain
10.1. Curcumin Nanoparticles
10.2. Curcumin Prodrugs
10.3. Drug Combination Approach
11. Potential Risks and Adverse Effects of Curcumin
12. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Holden, J.E.; Pizzi, J.A. The challenge of chronic pain. Adv. Drug Deliv. Rev. 2003, 55, 935–948. [Google Scholar] [CrossRef]
- Mills, S.E.E.; Nicolson, K.P.; Smith, B.H. Chronic pain: A review of its epidemiology and associated factors in population-based studies. Br. J. Anaesth. 2019, 123, e273–e283. [Google Scholar] [CrossRef]
- Gangadharan, V.; Kuner, R. Pain hypersensitivity mechanisms at a glance. Dis. Model. Mech. 2013, 6, 889–895. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ji, R.-R.; Chamessian, A.; Zhang, Y.-Q. Pain regulation by non-neuronal cells and inflammation. Science 2016, 354, 572–577. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Milligan, E.D.; Watkins, L.R. Pathological and protective roles of glia in chronic pain. Nat. Rev. Neurosci. 2009, 10, 23–36. [Google Scholar] [CrossRef] [PubMed]
- Reid, M.C.; Eccleston, C.; Pillemer, K. Management of chronic pain in older adults. Bmj 2015, 350, h532. [Google Scholar] [CrossRef] [Green Version]
- Varrassi, G.; Müller-Schwefe, G.; Pergolizzi, J.; Orónska, A.; Morlion, B.; Mavrocordatos, P.; Margarit, C.; Mangas, C.; Jaksch, W.; Huygen, F.; et al. Pharmacological treatment of chronic pain—The need for CHANGE. Curr. Med. Res. Opin. 2010, 26, 1231–1245. [Google Scholar] [CrossRef] [PubMed]
- Loggia, M.L.; Chonde, D.B.; Akeju, O.; Arabasz, G.; Catana, C.; Edwards, R.R.; Hill, E.; Hsu, S.; Izquierdo-Garcia, D.; Ji, R.-R.; et al. Evidence for brain glial activation in chronic pain patients. Brain 2015, 138, 604–615. [Google Scholar] [CrossRef] [PubMed]
- Albrecht, D.S.; Forsberg, A.; Sandström, A.; Bergan, C.; Kadetoff, D.; Protsenko, E.; Lampa, J.; Lee, Y.C.; Höglund, C.O.; Catana, C.; et al. Brain glial activation in fibromyalgia—A multi-site positron emission tomography investigation. Brain. Behav. Immun. 2019, 75, 72–83. [Google Scholar] [CrossRef]
- Jeon, S.Y.; Seo, S.; Lee, J.S.; Choi, S.-H.; Lee, D.-H.; Jung, Y.-H.; Song, M.-K.; Lee, K.-J.; Kim, Y.C.; Kwon, H.W.; et al. [11C]-(R)-PK11195 positron emission tomography in patients with complex regional pain syndrome: A pilot study. Medicine 2017, 96, e5735. [Google Scholar] [CrossRef] [PubMed]
- Del Valle, L.; Schwartzman, R.J.; Alexander, G. Spinal cord histopathological alterations in a patient with longstanding complex regional pain syndrome. Brain. Behav. Immun. 2009, 23, 85–91. [Google Scholar] [CrossRef]
- Ji, R.-R.; Berta, T.; Nedergaard, M. Glia and pain: Is chronic pain a gliopathy? Pain 2013, 154, S10–S28. [Google Scholar] [CrossRef]
- Ji, R.-R.; Xu, Z.-Z.; Gao, Y.-J. Emerging targets in neuroinflammation-driven chronic pain. Nat. Rev. Drug Discov. 2014, 13, 533–548. [Google Scholar] [CrossRef] [Green Version]
- Horvath, R.J.; Romero-Sandoval, E.A.; De Leo, J.A. Glial modulation in pain states. In Translational Pain Research: From Mouse to Man; CRC Press/Taylor & Francis: Boca Raton, FL, USA, 2010. [Google Scholar]
- Haight, E.S.; Forman, T.E.; Cordonnier, S.A.; James, M.L.; Tawfik, V.L. Microglial modulation as a target for chronic pain: From the bench to the bedside and back. Anesth. Analg. 2019, 128, 737–746. [Google Scholar] [CrossRef] [PubMed]
- Ghasemi, F.; Bagheri, H.; Barreto, G.E.; Read, M.I.; Sahebkar, A. Effects of curcumin on microglial cells. Neurotox. Res. 2019, 36, 12–26. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.-J.; Dai, L.; Zhao, L.-X.; Zhu, X.; Cao, S.; Gao, Y.-J. Intrathecal curcumin attenuates pain hypersensitivity and decreases spinal neuroinflammation in rat model of monoarthritis. Sci. Rep. 2015, 5, 10278. [Google Scholar] [CrossRef] [Green Version]
- Valverde, Y.; Benson, B.; Gupta, M.; Gupta, K. Spinal glial activation and oxidative stress are alleviated by treatment with curcumin or coenzyme Q in sickle mice. Haematologica 2016, 101, e44. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xie, W.; Xie, W.; Kang, Z.; Jiang, C.; Liu, N. Administration of curcumin alleviates neuropathic pain in a rat model of brachial plexus avulsion. Pharmacology 2019, 103, 324–332. [Google Scholar] [CrossRef] [PubMed]
- Ni, H.; Jin, W.; Zhu, T.; Wang, J.; Yuan, B.; Jiang, J.; Liang, W.; Ma, Z. Curcumin modulates TLR4/NF-κB inflammatory signaling pathway following traumatic spinal cord injury in rats. J. Spinal Cord Med. 2015, 38, 199–206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, X.; Li, Q.; Chang, R.; Yang, D.; Song, Z.; Guo, Q.; Huang, C. Curcumin alleviates neuropathic pain by inhibiting p300/CBP histone acetyltransferase activity-regulated expression of BDNF and Cox-2 in a rat model. PLoS ONE 2014, 9, e91303. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, S.; Li, Q.; Zhang, M.-T.; Mao-Ying, Q.-L.; Hu, L.-Y.; Wu, G.-C.; Mi, W.-L.; Wang, Y.-Q. Curcumin ameliorates neuropathic pain by down-regulating spinal IL-1β via suppressing astroglial NALP1 inflammasome and JAK2-STAT3 signalling. Sci. Rep. 2016, 6, 28956. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, Y.; Liu, D.B.; Liu, H.Y.; Hou, W.G.; Dong, Y.S. Curcumin attenuates diabetic neuropathic pain by downregulating TNF-alpha in a rat model. Int. J. Med. Sci. 2013, 10, 377–381. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, X.; Guan, Z.; Wang, X.; Sun, D.; Wang, D.; Li, Y.; Pei, B.; Ye, M.; Xu, J.; Yue, X. Curcumin alleviates oxaliplatin-induced peripheral neuropathic pain through inhibiting oxidative stress-mediated activation of NF-kappaB and mitigating inflammation. Biol. Pharm. Bull. 2020, 43, 348–355. [Google Scholar] [CrossRef] [Green Version]
- Lao, C.D.; Ruffin, M.T.; Normolle, D.; Heath, D.D.; Murray, S.I.; Bailey, J.M.; Boggs, M.E.; Crowell, J.; Rock, C.L.; Brenner, D.E. Dose escalation of a curcuminoid formulation. BMC Complement. Altern. Med. 2006, 6, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- US FDA Notice to US Food and Drug Administration of the Conclusion that the Intended Use of Curcumin is Generally Recognized as Safe. Available online: https://www.fda.gov/media/132575/download (accessed on 9 March 2021).
- Goel, A.; Kunnumakkara, A.B.; Aggarwal, B.B. Curcumin as “Curecumin”: From kitchen to clinic. Biochem. Pharmacol. 2008, 75, 787–809. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- National Center for Biotechnology Information PubChem Compound Summary for CID 969516, Curcumin. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Curcumin (accessed on 28 July 2021).
- Wang, Y.-J.; Pan, M.-H.; Cheng, A.-L.; Lin, L.-I.; Ho, Y.-S.; Hsieh, C.-Y.; Lin, J.-K. Stability of curcumin in buffer solutions and characterization of its degradation products. J. Pharm. Biomed. Anal. 1997, 15, 1867–1876. [Google Scholar] [CrossRef]
- Shen, L.; Ji, H.F. The pharmacology of curcumin: Is it the degradation products? Trends Mol. Med. 2012, 18, 138–144. [Google Scholar] [CrossRef] [PubMed]
- Price, L.C.; Buescher, R.W. Decomposition of turmeric curcuminoids as affected by light, solvent and oxygen. J. Food Biochem. 1996, 20, 125–133. [Google Scholar] [CrossRef]
- Priyadarsini, K.I. The chemistry of curcumin: From extraction to therapeutic agent. Molecules 2014, 19, 20091–20112. [Google Scholar] [CrossRef] [Green Version]
- Liu, W.; Zhai, Y.; Heng, X.; Che, F.Y.; Chen, W.; Sun, D.; Zhai, G. Oral bioavailability of curcumin: Problems and advancements. J. Drug Target. 2016, 24, 694–702. [Google Scholar] [CrossRef]
- Heger, M.; van Golen, R.F.; Broekgaarden, M.; Michel, M.C. The molecular basis for the pharmacokinetics and pharmacodynamics of curcumin and its metabolites in relation to cancer. Pharmacol. Rev. 2014, 66, 222–307. [Google Scholar] [CrossRef]
- Sundaram, J.R.; Poore, C.P.; Sulaimee, N.H.B.; Pareek, T.; Cheong, W.F.; Wenk, M.R.; Pant, H.C.; Frautschy, S.A.; Low, C.M.; Kesavapany, S. Curcumin ameliorates neuroinflammation, neurodegeneration, and memory deficits in p25 transgenic mouse model that bears hallmarks of Alzheimer’s Disease. J. Alzheimers Dis. 2017, 60, 1429–1442. [Google Scholar] [CrossRef] [PubMed]
- Qureshi, M.; Al-Suhaimi, E.A.; Wahid, F.; Shehzad, O.; Shehzad, A. Therapeutic potential of curcumin for multiple sclerosis. Neurol. Sci. Off. J. Ital. Neurol. Soc. Ital. Soc. Clin. Neurophysiol. 2018, 39, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Tripanichkul, W.; Jaroensuppaperch, E.O. Ameliorating effects of curcumin on 6-OHDA-induced dopaminergic denervation, glial response, and SOD1 reduction in the striatum of hemiparkinsonian mice. Eur. Rev. Med. Pharmacol. Sci. 2013, 17, 1360–1368. [Google Scholar] [PubMed]
- Chen, Y.; Feng, H. Prevention by curcumin of neuroinflammation in intracerebral hemorrhage. In Neuroprotective Effects of Phytochemicals in Neurological Disorders; Farooqui, T.F.A.A., Ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2017; pp. 489–500. ISBN 9781119155140. [Google Scholar]
- Kim, S.K.; Hayashi, H.; Ishikawa, T.; Shibata, K.; Shigetomi, E.; Shinozaki, Y.; Inada, H.; Roh, S.E.; Kim, S.J.; Lee, G.; et al. Cortical astrocytes rewire somatosensory cortical circuits for peripheral neuropathic pain. J. Clin. Investig. 2016, 126, 1983–1997. [Google Scholar] [CrossRef] [PubMed]
- Barcelon, E.E.; Cho, W.-H.; Jun, S.B.; Lee, S.J. Brain Microglial Activation in Chronic Pain-Associated Affective Disorder. Front. Neurosci. 2019, 13, 213. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ginhoux, F.; Lim, S.; Hoeffel, G.; Low, D.; Huber, T. Origin and differentiation of microglia. Front. Cell. Neurosci. 2013, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saijo, K.; Glass, C.K. Microglial cell origin and phenotypes in health and disease. Nat. Rev. Immunol. 2011, 11, 775–787. [Google Scholar] [CrossRef]
- Gosselin, R.-D.; Suter, M.R.; Ji, R.-R.; Decosterd, I. Glial cells and chronic pain. Neuroscientist 2010, 16, 519–531. [Google Scholar] [CrossRef] [Green Version]
- Hu, X.; Leak, R.K.; Shi, Y.; Suenaga, J.; Gao, Y.; Zheng, P.; Chen, J. Microglial and macrophage polarization—New prospects for brain repair. Nat. Rev. Neurol. 2015, 11, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Kierdorf, K.; Prinz, M. Factors regulating microglia activation. Front. Cell. Neurosci. 2013, 7, 44. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coull, J.A.M.; Beggs, S.; Boudreau, D.; Boivin, D.; Tsuda, M.; Inoue, K.; Gravel, C.; Salter, M.W.; De Koninck, Y. BDNF from microglia causes the shift in neuronal anion gradient underlying neuropathic pain. Nature 2005, 438, 1017–1021. [Google Scholar] [CrossRef] [PubMed]
- Pelvig, D.P.; Pakkenberg, H.; Stark, A.K.; Pakkenberg, B. Neocortical glial cell numbers in human brains. Neurobiol. Aging 2008, 29, 1754–1762. [Google Scholar] [CrossRef] [PubMed]
- Oberheim, N.A.; Goldman, S.A.; Nedergaard, M. Heterogeneity of Astrocytic Form and Function. In Astrocytes: Methods and Protocols; Milner, R., Ed.; Humana Press: Totowa, NJ, USA, 2012; pp. 23–45. ISBN 978-1-61779-452-0. [Google Scholar]
- Verkhratsky, A.; Nedergaard, M. The homeostatic astroglia emerges from evolutionary specialization of neural cells. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 2016, 371, 20150428. [Google Scholar] [CrossRef] [PubMed]
- Liddelow, S.A.; Barres, B.A. Reactive astrocytes: Production, function, and therapeutic potential. Immunity 2017, 46, 957–967. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, T.; Chen, X.; Zhang, C.; Zhang, Y.; Yao, W. An update on reactive astrocytes in chronic pain. J. Neuroinflammation 2019, 16, 140. [Google Scholar] [CrossRef]
- Hassanzadeh, S.; Jalessi, M.; Jameie, S.B.; Khanmohammadi, M.; Bagher, Z.; Namjoo, Z.; Davachi, S.M. More attention on glial cells to have better recovery after spinal cord injury. Biochem. Biophys. Rep. 2021, 25, 100905. [Google Scholar] [CrossRef]
- Sofroniew, M.V.; Vinters, H.V. Astrocytes: Biology and pathology. Acta Neuropathol. 2010, 119, 7–35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- White, F.A.; Bhangoo, S.K.; Miller, R.J. Chemokines: Integrators of pain and inflammation. Nat. Rev. Drug Discov. 2005, 4, 834–844. [Google Scholar] [CrossRef] [Green Version]
- Kiguchi, N.; Kobayashi, Y.; Kishioka, S. Chemokines and cytokines in neuroinflammation leading to neuropathic pain. Curr. Opin. Pharmacol. 2012, 12, 55–61. [Google Scholar] [CrossRef]
- Latremoliere, A.; Woolf, C.J. Central sensitization: A generator of pain hypersensitivity by central neural plasticity. J. Pain 2009, 10, 895–926. [Google Scholar] [CrossRef] [Green Version]
- Dodds, K.N.; Beckett, E.A.H.; Evans, S.F.; Grace, P.M.; Watkins, L.R.; Hutchinson, M.R. Glial contributions to visceral pain: Implications for disease etiology and the female predominance of persistent pain. Transl. Psychiatry 2016, 6, e888. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jin, C.-Y.; Lee, J.-D.; Park, C.; Choi, Y.-H.; Kim, G.-Y. Curcumin attenuates the release of pro-inflammatory cytokines in lipopolysaccharide-stimulated BV2 microglia. Acta Pharmacol. Sin. 2007, 28, 1645–1651. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kang, G.; Kong, P.-J.; Yuh, Y.-J.; Lim, S.-Y.; Yim, S.-V.; Chun, W.; Kim, S.-S. Curcumin suppresses lipopolysaccharide-induced cyclooxygenase-2 expression by inhibiting activator protein 1 and nuclear factor kappab bindings in BV2 microglial cells. J. Pharmacol. Sci. 2004, 94, 325–328. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cianciulli, A.; Calvello, R.; Porro, C.; Trotta, T.; Salvatore, R.; Panaro, M.A. PI3k/Akt signalling pathway plays a crucial role in the anti-inflammatory effects of curcumin in LPS-activated microglia. Int. Immunopharmacol. 2016, 36, 282–290. [Google Scholar] [CrossRef] [PubMed]
- Porro, C.; Cianciulli, A.; Trotta, T.; Lofrumento, D.D.; Panaro, M.A. Curcumin regulates anti-inflammatory responses by JAK/STAT/SOCS signaling pathway in BV-2 microglial cells. Biology 2019, 8, 51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karlstetter, M.; Lippe, E.; Walczak, Y.; Moehle, C.; Aslanidis, A.; Mirza, M.; Langmann, T. Curcumin is a potent modulator of microglial gene expression and migration. J. Neuroinflammation 2011, 8, 125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, L.; Xing, Y.; Pan, R.; Jiang, M.; Gong, Z.; Lin, L.; Wang, J.; Xiong, G.; Dong, J. Curcumin protects microglia and primary rat cortical neurons against HIV-1 gp120-mediated inflammation and apoptosis. PLoS ONE 2013, 8, e70565. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, H.; Bian, C.; Yuan, J.; Chu, W.; Xiang, X.; Chen, F.; Wang, C.; Feng, H.; Lin, J. Curcumin attenuates acute inflammatory injury by inhibiting the TLR4/MyD88/NF-κB signaling pathway in experimental traumatic brain injury. J. Neuroinflammation 2014, 11, 59. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.Y.; Park, E.J.; Joe, E.; Jou, I. Curcumin suppresses janus kinase-STAT inflammatory signaling through activation of src homology 2 domain-containing tyrosine phosphatase 2 in brain microglia. J. Immunol. 2003, 171, 6072. [Google Scholar] [CrossRef] [Green Version]
- Jin, M.; Park, S.Y.; Shen, Q.; Lai, Y.; Ou, X.; Mao, Z.; Lin, D.; Yu, Y.; Zhang, W. Anti-neuroinflammatory effect of curcumin on Pam3CSK4-stimulated microglial cells. Int. J. Mol. Med. 2018, 41, 521–530. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Shen, Q.; Lai, Y.; Park, S.Y.; Ou, X.; Lin, D.; Jin, M.; Zhang, W. Anti-inflammatory effects of curcumin in microglial cells. Front. Pharmacol. 2018, 9, 386. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tomita, M.; Holman, B.J.; Santoro, C.P.; Santoro, T.J. Astrocyte production of the chemokine macrophage inflammatory protein-2 is inhibited by the spice principle curcumin at the level of gene transcription. J. Neuroinflammation 2005, 2, 8. [Google Scholar] [CrossRef] [Green Version]
- Drion, C.M.; van Scheppingen, J.; Arena, A.; Geijtenbeek, K.W.; Kooijman, L.; van Vliet, E.A.; Aronica, E.; Gorter, J.A. Effects of rapamycin and curcumin on inflammation and oxidative stress in vitro and in vivo -in search of potential anti-epileptogenic strategies for temporal lobe epilepsy. J. Neuroinflammation 2018, 15, 212. [Google Scholar] [CrossRef] [Green Version]
- Seyedzadeh, M.H.; Safari, Z.; Zare, A.; Gholizadeh Navashenaq, J.; Razavi, S.A.; Kardar, G.A.; Khorramizadeh, M.R. Study of curcumin immunomodulatory effects on reactive astrocyte cell function. Int. Immunopharmacol. 2014, 22, 230–235. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.; Liu, W.; Zhu, H.; Chen, Y.; Zhang, X.; Li, L.; Chu, W.; Wen, Z.; Feng, H.; Lin, J. Curcumin inhibits glial scar formation by suppressing astrocyte-induced inflammation and fibrosis in vitro and in vivo. Brain Res. 2017, 1655, 90–103. [Google Scholar] [CrossRef] [PubMed]
- Soliman, K.F.; Mazzio, E.A. In vitro attenuation of nitric oxide production in C6 astrocyte cell culture by various dietary compounds. Proc. Soc. Exp. Biol. Med. 1998, 218, 390–397. [Google Scholar] [CrossRef]
- Daverey, A.; Agrawal, S.K. Pre and post treatment with curcumin and resveratrol protects astrocytes after oxidative stress. Brain Res. 2018, 1692, 45–55. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Tian, X.; Guo, Y.; Duan, W.; Bu, H.; Li, C. Activation of nuclear factor erythroid 2-related factor 2 cytoprotective signaling by curcumin protect primary spinal cord astrocytes against oxidative toxicity. Biol. Pharm. Bull. 2011, 34, 1194–1197. [Google Scholar] [CrossRef] [Green Version]
- Xu, M.X.; Yu, R.; Shao, L.F.; Zhang, Y.X.; Ge, C.X.; Liu, X.M.; Wu, W.Y.; Li, J.M.; Kong, L.D. Up-regulated fractalkine (FKN) and its receptor CX3CR1 are involved in fructose-induced neuroinflammation: Suppression by curcumin. Brain Behav. Immun. 2016, 58, 69–81. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Wang, X.; He, X.; Wang, Y.; Gao, S.; Ren, L.; Shi, Y. Curcumin exerts anti-inflammatory and antioxidative properties in 1-methyl-4-phenylpyridinium ion (MPP+)-stimulated mesencephalic astrocytes by interference with TLR4 and downstream signaling pathway. Cell Stress Chaperones 2016, 21, 697–705. [Google Scholar] [CrossRef] [Green Version]
- Xiao, L.; Ding, M.; Fernandez, A.; Zhao, P.; Jin, L.; Li, X. Curcumin alleviates lumbar radiculopathy by reducing neuroinflammation, oxidative stress and nociceptive factors. Eur. Cell Mater. 2017, 33, 279–293. [Google Scholar] [CrossRef]
- Zhu, Q.; Sun, Y.; Yun, X.; Ou, Y.; Zhang, W.; Li, J.-X. Antinociceptive effects of curcumin in a rat model of postoperative pain. Sci. Rep. 2014, 4, 4932. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.; Leong, D.J.; Xu, L.; He, Z.; Wang, A.; Navati, M.; Kim, S.J.; Hirsh, D.M.; Hardin, J.A.; Cobelli, N.J.; et al. Curcumin slows osteoarthritis progression and relieves osteoarthritis-associated pain symptoms in a post-traumatic osteoarthritis mouse model. Arthritis Res. Ther. 2016, 18, 128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, L.; Ding, X.; Wu, Z.; Wang, M.; Tian, M. Curcumin alleviates pain and improves cognitive impairment in a rat model of cobra venom-induced trigeminal neuralgia. J. Pain Res. 2018, 11, 1095–1104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cao, H.; Zheng, J.; Li, J.; Meng, B.; Li, J.; Ge, R. Effects of curcumin on pain threshold and on the expression of nuclear factor κ B and CX3C receptor 1 after sciatic nerve chronic constrictive injury in rats. Chin. J. Integr. Med. 2014, 20, 850–856. [Google Scholar] [CrossRef] [PubMed]
- Ji, F.T.; Liang, J.J.; Liu, L.; Cao, M.H.; Li, F. Curcumin exerts antinociceptive effects by inhibiting the activation of astrocytes in spinal dorsal horn and the intracellular extracellular signal-regulated kinase signaling pathway in rat model of chronic constriction injury. Chin. Med. J. 2013, 126, 1125–1131. [Google Scholar] [PubMed]
- Limcharoen, T.; Muangnoi, C.; Dasuni Wasana, P.W.; Hasriadi; Vajragupta, O.; Rojsitthisak, P.; Towiwat, P. Improved antiallodynic, antihyperalgesic and anti-inflammatory response achieved through potential prodrug of curcumin, curcumin diethyl diglutarate in a mouse model of neuropathic pain. Eur. J. Pharmacol. 2021, 899, 174008. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.K.; Vinayak, M. Curcumin Attenuates CFA Induced Thermal Hyperalgesia by Modulation of Antioxidant Enzymes and Down Regulation of TNF-α, IL-1β and IL-6. Neurochem. Res. 2015, 40, 463–472. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.S.; Lee, Y.H.; Chiu, W.T.; Hung, K.S. Curcumin provides neuroprotection after spinal cord injury. J. Surg. Res. 2011, 166, 280–289. [Google Scholar] [CrossRef]
- Lin, M.-S.; Sun, Y.-Y.; Chiu, W.-T.; Hung, C.-C.; Chang, C.-Y.; Shie, F.-S.; Tsai, S.-H.; Lin, J.-W.; Hung, K.-S.; Lee, Y.-H. Curcumin attenuates the expression and secretion of RANTES after spinal cord injury in vivo and lipopolysaccharide-induced astrocyte reactivation in vitro. J. Neurotrauma 2011, 28, 1259–1269. [Google Scholar] [CrossRef] [PubMed]
- Szymusiak, M.; Hu, X.; Leon Plata, P.A.; Ciupinski, P.; Wang, Z.J.; Liu, Y. Bioavailability of curcumin and curcumin glucuronide in the central nervous system of mice after oral delivery of nano-curcumin. Int. J. Pharm. 2016, 511, 415–423. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yeon, K.Y.; Kim, S.A.; Kim, Y.H.; Lee, M.K.; Ahn, D.K.; Kim, H.J.; Kim, J.S.; Jung, S.J.; Oh, S.B. Curcumin produces an antihyperalgesic effect via antagonism of TRPV1. J. Dent. Res. 2010, 89, 170–174. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Yang, J.; Han, X.; Gong, Y.; Rao, S.; Wu, B.; Yi, Z.; Zou, L.; Jia, T.; Li, L.; et al. Effects of nanoparticle-encapsulated curcumin on HIV-gp120-associated neuropathic pain induced by the P2 × 3 receptor in dorsal root ganglia. Brain Res. Bull. 2017, 135, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Jia, T.; Rao, J.; Zou, L.; Zhao, S.; Yi, Z.; Wu, B.; Li, L.; Yuan, H.; Shi, L.; Zhang, C.; et al. Nanoparticle-Encapsulated Curcumin Inhibits Diabetic Neuropathic Pain Involving the P2Y12 Receptor in the Dorsal Root Ganglia. Front. Neurosci. 2018, 11, 755. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zheng, J.; Zheng, C.; Cao, H.; Li, J.; Lian, Q. Curcumin down-regulates CX3CR1 expression in spinal cord dorsal horn and DRG in neuropathic pain rats. Zhongguo Zhongyao Zazhi China J. Chin. Mater. Med. 2011, 36, 2552–2556. [Google Scholar]
- Grace, P.M.; Rolan, P.E.; Hutchinson, M.R. Peripheral immune contributions to the maintenance of central glial activation underlying neuropathic pain. Brain. Behav. Immun. 2011, 25, 1322–1332. [Google Scholar] [CrossRef] [PubMed]
- Prinz, M.; Priller, J. The role of peripheral immune cells in the CNS in steady state and disease. Nat. Neurosci. 2017, 20, 136–144. [Google Scholar] [CrossRef]
- Bruce, M.; Streifel, K.M.; Boosalis, C.A.; Heuer, L.; González, E.A.; Li, S.; Harvey, D.J.; Lein, P.J.; Van de Water, J. Acute peripheral immune activation alters cytokine expression and glial activation in the early postnatal rat brain. J. Neuroinflammation 2019, 16, 200. [Google Scholar] [CrossRef] [Green Version]
- Mohammadi, A.; Blesso, C.N.; Barreto, G.E.; Banach, M.; Majeed, M.; Sahebkar, A. Macrophage plasticity, polarization and function in response to curcumin, a diet-derived polyphenol, as an immunomodulatory agent. J. Nutr. Biochem. 2019, 66, 1–16. [Google Scholar] [CrossRef]
- Dworsky-Fried, Z.; Kerr, B.J.; Taylor, A.M.W. Microbes, microglia, and pain. Neurobiol. Pain 2020, 7, 100045. [Google Scholar] [CrossRef] [PubMed]
- Sanmarco, L.M.; Wheeler, M.A.; Gutiérrez-Vázquez, C.; Polonio, C.M.; Linnerbauer, M.; Pinho-Ribeiro, F.A.; Li, Z.; Giovannoni, F.; Batterman, K.V.; Scalisi, G.; et al. Gut-licensed IFNγ+ NK cells drive LAMP1+TRAIL+ anti-inflammatory astrocytes. Nature 2021, 590, 473–479. [Google Scholar] [CrossRef]
- Feng, W.; Wang, H.; Zhang, P.; Gao, C.; Tao, J.; Ge, Z.; Zhu, D.; Bi, Y. Modulation of gut microbiota contributes to curcumin-mediated attenuation of hepatic steatosis in rats. Biochim. Biophys. Acta. Gen. Subj. 2017, 1861, 1801–1812. [Google Scholar] [CrossRef] [PubMed]
- Bereswill, S.; Muñoz, M.; Fischer, A.; Plickert, R.; Haag, L.-M.; Otto, B.; Kühl, A.A.; Loddenkemper, C.; Göbel, U.B.; Heimesaat, M.M. Anti-inflammatory effects of resveratrol, curcumin and simvastatin in acute small intestinal inflammation. PLoS ONE 2010, 5, e15099. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.-J.; Zhao, L.-X.; Cao, D.-L.; Zhang, X.; Gao, Y.-J.; Xia, C. Curcumin inhibits LPS-induced CCL2 expression via JNK pathway in C6 rat astrocytoma cells. Cell. Mol. Neurobiol. 2012, 32, 1003–1010. [Google Scholar] [CrossRef] [PubMed]
- Limcharoen, T.; Dasuni Wasana, P.W.; Hasriadi; Muangnoi, C.; Vajragupta, O.; Rojsitthisak, P.; Towiwat, P. Curcumin Diglutaric Acid, a Prodrug of Curcumin Reduces Pain Hypersensitivity in Chronic Constriction Injury of Sciatic Nerve Induced-Neuropathy in Mice. Pharmaceuticals 2020, 13, 212. [Google Scholar] [CrossRef]
- Pieretti, S.; Ranjan, A.P.; Di Giannuario, A.; Mukerjee, A.; Marzoli, F.; Di Giovannandrea, R.; Vishwanatha, J.K. Curcumin-loaded poly (d, l-lactide-co-glycolide) nanovesicles induce antinociceptive effects and reduce pronociceptive cytokine and BDNF release in spinal cord after acute administration in mice. Colloids Surf. B Biointerfaces 2017, 158, 379–386. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Huang, F.; Szymusiak, M.; Tian, X.; Liu, Y.; Wang, Z.J. PLGA-Curcumin Attenuates Opioid-Induced Hyperalgesia and Inhibits Spinal CaMKIIα. PLoS ONE 2016, 11, e0146393. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bäckryd, E.; Lind, A.-L.; Thulin, M.; Larsson, A.; Gerdle, B.; Gordh, T. High levels of cerebrospinal fluid chemokines point to the presence of neuroinflammation in peripheral neuropathic pain: A cross-sectional study of 2 cohorts of patients compared with healthy controls. Pain 2017, 158, 2487–2495. [Google Scholar] [CrossRef] [PubMed]
- Bjurström, M.F.; Bodelsson, M.; Montgomery, A.; Harsten, A.; Waldén, M.; Janelidze, S.; Hall, S.; Hansson, O.; Irwin, M.R.; Mattsson-Carlgren, N. Differential expression of cerebrospinal fluid neuroinflammatory mediators depending on osteoarthritis pain phenotype. Pain 2020, 161, 2142–2154. [Google Scholar] [CrossRef]
- Das, B.; Conroy, M.; Moore, D.; Lysaght, J.; McCrory, C.; Human Dorsal Root Das, B.; Conroy, M.; Moore, D.; Lysaght, J.; McCrory, C. Human dorsal root ganglion pulsed radiofrequency treatment modulates cerebrospinal fluid lymphocytes and neuroinflammatory markers in chronic radicular pain. Brain. Beh. Brain. Behav. Immun. 2018, 70, 157–165. [Google Scholar] [CrossRef] [PubMed]
- Kuptniratsaikul, V.; Thanakhumtorn, S.; Chinswangwatanakul, P.; Wattanamongkonsil, L.; Thamlikitkul, V. Efficacy and safety of Curcuma domestica extracts in patients with knee osteoarthritis. J. Altern. Complement. Med. 2009, 15, 891–897. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Panahi, Y.; Rahimnia, A.-R.; Sharafi, M.; Alishiri, G.; Saburi, A.; Sahebkar, A. Curcuminoid treatment for knee osteoarthritis: A randomized double-blind placebo-controlled trial. Phytother. Res. 2014, 28, 1625–1631. [Google Scholar] [CrossRef] [PubMed]
- Belcaro, G.; Dugall, M.; Luzzi, R.; Ledda, A.; Pellegrini, L.; Cesarone, M.R.; Hosoi, M.; Errichi, M. Meriva®+Glucosamine versus Condroitin+Glucosamine in patients with knee osteoarthritis: An observational study. Eur. Rev. Med. Pharmacol. Sci. 2014, 18, 3959–3963. [Google Scholar]
- Nakagawa, Y.; Mukai, S.; Yamada, S.; Matsuoka, M.; Tarumi, E.; Hashimoto, T.; Tamura, C.; Imaizumi, A.; Nishihira, J.; Nakamura, T. Short-term effects of highly-bioavailable curcumin for treating knee osteoarthritis: A randomized, double-blind, placebo-controlled prospective study. J. Orthop. Sci. Off. J. Jpn. Orthop. Assoc. 2014, 19, 933–939. [Google Scholar] [CrossRef] [Green Version]
- Chandran, B.; Goel, A. A randomized, pilot study to assess the efficacy and safety of curcumin in patients with active rheumatoid arthritis. Phyther. Res. 2012, 26, 1719–1725. [Google Scholar] [CrossRef]
- Amalraj, A.; Varma, K.; Jacob, J.; Divya, C.; Kunnumakkara, A.B.; Stohs, S.J.; Gopi, S. A novel highly bioavailable curcumin formulation improves symptoms and diagnostic indicators in rheumatoid arthritis patients: A randomized, double-blind, placebo-controlled, two-dose, three-arm, and parallel-group study. J. Med. Food 2017, 20, 1022–1030. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, K.A.; Tripathi, C.D.; Agarwal, B.B.; Saluja, S. Efficacy of turmeric (curcumin) in pain and postoperative fatigue after laparoscopic cholecystectomy: A double-blind, randomized placebo-controlled study. Surg. Endosc. 2011, 25, 3805–3810. [Google Scholar] [CrossRef] [PubMed]
- Satoskar, R.R.; Shah, S.J.; Shenoy, S.G. Evaluation of anti-inflammatory property of curcumin (diferuloyl methane) in patients with postoperative inflammation. Int. J. Clin. Pharmacol. Ther. Toxicol. 1986, 24, 651–654. [Google Scholar]
- Lal, B.; Kapoor, A.K.; Agrawal, P.K.; Asthana, O.P.; Srimal, R.C. Role of curcumin in idiopathic inflammatory orbital pseudotumours. Phyther. Res. 2000, 14, 443–447. [Google Scholar] [CrossRef]
- Cowan, K.J.; Storey, K.B. Mitogen-activated protein kinases: New signaling pathways functioning in cellular responses to environmental stress. J. Exp. Biol. 2003, 206, 1107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ji, R.R.; Gereau, R.W.; Malcangio, M.; Strichartz, G.R. MAP kinase and pain. Brain Res. Rev. 2009, 60, 135–148. [Google Scholar] [CrossRef] [PubMed]
- Anand, P.; Shenoy, R.; Palmer, J.E.; Baines, A.J.; Lai, R.Y.K.; Robertson, J.; Bird, N.; Ostenfeld, T.; Chizh, B.A. Clinical trial of the p38 MAP kinase inhibitor dilmapimod in neuropathic pain following nerve injury. Eur. J. Pain 2011, 15, 1040–1048. [Google Scholar] [CrossRef] [PubMed]
- Nikodemova, M.; Duncan, I.D.; Watters, J.J. Minocycline exerts inhibitory effects on multiple mitogen-activated protein kinases and IkappaBalpha degradation in a stimulus-specific manner in microglia. J. Neurochem. 2006, 96, 314–323. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Zhuang, Z.; Lu, Y.; Tao, T.; Zhou, Y.; Liu, G.; Wang, H.; Zhang, D.; Wu, L.; Dai, H.; et al. Curcumin Mitigates Neuro-Inflammation by Modulating Microglia Polarization Through Inhibiting TLR4 Axis Signaling Pathway Following Experimental Subarachnoid Hemorrhage. Front. Neurosci. 2019, 13, 1223. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, Z.-Y.; Kawasaki, Y.; Tan, P.-H.; Wen, Y.-R.; Huang, J.; Ji, R.-R. Role of the CX3CR1/p38 MAPK pathway in spinal microglia for the development of neuropathic pain following nerve injury-induced cleavage of fractalkine. Brain. Behav. Immun. 2007, 21, 642–651. [Google Scholar] [CrossRef] [Green Version]
- Meng, X.-W.; Gao, J.-L.; Zuo, J.-L.; Wang, L.-N.; Liu, S.-L.; Jin, X.-H.; Yao, M.; Namaka, M. Toll-like receptor-4/p38 MAPK signaling in the dorsal horn contributes to P2 × 4 receptor activation and BDNF over-secretion in cancer induced bone pain. Neurosci. Res. 2017, 125, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.-C. NF-κB signaling in inflammation. Signal Transduct. Target. Ther. 2017, 2, 17023. [Google Scholar] [CrossRef] [Green Version]
- Xu, L.; Liu, Y.; Sun, Y.; Li, H.; Mi, W.; Jiang, Y. Analgesic effects of TLR4/NF-κB signaling pathway inhibition on chronic neuropathic pain in rats following chronic constriction injury of the sciatic nerve. Biomed. Pharmacother. 2018, 107, 526–533. [Google Scholar] [CrossRef]
- Yin, Q.; Fan, Q.; Zhao, Y.; Cheng, M.-Y.; Liu, H.; Li, J.; Lu, F.-F.; Jia, J.-T.; Cheng, W.; Yan, C.-D. Spinal NF-κB and chemokine ligand 5 expression during spinal glial cell activation in a neuropathic pain model. PLoS ONE 2015, 10, e0115120. [Google Scholar] [CrossRef] [Green Version]
- Shih, V.F.-S.; Tsui, R.; Caldwell, A.; Hoffmann, A. A single NFκB system for both canonical and non-canonical signaling. Cell Res. 2011, 21, 86–102. [Google Scholar] [CrossRef] [Green Version]
- Giridharan, S.; Srinivasan, M. Mechanisms of NF-kappaB p65 and strategies for therapeutic manipulation. J. Inflamm. Res. 2018, 11, 407–419. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vallabhapurapu, S.; Karin, M. Regulation and Function of NF-κB Transcription Factors in the Immune System. Annu. Rev. Immunol. 2009, 27, 693–733. [Google Scholar] [CrossRef] [PubMed]
- Roman-Blas, J.A.; Jimenez, S.A. NF-κB as a potential therapeutic target in osteoarthritis and rheumatoid arthritis. Osteoarthr. Cartil. 2006, 14, 839–848. [Google Scholar] [CrossRef] [Green Version]
- Kobayashi, K.; Imagama, S.; Ohgomori, T.; Hirano, K.; Uchimura, K.; Sakamoto, K.; Hirakawa, A.; Takeuchi, H.; Suzumura, A.; Ishiguro, N.; et al. Minocycline selectively inhibits M1 polarization of microglia. Cell Death Dis. 2013, 4, e525. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.; Cleeland, C.; Shi, Q.; Prasad, S.; Bokhari, R.; Orlowski, R. Minocycline to reduce cancer pain in patients with multiple myeloma: A phase II randomized trial. J. Clin. Oncol. 2017, 35, 10100. [Google Scholar] [CrossRef]
- Zhang, J.; Zheng, Y.; Luo, Y.; Du, Y.; Zhang, X.; Fu, J. Curcumin inhibits LPS-induced neuroinflammation by promoting microglial M2 polarization via TREM2/TLR4/NF-κB pathways in BV2 cells. Mol. Immunol. 2019, 116, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Kanda, H.; Kobayashi, K.; Yamanaka, H.; Okubo, M.; Noguchi, K. Microglial TNFα Induces COX2 and PGI2 Synthase Expression in Spinal Endothelial Cells during Neuropathic Pain. eNeuro 2017, 4. [Google Scholar] [CrossRef]
- Wojdasiewicz, P.; Poniatowski, Ł.A.; Kotela, A.; Deszczyński, J.; Kotela, I.; Szukiewicz, D. The Chemokine CX3CL1 (Fractalkine) and its Receptor CX3CR1: Occurrence and Potential Role in Osteoarthritis. Arch. Immunol. Ther. Exp. 2014, 62, 395–403. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Z.-C.; Li, L.-H.; Bian, C.; Yang, L.; Lv, N.; Zhang, Y.-Q. Involvement of NF-κB and the CX3CR1 Signaling Network in Mechanical Allodynia Induced by Tetanic Sciatic Stimulation. Neurosci. Bull. 2018, 34, 64–73. [Google Scholar] [CrossRef]
- Rawlings, J.S.; Rosler, K.M.; Harrison, D.A. The JAK/STAT signaling pathway. J. Cell Sci. 2004, 117, 1281–1283. [Google Scholar] [CrossRef] [Green Version]
- Dominguez, E.; Mauborgne, A.; Mallet, J.; Desclaux, M.; Pohl, M. SOCS3-mediated blockade of JAK/STAT3 signaling pathway reveals its major contribution to spinal cord neuroinflammation and mechanical allodynia after peripheral nerve injury. J. Neurosci. 2010, 30, 5754–5766. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dominguez, E.; Rivat, C.; Pommier, B.; Mauborgne, A.; Pohl, M. JAK/STAT3 pathway is activated in spinal cord microglia after peripheral nerve injury and contributes to neuropathic pain development in rat. J. Neurochem. 2008, 107, 50–60. [Google Scholar] [CrossRef] [PubMed]
- Tsuda, M.; Kohro, Y.; Yano, T.; Tsujikawa, T.; Kitano, J.; Tozaki-Saitoh, H.; Koyanagi, S.; Ohdo, S.; Ji, R.-R.; Salter, M.W.; et al. JAK-STAT3 pathway regulates spinal astrocyte proliferation and neuropathic pain maintenance in rats. Brain 2011, 134, 1127–1139. [Google Scholar] [CrossRef] [PubMed]
- Li, S.F.; Ouyang, B.S.; Zhao, X.; Wang, Y.P. Analgesic effect of AG490, a Janus kinase inhibitor, on oxaliplatin-induced acute neuropathic pain. Neural. Regen. Res. 2018, 13, 1471–1476. [Google Scholar] [CrossRef]
- Levy, D.E.; Darnell, J.E. STATs: Transcriptional control and biological impact. Nat. Rev. Mol. Cell Biol. 2002, 3, 651–662. [Google Scholar] [CrossRef]
- Kawashima, T.; Bao, Y.C.; Nomura, Y.; Moon, Y.; Tonozuka, Y.; Minoshima, Y.; Hatori, T.; Tsuchiya, A.; Kiyono, M.; Nosaka, T.; et al. Rac1 and a GTPase-activating protein, MgcRacGAP, are required for nuclear translocation of STAT transcription factors. J. Cell Biol. 2006, 175, 937–946. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salas, A.; Hernandez-Rocha, C.; Duijvestein, M.; Faubion, W.; McGovern, D.; Vermeire, S.; Vetrano, S.; Vande Casteele, N. JAK–STAT pathway targeting for the treatment of inflammatory bowel disease. Nat. Rev. Gastroenterol. Hepatol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Kiu, H.; Nicholson, S.E. Biology and significance of the JAK/STAT signalling pathways. Growth Factors 2012, 30, 88–106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zu, J.; Wang, Y.; Xu, G.; Zhuang, J.; Gong, H.; Yan, J. Curcumin improves the recovery of motor function and reduces spinal cord edema in a rat acute spinal cord injury model by inhibiting the JAK/STAT signaling pathway. Acta Histochem. 2014, 116, 1331–1336. [Google Scholar] [CrossRef] [PubMed]
- Penas, C.; Navarro, X. Epigenetic Modifications Associated to Neuroinflammation and Neuropathic Pain After Neural Trauma. Front. Cell. Neurosci. 2018, 12, 158. [Google Scholar] [CrossRef] [Green Version]
- Buchheit, T.; Van de Ven, T.; Shaw, A. Epigenetics and the transition from acute to chronic pain. Pain Med. 2012, 13, 1474–1490. [Google Scholar] [CrossRef] [PubMed]
- Boyanapalli, S.S.S.; Kong, A.-N.T. “Curcumin, the King of Spices”: Epigenetic Regulatory Mechanisms in the Prevention of Cancer, Neurological, and Inflammatory Diseases. Curr. Pharmacol. Rep. 2015, 1, 129–139. [Google Scholar] [CrossRef] [Green Version]
- Yun, J.-M.; Jialal, I.; Devaraj, S. Epigenetic regulation of high glucose-induced proinflammatory cytokine production in monocytes by curcumin. J. Nutr. Biochem. 2011, 22, 450–458. [Google Scholar] [CrossRef] [Green Version]
- Yuan, Z.; Syed, M.A.; Panchal, D.; Rogers, D.; Joo, M.; Sadikot, R.T. Curcumin mediated epigenetic modulation inhibits TREM-1 expression in response to lipopolysaccharide. Int. J. Biochem. Cell Biol. 2012, 44, 2032–2043. [Google Scholar] [CrossRef]
- Wu, B.; Yao, X.; Nie, X.; Xu, R. Epigenetic reactivation of RANK in glioblastoma cells by curcumin: Involvement of STAT3 inhibition. DNA Cell Biol. 2013, 32, 292–297. [Google Scholar] [CrossRef]
- Kichev, A.; Eede, P.; Gressens, P.; Thornton, C.; Hagberg, H. Implicating Receptor Activator of NF-κB (RANK)/RANK Ligand Signalling in Microglial Responses to Toll-Like Receptor Stimuli. Dev. Neurosci. 2017, 39, 192–206. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Tian, Y.; Wang, Z.-F.; Liu, S.-B.; Mi, W.-L.; Ma, H.-J.; Wu, G.-C.; Wang, J.; Yu, J.; Wang, Y.-Q. Involvement of the spinal NALP1 inflammasome in neuropathic pain and aspirin-triggered-15-epi-lipoxin A4 induced analgesia. Neuroscience 2013, 254, 230–240. [Google Scholar] [CrossRef] [PubMed]
- de Rivero Vaccari, J.P.; Bastien, D.; Yurcisin, G.; Pineau, I.; Dietrich, W.D.; De Koninck, Y.; Keane, R.W.; Lacroix, S. P2 × 4 receptors influence inflammasome activation after spinal cord injury. J. Neurosci. 2012, 32, 3058–3066. [Google Scholar] [CrossRef] [PubMed]
- Cordero, M.D. The inflammasome in fibromyalgia and CRPS: A microglial hypothesis? Nat. Rev. Rheumatol. 2015, 11, 630. [Google Scholar] [CrossRef] [PubMed]
- Khan, N.; Kuo, A.; Brockman, D.A.; Cooper, M.A.; Smith, M.T. Pharmacological inhibition of the NLRP3 inflammasome as a potential target for multiple sclerosis induced central neuropathic pain. Inflammopharmacology 2018, 26, 77–86. [Google Scholar] [CrossRef]
- Jiao, J.; Zhao, G.; Wang, Y.; Ren, P.; Wu, M. MCC950, a Selective Inhibitor of NLRP3 Inflammasome, Reduces the Inflammatory Response and Improves Neurological Outcomes in Mice Model of Spinal Cord Injury. Front. Mol. Biosci. 2020, 7, 37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yin, H.; Guo, Q.; Li, X.; Tang, T.; Li, C.; Wang, H.; Sun, Y.; Feng, Q.; Ma, C.; Gao, C.; et al. Curcumin Suppresses IL-1β Secretion and Prevents Inflammation through Inhibition of the NLRP3 Inflammasome. J. Immunol. 2018, 200, 2835–2846. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hasanzadeh, S.; Read, M.I.; Bland, A.R.; Majeed, M.; Jamialahmadi, T.; Sahebkar, A. Curcumin: An inflammasome silencer. Pharmacol. Res. 2020, 159, 104921. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Ren, W.; Zhao, F.; Han, Y.; Liu, C.; Jia, K. Curcumin amends Ca(2+) dysregulation in microglia by suppressing the activation of P2 × 7 receptor. Mol. Cell. Biochem. 2020, 465, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Pelegrin, P. P2 × 7 receptor and the NLRP3 inflammasome: Partners in crime. Biochem. Pharmacol. 2021, 187, 114385. [Google Scholar] [CrossRef] [PubMed]
- He, Q.; Jiang, L.; Man, S.; Wu, L.; Hu, Y.; Chen, W. Curcumin Reduces Neuronal Loss and Inhibits the NLRP3 Inflammasome Activation in an Epileptic Rat Model. Curr. Neurovasc. Res. 2018, 15, 186–192. [Google Scholar] [CrossRef]
- Zhang, W.; Guo, Y.; Han, W.; Yang, M.; Wen, L.; Wang, K.; Jiang, P. Curcumin relieves depressive-like behaviors via inhibition of the NLRP3 inflammasome and kynurenine pathway in rats suffering from chronic unpredictable mild stress. Int. Immunopharmacol. 2019, 67, 138–144. [Google Scholar] [CrossRef] [PubMed]
- Ji, H.; Tang, J.; Li, M.; Ren, J.; Zheng, N.; Wu, L. Curcumin-loaded solid lipid nanoparticles with Brij78 and TPGS improved in vivo oral bioavailability and in situ intestinal absorption of curcumin. Drug Deliv. 2016, 23, 459–470. [Google Scholar] [CrossRef] [PubMed]
- Tian, C.; Asghar, S.; Wu, Y.; Chen, Z.; Jin, X.; Yin, L.; Huang, L.; Ping, Q.; Xiao, Y. Improving intestinal absorption and oral bioavailability of curcumin via taurocholic acid-modified nanostructured lipid carriers. Int. J. Nanomed. 2017, 12, 7897–7911. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Ma, W.; Tu, P. The mechanism of self-assembled mixed micelles in improving curcumin oral absorption: In vitro and in vivo. Colloids Surf. B. Biointerfaces 2015, 133, 108–119. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Huang, Q. Improving the oral bioavailability of curcumin using novel organogel-based nanoemulsions. J. Agric. Food Chem. 2012, 60, 5373–5379. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Huang, Q. Investigation of the absorption mechanism of solubilized curcumin using Caco-2 cell monolayers. J. Agric. Food Chem. 2011, 59, 9120–9126. [Google Scholar] [CrossRef] [PubMed]
- Press, B.; Di Grandi, D. Permeability for intestinal absorption: Caco-2 assay and related issues. Curr. Drug Metab. 2008, 9, 893–900. [Google Scholar] [CrossRef]
- Metzler, M.; Pfeiffer, E.; Schulz, S.I.; Dempe, J.S. Curcumin uptake and metabolism. Biofactors 2013, 39, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Pan, M.H.; Huang, T.M.; Lin, J.K. Biotransformation of curcumin through reduction and glucuronidation in mice. Drug Metab. Dispos. 1999, 27, 486–494. [Google Scholar] [PubMed]
- Ireson, C.; Orr, S.; Jones, D.J.; Verschoyle, R.; Lim, C.K.; Luo, J.L.; Howells, L.; Plummer, S.; Jukes, R.; Williams, M.; et al. Characterization of metabolites of the chemopreventive agent curcumin in human and rat hepatocytes and in the rat in vivo, and evaluation of their ability to inhibit phorbol ester-induced prostaglandin E2 production. Cancer Res. 2001, 61, 1058–1064. [Google Scholar]
- Holder, G.M.; Plummer, J.L.; Ryan, A.J. The metabolism and excretion of curcumin (1,7-bis-(4-hydroxy-3-methoxyphenyl)-1,6-heptadiene-3,5-dione) in the rat. Xenobiotica 1978, 8, 761–768. [Google Scholar] [CrossRef] [PubMed]
- Marczylo, T.H.; Steward, W.P.; Gescher, A.J. Rapid analysis of curcumin and curcumin metabolites in rat biomatrices using a novel ultraperformance liquid chromatography (UPLC) method. J. Agric. Food Chem. 2009, 57, 797–803. [Google Scholar] [CrossRef]
- Burgos-Morón, E.; Calderón-Montaño, J.M.; Salvador, J.; Robles, A.; López-Lázaro, M. The dark side of curcumin. Int. J. Cancer 2010, 126, 1771–1775. [Google Scholar] [CrossRef] [PubMed]
- Wahlström, B.; Blennow, G. A study on the fate of curcumin in the rat. Acta Pharmacol. Toxicol. 1978, 43, 86–92. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.A.; Euden, S.A.; Platton, S.L.; Cooke, D.N.; Shafayat, A.; Hewitt, H.R.; Marczylo, T.H.; Morgan, B.; Hemingway, D.; Plummer, S.M.; et al. Phase I clinical trial of oral curcumin. Clin. Cancer Res. 2004, 10, 6847. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsai, Y.-M.; Chang-Liao, W.-L.; Chien, C.-F.; Lin, L.-C.; Tsai, T.-H. Effects of polymer molecular weight on relative oral bioavailability of curcumin. Int. J. Nanomed. 2012, 7, 2957–2966. [Google Scholar] [CrossRef] [Green Version]
- Ravindranath, V.; Chandrasekhara, N. Absorption and tissue distribution of curcumin in rats. Toxicology 1980, 16, 259–265. [Google Scholar] [CrossRef]
- Tian, C.; Asghar, S.; Wu, Y.; Kambere Amerigos, D.; Chen, Z.; Zhang, M.; Yin, L.; Huang, L.; Ping, Q.; Xiao, Y. N-acetyl-L-cysteine functionalized nanostructured lipid carrier for improving oral bioavailability of curcumin: Preparation, in vitro and in vivo evaluations. Drug Deliv. 2017, 24, 1605–1616. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xie, X.; Tao, Q.; Zou, Y.; Zhang, F.; Guo, M.; Wang, Y.; Wang, H.; Zhou, Q.; Yu, S. PLGA nanoparticles improve the oral bioavailability of curcumin in rats: Characterizations and mechanisms. J. Agric. Food Chem. 2011, 59, 9280–9289. [Google Scholar] [CrossRef]
- Onoue, S.; Takahashi, H.; Kawabata, Y.; Seto, Y.; Hatanaka, J.; Timmermann, B.; Yamada, S. Formulation design and photochemical studies on nanocrystal solid dispersion of curcumin with improved oral bioavailability. J. Pharm. Sci. 2010, 99, 1871–1881. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.-Y.; Lin, L.-C.; Tseng, T.-Y.; Wang, S.-C.; Tsai, T.-H. Oral bioavailability of curcumin in rat and the herbal analysis from Curcuma longa by LC-MS/MS. J. Chromatogr. B. Analyt. Technol. Biomed. Life Sci. 2007, 853, 183–189. [Google Scholar] [CrossRef]
- Tsai, Y.-M.; Jan, W.-C.; Chien, C.-F.; Lee, W.-C.; Lin, L.-C.; Tsai, T.-H. Optimised nano-formulation on the bioavailability of hydrophobic polyphenol, curcumin, in freely-moving rats. Food Chem. 2011, 127, 918–925. [Google Scholar] [CrossRef] [PubMed]
- Cheng, A.L.; Hsu, C.H.; Lin, J.K.; Hsu, M.M.; Ho, Y.F.; Shen, T.S.; Ko, J.Y.; Lin, J.T.; Lin, B.R.; Ming-Shiang, W.; et al. Phase I clinical trial of curcumin, a chemopreventive agent, in patients with high-risk or pre-malignant lesions. Anticancer. Res. 2001, 21, 2895–2900. [Google Scholar] [PubMed]
- Sharma, R.A.; McLelland, H.R.; Hill, K.A.; Ireson, C.R.; Euden, S.A.; Manson, M.M.; Pirmohamed, M.; Marnett, L.J.; Gescher, A.J.; Steward, W.P. Pharmacodynamic and pharmacokinetic study of oral Curcuma extract in patients with colorectal cancer. Clin. Cancer Res. 2001, 7, 1894–1900. [Google Scholar] [PubMed]
- Cuomo, J.; Appendino, G.; Dern, A.S.; Schneider, E.; McKinnon, T.P.; Brown, M.J.; Togni, S.; Dixon, B.M. Comparative absorption of a standardized curcuminoid mixture and its lecithin formulation. J. Nat. Prod. 2011, 74, 664–669. [Google Scholar] [CrossRef]
- Sasaki, H.; Sunagawa, Y.; Takahashi, K.; Imaizumi, A.; Fukuda, H.; Hashimoto, T.; Wada, H.; Katanasaka, Y.; Kakeya, H.; Fujita, M.; et al. Innovative preparation of curcumin for improved oral bioavailability. Biol. Pharm. Bull. 2011, 34, 660–665. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schiborr, C.; Kocher, A.; Behnam, D.; Jandasek, J.; Toelstede, S.; Frank, J. The oral bioavailability of curcumin from micronized powder and liquid micelles is significantly increased in healthy humans and differs between sexes. Mol. Nutr. Food Res. 2014, 58, 516–527. [Google Scholar] [CrossRef] [PubMed]
- Schiborr, C.; Eckert, G.P.; Rimbach, G.; Frank, J. A validated method for the quantification of curcumin in plasma and brain tissue by fast narrow-bore high-performance liquid chromatography with fluorescence detection. Anal. Bioanal. Chem. 2010, 397, 1917–1925. [Google Scholar] [CrossRef]
- Tsai, Y.M.; Chien, C.F.; Lin, L.C.; Tsai, T.H. Curcumin and its nano-formulation: The kinetics of tissue distribution and blood-brain barrier penetration. Int. J. Pharm. 2011, 416, 331–338. [Google Scholar] [CrossRef] [PubMed]
- Sorrenti, V.; Contarini, G.; Sut, S.; Dall’Acqua, S.; Confortin, F.; Pagetta, A.; Giusti, P.; Zusso, M. Curcumin Prevents Acute Neuroinflammation and Long-Term Memory Impairment Induced by Systemic Lipopolysaccharide in Mice. Front. Pharmacol. 2018, 9, 183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Varatharaj, A.; Galea, I. The blood-brain barrier in systemic inflammation. Brain. Behav. Immun. 2017, 60, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Brooks, T.A.; Hawkins, B.T.; Huber, J.D.; Egleton, R.D.; Davis, T.P. Chronic inflammatory pain leads to increased blood-brain barrier permeability and tight junction protein alterations. Am. J. Physiol. Heart Circ. Physiol. 2005, 289, H738–H743. [Google Scholar] [CrossRef] [Green Version]
- Cahill, L.S.; Laliberté, C.L.; Liu, X.J.; Bishop, J.; Nieman, B.J.; Mogil, J.S.; Sorge, R.E.; Jones, C.D.; Salter, M.W.; Henkelman, R.M. Quantifying blood-spinal cord barrier permeability after peripheral nerve injury in the living mouse. Mol. Pain 2014, 10, 60. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huber, J.D.; Hau, V.S.; Borg, L.; Campos, C.R.; Egleton, R.D.; Davis, T.P. Blood-brain barrier tight junctions are altered during a 72-h exposure to lambda-carrageenan-induced inflammatory pain. Am. J. Physiol. Heart Circ. Physiol. 2002, 283, H1531–H1537. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.; Ma, C.; Wu, Z.; Liang, H.; Yan, P.; Song, J.; Ma, N.; Zhao, Q. Enhanced Bioavailability and Anticancer Effect of Curcumin-Loaded Electrospun Nanofiber: In Vitro and In Vivo Study. Nanoscale Res. Lett. 2015, 10, 439. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, S.; Kesharwani, S.S.; Mathur, H.; Tyagi, M.; Bhat, G.J.; Tummala, H. Molecular complexation of curcumin with pH sensitive cationic copolymer enhances the aqueous solubility, stability and bioavailability of curcumin. Eur. J. Pharm. Sci. Off. J. Eur. Fed. Pharm. Sci. 2016, 82, 86–96. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Yang, X.; Wu, W.; Long, Z.; Xiao, H.; Luo, F.; Shen, Y.; Lin, Q. Elaboration of curcumin-loaded rice bran albumin nanoparticles formulation with increased in vitro bioactivity and in vivo bioavailability. Food Hydrocoll. 2018, 77, 834–842. [Google Scholar] [CrossRef]
- Baek, J.-S.; Cho, C.-W. Surface modification of solid lipid nanoparticles for oral delivery of curcumin: Improvement of bioavailability through enhanced cellular uptake, and lymphatic uptake. Eur. J. Pharm. Biopharm. Off. J. Arb. fur Pharm. Verfahr. e.V 2017, 117, 132–140. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, C.; Zhao, J.; Ding, Y.; Li, L. A cost-effective method to prepare curcumin nanosuspensions with enhanced oral bioavailability. J. Colloid Interface Sci. 2017, 485, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Wan, S.; Sun, Y.; Qi, X.; Tan, F. Improved bioavailability of poorly water-soluble drug curcumin in cellulose acetate solid dispersion. AAPS PharmSciTech 2012, 13, 159–166. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chaurasia, S.; Patel, R.R.; Chaubey, P.; Kumar, N.; Khan, G.; Mishra, B. Lipopolysaccharide based oral nanocarriers for the improvement of bioavailability and anticancer efficacy of curcumin. Carbohydr. Polym. 2015, 130, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Khalil, N.M.; do Nascimento, T.C.F.; Casa, D.M.; Dalmolin, L.F.; de Mattos, A.C.; Hoss, I.; Romano, M.A.; Mainardes, R.M. Pharmacokinetics of curcumin-loaded PLGA and PLGA-PEG blend nanoparticles after oral administration in rats. Colloids Surf. B. Biointerfaces 2013, 101, 353–360. [Google Scholar] [CrossRef] [PubMed]
- Peng, S.; Li, Z.; Zou, L.; Liu, W.; Liu, C.; McClements, D.J. Enhancement of Curcumin Bioavailability by Encapsulation in Sophorolipid-Coated Nanoparticles: An in Vitro and in Vivo Study. J. Agric. Food Chem. 2018, 66, 1488–1497. [Google Scholar] [CrossRef]
- Shukla, M.; Jaiswal, S.; Sharma, A.; Srivastava, P.K.; Arya, A.; Dwivedi, A.K.; Lal, J. A combination of complexation and self-nanoemulsifying drug delivery system for enhancing oral bioavailability and anticancer efficacy of curcumin. Drug Dev. Ind. Pharm. 2017, 43, 847–861. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Ahuja, A.; Ali, J.; Baboota, S. Curcumin-loaded lipid nanocarrier for improving bioavailability, stability and cytotoxicity against malignant glioma cells. Drug Deliv. 2016, 23, 214–229. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Polyakov, N.E.; Chistyachenko, Y.S.; Khvostov, M.V.; Frolova, T.S.; Tolstikova, T.G.; Dushkin, A.V.; Su, W. Preparation of curcumin self-micelle solid dispersion with enhanced bioavailability and cytotoxic activity by mechanochemistry. Drug Deliv. 2018, 25, 198–209. [Google Scholar] [CrossRef] [PubMed]
- Shaikh, J.; Ankola, D.D.; Beniwal, V.; Singh, D.; Kumar, M.N.V.R. Nanoparticle encapsulation improves oral bioavailability of curcumin by at least 9-fold when compared to curcumin administered with piperine as absorption enhancer. Eur. J. Pharm. Sci. Off. J. Eur. Fed. Pharm. Sci. 2009, 37, 223–230. [Google Scholar] [CrossRef] [PubMed]
- Joshi, R.P.; Negi, G.; Kumar, A.; Pawar, Y.B.; Munjal, B.; Bansal, A.K.; Sharma, S.S. SNEDDS curcumin formulation leads to enhanced protection from pain and functional deficits associated with diabetic neuropathy: An insight into its mechanism for neuroprotection. Nanomedicine 2013, 9, 776–785. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, C.C.C.; Mendonça, L.M.; Bergamaschi, M.M.; Queiroz, R.H.C.; Souza, G.E.P.; Antunes, L.M.G.; Freitas, L.A.P. Microparticles Containing Curcumin Solid Dispersion: Stability, Bioavailability and Anti-Inflammatory Activity. AAPS PharmSciTech 2016, 17, 252–261. [Google Scholar] [CrossRef] [Green Version]
- Hu, L.; Shi, Y.; Li, J.H.; Gao, N.; Ji, J.; Niu, F.; Chen, Q.; Yang, X.; Wang, S. Enhancement of Oral Bioavailability of Curcumin by a Novel Solid Dispersion System. AAPS PharmSciTech 2015, 16, 1327–1334. [Google Scholar] [CrossRef] [Green Version]
- Chang, M.-T.; Tsai, T.-R.; Lee, C.-Y.; Wei, Y.-S.; Chen, Y.-J.; Chen, C.-R.; Tzen, J.T.C. Elevating bioavailability of curcumin via encapsulation with a novel formulation of artificial oil bodies. J. Agric. Food Chem. 2013, 61, 9666–9671. [Google Scholar] [CrossRef] [PubMed]
- Gupta, N.K.; Dixit, V.K. Bioavailability enhancement of curcumin by complexation with phosphatidyl choline. J. Pharm. Sci. 2011, 100, 1987–1995. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Jia, Y.; Niu, F.; Jia, Z.; Yang, X.; Jiao, K. Preparation and enhancement of oral bioavailability of curcumin using microemulsions vehicle. J. Agric. Food Chem. 2012, 60, 7137–7141. [Google Scholar] [CrossRef] [PubMed]
- IM, K.; Maliakel, A.; Kumar, D.; Maliakel, B.; Kuttan, R. Improved blood–brain-barrier permeability and tissue distribution following the oral administration of a food-grade formulation of curcumin with fenugreek fibre. J. Funct. Foods 2015, 14, 215–225. [Google Scholar] [CrossRef]
- Sun, M.; Gao, Y.; Guo, C.; Cao, F.; Song, Z.; Xi, Y.; Yu, A.; Li, A.; Zhai, G. Enhancement of transport of curcumin to brain in mice by poly(n-butylcyanoacrylate) nanoparticle. J. Nanoparticle Res. 2010, 12, 3111–3122. [Google Scholar] [CrossRef]
- Dende, C.; Meena, J.; Nagarajan, P.; Nagaraj, V.A.; Panda, A.K.; Padmanaban, G. Nanocurcumin is superior to native curcumin in preventing degenerative changes in Experimental Cerebral Malaria. Sci. Rep. 2017, 7, 10062. [Google Scholar] [CrossRef] [Green Version]
- Mancuso, C.; Barone, E. Curcumin in clinical practice: Myth or reality? Trends Pharmacol. Sci. 2009, 30, 333–334. [Google Scholar] [CrossRef] [PubMed]
- Krupa, P.; Svobodova, B.; Dubisova, J.; Kubinova, S.; Jendelova, P.; Machova Urdzikova, L. Nano-formulated curcumin (LipodisqTM) modulates the local inflammatory response, reduces glial scar and preserves the white matter after spinal cord injury in rats. Neuropharmacology 2019, 155, 54–64. [Google Scholar] [CrossRef] [PubMed]
- Ganesan, P.; Kim, B.; Ramalaingam, P.; Karthivashan, G.; Revuri, V.; Park, S.; Kim, J.S.; Ko, Y.T.; Choi, D.-K. Antineuroinflammatory Activities and Neurotoxicological Assessment of Curcumin Loaded Solid Lipid Nanoparticles on LPS-Stimulated BV-2 Microglia Cell Models. Molecules 2019, 24, 1170. [Google Scholar] [CrossRef] [Green Version]
- Hu, X.; Huang, F.; Wang, Z. CaMKIIα mechanism for pain in multiple sclerosis. J. Pain 2014, 15, S46. [Google Scholar] [CrossRef]
- Wang, Y.; Luo, J.; Li, S.-Y. Nano-Curcumin Simultaneously Protects the Blood–Brain Barrier and Reduces M1 Microglial Activation During Cerebral Ischemia–Reperfusion Injury. ACS Appl. Mater. Interfaces 2019, 11, 3763–3770. [Google Scholar] [CrossRef] [PubMed]
- Ratnatilaka Na Bhuket, P.; El-Magboub, A.; Haworth, I.S.; Rojsitthisak, P. Enhancement of Curcumin Bioavailability Via the Prodrug Approach: Challenges and Prospects. Eur. J. Drug Metab. Pharmacokinet. 2017, 42, 341–353. [Google Scholar] [CrossRef] [PubMed]
- Panda, S.S.; Girgis, A.S.; Thomas, S.J.; Capito, J.E.; George, R.F.; Salman, A.; El-Manawaty, M.A.; Samir, A. Synthesis, pharmacological profile and 2D-QSAR studies of curcumin-amino acid conjugates as potential drug candidates. Eur. J. Med. Chem. 2020, 196, 112293. [Google Scholar] [CrossRef] [PubMed]
- Parvathy, K.S.; Negi, P.S.; Srinivas, P. Curcumin–amino acid conjugates: Synthesis, antioxidant and antimutagenic attributes. Food Chem. 2010, 120, 523–530. [Google Scholar] [CrossRef]
- Laali, K.K.; Zwarycz, A.T.; Beck, N.; Borosky, G.L.; Nukaya, M.; Kennedy, G.D. Curcumin conjugates of non-steroidal anti-inflammatory drugs: Synthesis, structures, anti-proliferative assays, computational docking, and inflammatory response. ChemistryOpen 2020, 9, 822–834. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Li, Y.; Yue, Y.; Zhang, K.; Chen, Q.; Wang, H.; Lu, Y.; Huang, M.-T.; Zheng, X.; Du, Z. Synthesis and biological evaluation of curcumin derivatives containing NSAIDs for their anti-inflammatory activity. Bioorg. Med. Chem. Lett. 2015, 25, 3044–3051. [Google Scholar] [CrossRef] [PubMed]
- Muangnoi, C.; Ratnatilaka Na Bhuket, P.; Jithavech, P.; Supasena, W.; Paraoan, L.; Patumraj, S.; Rojsitthisak, P. Curcumin diethyl disuccinate, a prodrug of curcumin, enhances anti-proliferative effect of curcumin against HepG2 cells via apoptosis induction. Sci. Rep. 2019, 9, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wongsrisakul, J.; Wichitnithad, W.; Rojsitthisak, P.; Towiwat, P. Antinociceptive effects of curcumin diethyl disuccinate in animal models. J. Health Res. 2010, 24, 175–180. [Google Scholar]
- De Paz-Campos, M.A.; Ortiz, M.I.; Chávez Piña, A.E.; Zazueta-Beltrán, L.; Castañeda-Hernández, G. Synergistic effect of the interaction between curcumin and diclofenac on the formalin test in rats. Phytomedicine 2014, 21, 1543–1548. [Google Scholar] [CrossRef] [PubMed]
- Leksiri, S.; Hasriadi; Dasuni Wasana, P.W.; Vajragupta, O.; Rojsitthisak, P.; Towiwat, P. Co-administration of Pregabalin and Curcumin Synergistically Decreases Pain-Like Behaviors in Acute Nociceptive Pain Murine Models. Molecules 2020, 25, 4172. [Google Scholar] [CrossRef] [PubMed]
- National Toxicology Program NTP toxicology and carcinogenesis studies of turmeric oleoresin (CAS No. 8024-37-1) (major component 79–85% curcumin, CAS No. 458-37-7) in F344/N rats and B6C3F1 mice (Feed Studies). Natl Toxicol Progr. Tech Rep Ser 1993, 427, 1–275.
- Eaton, J.E.; Nelson, K.M.; Gossard, A.A.; Carey, E.J.; Tabibian, J.H.; Lindor, K.D.; LaRusso, N.F. Efficacy and safety of curcumin in primary sclerosing cholangitis: An open label pilot study. Scand. J. Gastroenterol. 2019, 54, 633–639. [Google Scholar] [CrossRef] [PubMed]
- Lukefahr, A.L.; McEvoy, S.; Alfafara, C.; Funk, J.L. Drug-induced autoimmune hepatitis associated with turmeric dietary supplement use. BMJ Case Rep. 2018, 2018. [Google Scholar] [CrossRef]
- Watkins, L.R.; Hutchinson, M.R.; Ledeboer, A.; Wieseler-Frank, J.; Milligan, E.D.; Maier, S.F. Glia as the “bad guys”: Implications for improving clinical pain control and the clinical utility of opioids. Brain. Behav. Immun. 2007, 21, 131–146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Begum, A.N.; Jones, M.R.; Lim, G.P.; Morihara, T.; Kim, P.; Heath, D.D.; Rock, C.L.; Pruitt, M.A.; Yang, F.; Hudspeth, B.; et al. Curcumin structure-function, bioavailability, and efficacy in models of neuroinflammation and Alzheimer’s disease. J. Pharmacol. Exp. Ther. 2008, 326, 196–208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mátyus, P.; Chai, C.L.L. Metabolism-Activated Multitargeting (MAMUT): An Innovative Multitargeting Approach to Drug Design and Development. ChemMedChem 2016, 11, 1197–1198. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hasriadi; Dasuni Wasana, P.W.; Vajragupta, O.; Rojsitthisak, P.; Towiwat, P. Mechanistic Insight into the Effects of Curcumin on Neuroinflammation-Driven Chronic Pain. Pharmaceuticals 2021, 14, 777. https://doi.org/10.3390/ph14080777
Hasriadi, Dasuni Wasana PW, Vajragupta O, Rojsitthisak P, Towiwat P. Mechanistic Insight into the Effects of Curcumin on Neuroinflammation-Driven Chronic Pain. Pharmaceuticals. 2021; 14(8):777. https://doi.org/10.3390/ph14080777
Chicago/Turabian StyleHasriadi, Peththa Wadu Dasuni Wasana, Opa Vajragupta, Pornchai Rojsitthisak, and Pasarapa Towiwat. 2021. "Mechanistic Insight into the Effects of Curcumin on Neuroinflammation-Driven Chronic Pain" Pharmaceuticals 14, no. 8: 777. https://doi.org/10.3390/ph14080777
APA StyleHasriadi, Dasuni Wasana, P. W., Vajragupta, O., Rojsitthisak, P., & Towiwat, P. (2021). Mechanistic Insight into the Effects of Curcumin on Neuroinflammation-Driven Chronic Pain. Pharmaceuticals, 14(8), 777. https://doi.org/10.3390/ph14080777