Chemical Composition and Antimicrobial Activity of a New Olive Pomace Functional Ingredient
Abstract
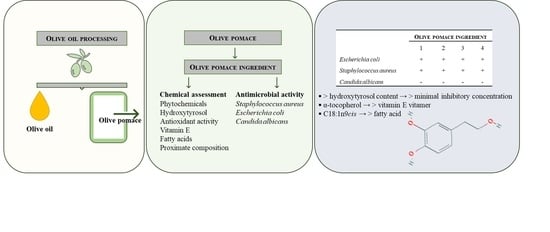
:1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. Chemicals and Reagents
3.2. Sampling and Preparation of the Olive Pomace Functional Ingredient
3.3. Total Fat
3.4. Total Protein
3.5. Ash
3.6. Phytochemicals and Antioxidant Activity Assessment
3.6.1. Total Phenolics
3.6.2. Total Flavonoids
3.6.3. Ferric-Reducing Antioxidant Power (FRAP)
3.6.4. 2,2-Diphenyl-1-Picrylhydrazyl Radical (DPPH•) Scavenging Ability (DPPH)
3.6.5. 2,2′-Azinobis(3-Ethylenbenzothiazoline-6-Sulfonic Acid) Cation Radical (ABTS•+) Scavenging Ability (ABTS)
3.7. Hydroxytyrosol Content Analysis by HPLC-DAD-FLD
3.8. Vitamin E Profile by HPLC-DAD-FLD
3.9. Fatty Acids Profile by GC-FID
3.10. pH
3.11. Antimicrobial Activity
3.11.1. Incorporation Method
3.11.2. Surface Spreading Method
3.11.3. Agar Diffusion Method
3.11.4. Microdilution Method
3.12. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Aguilar, C.N.; Ruiz, H.A.; Rios, A.R.; Chávez-González, M.; Sepúlveda, L.; Rodríguez-Jasso, R.M.; Loredo-Treviño, A.; Flores-Gallegos, A.C.; Govea-Salas, M.; Ascacio-Valdes, J.A. Emerging strategies for the development of food industries. Bioengineered 2019, 10, 522–537. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, K.; Yadav, A.N.; Kumar, V.; Vyas, P.; Dhaliwal, H.S. Food waste: A potential bioresource for extraction of nutraceuticals and bioactive compounds. Bioresour. Bioprocess 2017, 4, 1–14. [Google Scholar] [CrossRef] [Green Version]
- European Commission. Olive Oil—Detailed Information on the Market Situation, Price Developments, Balance Sheets, Production and Trade. 2020. Available online: https://ec.europa.eu/info/food-farming-fisheries/farming/facts-and-figures/markets/prices/price-monitoring-sector/plant-products/olive-oil_en (accessed on 26 March 2020).
- Di Giovacchino, L.; Preziuso, S.M.; Di Serio, M.G.; Mucciarella, M.R.; Di Loreto, G.; Lanza, B. Double extraction of olive oil in large oil mills of Southern Italy: Effects on extraction efficiency, oil quality, and economy of the process. Eur. J. Lipid Sci. Technol. 2017, 119, 1600161. [Google Scholar] [CrossRef]
- Nunes, M.A.; Costa, A.S.G.; Bessada, S.; Santos, J.; Puga, H.; Alves, R.C.; Freitas, V.; Oliveira, M.B.P.P. Olive pomace as a valuable source of bioactive compounds: A study regarding its lipid- and water-soluble components. Sci. Total Environ. 2018, 644, 229–236. [Google Scholar] [CrossRef]
- Gullón, P.; Gullón, B.; Astray, G.; Carpena, M.; Fraga-Corral, M.; Prieto, M.A.; Simal-Gandara, J. Valorization of by-products from olive oil industry and added-value applications for innovative eco-foods. Food Res. Int. 2020, 137, 109683. [Google Scholar] [CrossRef]
- Ng, K.R.; Lyu, X.; Mark, R.; Chen, W.N. Antimicrobial and antioxidant activities of phenolic metabolites from flavonoid-producing yeast: Potential as natural food preservatives. Food Chem. 2019, 270, 123–129. [Google Scholar] [CrossRef]
- de Carvalho, B.L.; Salgueiro, M.d.F.; Rita, P. Consumer sustainability consciousness: A five dimensional construct. Ecol. Indic. 2015, 58, 402–410. [Google Scholar] [CrossRef]
- Olszewska, M.A.; Gędas, A.; Simões, M. Antimicrobial polyphenol-rich extracts: Applications and limitations in the food industry. Food Res. Int. 2020, 134, 109214. [Google Scholar] [CrossRef]
- Korukluoglu, M.; Sahan, Y.; Yigit, A. Antifungal properties of olive leaf extracts and their phenolic compounds. J. Food Saf. 2008, 28, 76–87. [Google Scholar] [CrossRef]
- Gyawali, R.; Ibrahim, S.A. Natural products as antimicrobial agents. Food Control 2014, 46, 412–429. [Google Scholar] [CrossRef]
- Mbaveng, A.T.; Sandjo, L.P.; Tankeo, S.B.; Ndifor, A.R.; Pantaleon, A.; Nagdjui, B.T.; Kuete, V. Antibacterial activity of nineteen selected natural products against multi-drug resistant Gram-negative phenotypes. SpringerPlus 2015, 4, 823. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Diallinas, G.; Rafailidou, N.; Kalpaktsi, I.; Komianou, A.C.; Tsouvali, V.; Zantza, I.; Mikros, E.; Skaltsounis, A.L.; Kostakis, I.K. Hydroxytyrosol (HT) analogs act as potent antifungals by direct disruption of the fungal cell membrane. Front. Microbiol. 2018, 9, 2624. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xue, J.; Davidson, P.M.; Zhong, Q. Thymol nanoemulsified by whey protein-maltodextrin conjugates: The enhanced emulsifying capacity and antilisterial properties in milk by propylene glycol. J. Agric. Food Chem. 2013, 61, 12720–12726. [Google Scholar] [CrossRef]
- Peralbo-Molina, Á.; Priego-Capote, F.; de Castro, M.D.L. Tentative identification of phenolic compounds in olive pomace extracts using liquid chromatography-tandem mass spectrometry with a quadrupole-quadrupole-time-of-flight mass detector. J. Agric. Food Chem. 2012, 60, 11542–11550. [Google Scholar] [CrossRef]
- Cushnie, T.P.T.; Lamb, A.J. Recent advances in understanding the antibacterial properties of flavonoids. Int. J. Antimicrob. Agents 2011, 38, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Cueva, C.; Moreno-Arribas, M.V.; Martín-Álvarez, P.J.; Bills, G.; Vicente, M.F.; Basilio, A.; Rivas, C.L.; Requena, T.; Rodríguez, J.M.; Bartolomé, B. Antimicrobial activity of phenolic acids against commensal, probiotic and pathogenic bacteria. Res. Microbiol. 2010, 161, 372–382. [Google Scholar] [CrossRef]
- Stojković, D.; Petrović, J.; Soković, M.; Glamočlija, J.; Kukić-Marković, J.; Petrović, S. In situ antioxidant and antimicrobial activities of naturally occurring caffeic acid, p-coumaric acid and rutin, using food systems. J. Sci Food Agric. 2013, 93, 3205–3208. [Google Scholar] [CrossRef]
- Alghazeer, R.; Elmansori, A.; Sidati, M.; Gammoudi, F.; Azwai, S.; Naas, H.; Garbaj, A.; Eldaghayes, I. In vitro antibacterial activity of flavonoid extracts of two selected Libyan algae against multi-drug resistant bacteria isolated from food products. J Biosci. Med. 2017, 5, 26–48. [Google Scholar] [CrossRef] [Green Version]
- Yangui, T.; Dhouib, A.; Rhouma, A.; Sayadi, S. Potential of hydroxytyrosol-rich composition from olive mill wastewater as a natural disinfectant and its effect on seeds vigour response. Food Chem. 2009, 117, 1–8. [Google Scholar] [CrossRef]
- Guo, L.; Gong, S.; Wang, Y.; Sun, Q.; Duo, K.; Fei, P. Antibacterial activity of olive oil polyphenol extract against Salmonella Typhimurium and Staphylococcus aureus: Possible mechanisms. Foodborne Pathog. Dis. 2020, 17, 396–403. [Google Scholar] [CrossRef]
- Andrade, J.C.; Morais-Braga, M.F.B.; Guedes, G.M.M.; Tintino, S.R.; Freitas, M.A.; Menezes, I.R.A.; Coutinho, H.D.M. Enhancement of the antibiotic activity of aminoglycosides by alpha-tocopherol and other cholesterol derivates. Biomed. Pharmacother. 2014, 68, 1065–1069. [Google Scholar] [CrossRef] [PubMed]
- Yoon, B.K.; Jackman, J.A.; Valle-González, E.R.; Cho, N.-J. Antibacterial free fatty acids and monoglycerides: Biological activities, experimental testing, and therapeutic applications. Int. J. Mol. Sci. 2018, 19, 1114. [Google Scholar] [CrossRef] [Green Version]
- Zhou, J.; Velliou, E.; Hong, S.H. Investigating the effects of nisin and free fatty acid combined treatment on Listeria monocytogenes inactivation. LWT-Food Sci. Technol. 2020, 133, 110115. [Google Scholar] [CrossRef]
- Klen, T.J.; Vodopivec, B.M. The fate of olive fruit phenols during commercial olive oil processing: Traditional press versus continuous two- and three-phase centrifuge. LWT-Food Sci. Technol. 2012, 49, 267–274. [Google Scholar] [CrossRef]
- Klen, T.J.; Wondra, A.G.; Vrhovšek, U.; Sivilotti, P.; Vodopivec, B.M. Olive fruit phenols transfer, transformation, and partition trail during laboratory-scale olive oil processing. J. Agric. Food Chem. 2015, 63, 4570–4579. [Google Scholar] [CrossRef]
- Tintino, S.R.; Morais-Tintino, C.D.; Campina, F.F.; Pereira, R.L.; Costa, M.d.S.; Braga, M.F.B.M.; Limaverde, P.W.; Andrade, J.C.; Siqueira-Junior, J.P.; Coutinho, H.D.M.; et al. Action of cholecalciferol and alpha-tocopherol on Staphylococcus aureus efflux pumps. EXCLI J. 2016, 15, 315–322. [Google Scholar] [CrossRef] [PubMed]
- Bintsis, T. Foodborne pathogens. AIMS Microbiol. 2017, 3, 529–563. [Google Scholar] [CrossRef] [PubMed]
- Denayer, S.; Delbrassinne, L.; Nia, Y.; Botteldoorn, N. Foodborne outbreak investigation and molecular typing: High diversity of Staphylococcus aureus strains and importance of toxin detection. Toxins 2017, 9, 407. [Google Scholar] [CrossRef] [Green Version]
- Palmeira, J.D.; Haenni, M.; Metayer, V.; Madec, J.-Y.; Ferreira, H.M.N. Epidemic spread of IncI1/pST113 plasmid carrying the Extended-Spectrum Beta-Lactamase (ESBL) blaCTX-M-8 gene in Escherichia coli of Brazilian cattle. Vet. Microbiol 2020, 243, 108629. [Google Scholar] [CrossRef]
- Mota, R.; Pinto, M.; Palmeira, J.; Gonçalves, D.; Ferreira, H. Multidrug-resistant bacteria as intestinal colonizers and evolution of intestinal colonization in healthy university students in Portugal. Access Microbiol. 2020, 3, 000182. [Google Scholar] [CrossRef]
- Rajkowska, K.; Kunicka-Styczyńska, A. Typing and virulence factors of foodborne Candida spp. isolates. Int. J. Food Microbiol. 2018, 279, 57–63. [Google Scholar] [CrossRef]
- da Silva Dantas, A.; Lee, K.K.; Raziunaite, I.; Schaefer, K.; Wagener, J.; Yadav, B.; Gow, N.A.R. Cell biology of Candida albicans–host interactions. Curr. Opin. Microbiol. 2016, 34, 111–118. [Google Scholar] [CrossRef] [Green Version]
- Zorić, N.; Kopjar, N.; Bobnjarić, I.; Horvat, I.; Tomić, S.; Kosalec, I. Antifungal activity of oleuropein against Candida albicans—The in vitro study. Molecules 2016, 21, 1631. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, X.; Liu, Y.; Jia, Q.; LaMacchia, V.; O’Donoghue, K.; Huang, Z. A systems biology approach to investigate the antimicrobial activity of oleuropein. J. Ind. Microbiol. Biotechnol. 2016, 43, 1705–1717. [Google Scholar] [CrossRef] [PubMed]
- Medina-Martínez, M.S.; Truchado, P.; Castro-Ibáñez, I.; Allende, A. Antimicrobial activity of hydroxytyrosol: A current controversy. Biosci. Biotechnol. Biochem. 2016, 80, 801–810. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nunes, M.A.; Costa., A.S.G.; Oliveira, M.B.P.P.; Applicant: University of Porto. Foodstuff Composition Comprising a Derivate of Olive Pomace Patent International Application Number PCT/IB2018/060111, 2019.
- Manirakiza, P.; Covaci, A.; Schepens, P. Comparative study on total lipid determination using Soxhlet, Roese-Gottlieb, Bligh & Dyer, and modified Bligh & Dyer extraction methods. J. Food Compost. Anal. 2001, 14, 93–100. [Google Scholar] [CrossRef]
- Official Methods of Analysis, 19th ed.; AOAC International: Rockville, MD, USA, 2012.
- Tontisirin, K. Chapter 2: Methods of food analysis. In Food Energy: Methods of Analysis and Conversion Factors: Report of a Technical Workshop; Food and Agriculture Organization of the United Nations: Rome, Italy, 2003; pp. 7–17. [Google Scholar]
- Costa, A.S.G.; Alves, R.C.; Vinha, A.F.; Costa, E.; Costa, C.S.G.; Nunes, M.A.; Almeida, A.A.; Santos-Silva, A.; Oliveira, M.B.P.P. Nutritional, chemical and antioxidant/pro-oxidant profiles of silverskin, a coffee roasting by-product. Food Chem. 2018, 267, 28–35. [Google Scholar] [CrossRef]
- Seiquer, I.; Rueda, A.; Olalla, M.; Cabrera-Vique, C. Assessing the bioavailability of polyphenols and antioxidant properties of extra virgin argan oil by simulated digestion and Caco-2 cell assays. Comparative study with extra virgin olive oil. Food Chem. 2015, 188, 496–503. [Google Scholar] [CrossRef]
- Alves, R.C.; Casal, S.; Oliveira, M.B.P.P. Determination of vitamin E in coffee beans by HPLC using a micro-extraction method. Food Sci. Technol. Int. 2009, 15, 57–63. [Google Scholar] [CrossRef]
- ISO 12966-2:2011—Animal and vegetable fats and oils—Gas chromatography of fatty acid methyl esters—Part 2: Preparation of methyl esters of fatty acids.
- Almeida, D.; Pinto, D.; Santos, J.; Vinha, A.F.; Palmeira, J.; Ferreira, H.N.; Rodrigues, F.; Oliveira, M.B.P.P. Hardy kiwifruit leaves (Actinidia arguta): An extraordinary source of value-added compounds for food industry. Food Chem. 2018, 259, 113–121. [Google Scholar] [CrossRef]
- Palmeira, J.D.; Ferreira, S.B.; de Souza, J.H.; de Almeida, J.M.; Figueiredo, M.C.; Pequeno, A.S.; Arruda, T.A.; Antunes, R.M.P.; Catão, R.M.R. Evaluation of the antimicrobial activity in vitro and determination of minimal the inhibitory concentration of hidroalcoholicoc extracts of angico in strains Staphylococcus aureus. Br. J. Clin. Anal. 2010, 42, 33–37. [Google Scholar]

| Portugal Regions | Olive Pomace Geographical Origin | Predominant Olive Varieties |
|---|---|---|
| Northeast region (Trás-Os-Montes) | Alfândega da Fé | Cobrançosa |
| Madural | ||
| Verdeal Transmontana | ||
| Cordovil | ||
| Valpaços | Madural | |
| Cordovil | ||
| South (Alentejo) | Beja | Cobrançosa |
| Ferreira do Alentejo | Arbosana |
| Phytochemicals | Antioxidant Activity | ||||||
|---|---|---|---|---|---|---|---|
| Samples | TPC | TF | Hydroxytyrosol | FRAP | DPPH | ABTS | |
| g GAE/100 g | g HE/100 g | g CE/100 g | mg/100 g | g FSE/100 g | g TE/100 g | g TE/100 g | |
| O1 | 3.49 ± 0.28 a | 2.63 ± 0.29 a | 2.47 ± 0.29 b | 220 ± 20.57 a | 2.76 ± 0.52 a | 0.6 ± 0.07 b | 1.13 ± 0.07 a |
| O2 | 3.05 ± 0.07 b | 2.34 ± 0.12 b | 2.29 ± 0.12 b | 122.39 ± 1.92 c | 2.46 ± 0.13 a | 0.96 ± 0.21 a | 0.85 ± 0.03 b |
| O3 | 3.83 ± 0.2 a | 2.8 ± 0.11 a | 3.17 ± 0.11 a | 172.7 ± 2.09 b | 2.76 ± 0.16 a | 0.91 ± 0.05 a | 1.1 ± 0.1 a |
| O4 | 3.06 ± 0.23 b | 2.23 ± 0.2 b | 1.96 ± 0.09 c | 63.33 ± 3.59 d | 2.65 ± 0.51 a | 0.68 ± 0.07 b | 0.8 ± 0.04 b |
| Olive Pomace Extracts | ||||||
|---|---|---|---|---|---|---|
| Microorganism | Strain | Type | O1 | O2 | O3 | O4 |
| Escherichia coli | ATCC® 25922 | Gram-negative | + | + | + | + |
| Staphylococcus aureus | ATCC® 25923 | Gram-positive | + | + | + | + |
| Candida albicans | ATCC® 10231 | Yeast | − | − | − | − |
| O1 | O2 | O3 | O4 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Microorganism | E. coli | S. aureus | E. coli | S. aureus | E. coli | S. aureus | E. coli | S. aureus | |
| Method | Incorporation (IZ mm) | 10 | 15 | 10 | 13 | 12 | 14 | 10 | 13 |
| Surface spreading (IZ mm) | 10 | 15 | 10 | 14 | 9 | 14 | 0 | 12 | |
| Disk diffusion (IZ mm) | 0 | 8 | 0 | 8 | 0 | 8 | 0 | 7 | |
| MIC (mg/mL) | 62.5 | 31.25 | 125.0 | 62.5 | 125.0 | 31.25 | 125 | 125 | |
| O1 | O2 | O3 | O4 | |
|---|---|---|---|---|
| Total fat (g/100 g) | 5.55 ± 0.02 b | 10.54 ± 1.99 a | 4.62 ± 0.24 b | 5.86 ± 0.06 b |
| Fatty acids (relative %) | ||||
| C16:0 (Palmitic) | 11.77 ± 0.0 c | 11.59 ± 0.04 d | 13.22 ± 0.03 b | 14.35 ± 0.02 a |
| C16:1 (Palmitoleic) | 0.74 ± 0.01 c | 0.62 ± 0.02 d | 0.84 ± 0.05 b | 1.28 ± 0.01 a |
| C17:0 (Heptadecanoic) | 0.19 ± 0.00 a | 0.16 ± 0.00 b | 0.12 ± 0.01 d | 0.14 ± 0.00 c |
| C18:0 (Stearic) | 3.16 ± 0.01 c | 3.65 ± 0.01 a | 3.44 ± 0.02 b | 2.28 ± 0.01 d |
| C18:1n9cis (Oleic) | 72.94 ± 0.03 a | 71.7 ± 0.05 c | 71.82 ± 0.05 b | 71.78 ± 0.03 b |
| C18:2n6cis (Linoleic) | 9.44 ± 0.03 b | 10.48 ± 0.02 a | 8.75 ± 0.02 c | 8.4 ± 0.02 d |
| C20:0 (Arachidic) | 0.43 ± 0.01 b | 0.44 ± 0.02 ab | 0.45 ± 0.02 ab | 0.47 ± 0.01 a |
| C18:3n3 (α-Linolenic) | 0.83 ± 0.01 b | 0.9 ± 0.02 a | 0.9 ± 0.01 a | 0.72 ± 0.00 c |
| C20:1n9 (cis-11-Eicosenoic) | 0.28 ± 0.01 a | 0.25 ± 0.01 b | 0.22 ± 0.01 c | 0.3 ± 0.01 a |
| C22:0 (Behenic) | 0.16 ± 0.00 b | 0.15 ± 0.00 b | 0.16 ± 0.01 b | 0.21 ± 0.01 a |
| C24:0 (Lignoceric) | 0.06 ± 0.01 b | 0.06 ± 0.00 b | 0.07 ± 0.01 a | 0.08 ± 0.00 a |
| Total vitamin E (mg/100 g) | 1.83 ± 0.01 a | 1.99 ± 0.44 a | 0.87 ± 0.06 b | 2.25 ± 0.17 a |
| α-Tocopherol | 1.69 ± 0.01 a | 1.78 ± 0.42 a | 0.77 ± 0.06 b | 1.96 ± 0.15 a |
| α-Tocotrienol | 0.04 ± 0.00 c | 0.07 ± 0.00 b | 0.04 ± 0.00 c | 0.21 ± 0.01 a |
| β-Tocopherol | 0.03 ± 0.00 c | 0.05 ± 0.00 a | 0.02 ± 0.00 d | 0.04 ± 0.00 b |
| γ-Tocopherol | 0.07 ± 0.00 b | 0.09 ± 0.01 a | 0.04 ± 0.00 c | 0.04 ± 0.00 c |
| Total protein (g/100 g) | 0.88 ± 0.01 c | 0.88 ± 0.03 c | 3.16 ± 0.03 b | 4.44 ± 0.01 a |
| Ash (g/100 g) | 11.25 ± 0.65 b | 10.63 ± 1.16 b | 9.93 ± 0.30 b | 16.68 ± 0.18 a |
| pH | 5.23 ± 0.02 c | 5.36 ± 0.04 b | 5.26 ± 0.01 c | 5.63 ± 0.01 a |
| Compounds | Proposed Mechanism | References |
|---|---|---|
| Phenolics | Disruption of the membranes structure and leakage of the cellular components Hydroxyl groups promote the delocalization of electrons, leading to the reduction of the gradient across membranes Reduction of the redox potential of the growth medium, leading to microbial growth constraints | [14,17,18] |
| Flavonoids | Inactivation of the microbial adhesion, enzymes and cell envelope transport proteins Disruption of microbial membranes (lipophilic flavonoids) Perforation and/or a reduction of the membrane fluidity Inhibition of nucleic acid synthesis, energy metabolism and cell membrane synthesis | [16,19] |
| Hydroxytyrosol | Capability of chelating transition metals, reducing the reactivity of iron and copper by forming an inert metal–ligand complex, which decreases the bioavailability for bacterial growth Reduction of intracellular ATP concentrations Cell membrane depolarization Reduction of the bacterial protein content | [20,21] |
| Vitamin E (α-tocopherol) | Damage in the cell membrane affecting the essential components for the integrity of the membrane (reduction in membrane potential and loss of ions, cytochrome C, proteins and radicals, followed by the collapse of proton pumps and decrease in ATP), increasing the membrane permeability Interaction with the lipid bilayer of the bacteria cell membrane modifying the respiratory chain and energy production Capacity of acting in the cell envelope resulting in an imbalance in the fluid mosaic nature of the bacterial membrane | [22] |
| Fatty acids | Disruption of the electron transport chain by binding to electron carriers Leakage of cell metabolites via cell lysis Inhibition of nutrient uptake Formation of peroxidation/auto-oxidation products resulting in cell deactivation | [23,24] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nunes, M.A.; Palmeira, J.D.; Melo, D.; Machado, S.; Lobo, J.C.; Costa, A.S.G.; Alves, R.C.; Ferreira, H.; Oliveira, M.B.P.P. Chemical Composition and Antimicrobial Activity of a New Olive Pomace Functional Ingredient. Pharmaceuticals 2021, 14, 913. https://doi.org/10.3390/ph14090913
Nunes MA, Palmeira JD, Melo D, Machado S, Lobo JC, Costa ASG, Alves RC, Ferreira H, Oliveira MBPP. Chemical Composition and Antimicrobial Activity of a New Olive Pomace Functional Ingredient. Pharmaceuticals. 2021; 14(9):913. https://doi.org/10.3390/ph14090913
Chicago/Turabian StyleNunes, Maria Antónia, Josman Dantas Palmeira, Diana Melo, Susana Machado, Joana Correia Lobo, Anabela Sílvia Guedes Costa, Rita Carneiro Alves, Helena Ferreira, and Maria Beatriz Prior Pinto Oliveira. 2021. "Chemical Composition and Antimicrobial Activity of a New Olive Pomace Functional Ingredient" Pharmaceuticals 14, no. 9: 913. https://doi.org/10.3390/ph14090913
APA StyleNunes, M. A., Palmeira, J. D., Melo, D., Machado, S., Lobo, J. C., Costa, A. S. G., Alves, R. C., Ferreira, H., & Oliveira, M. B. P. P. (2021). Chemical Composition and Antimicrobial Activity of a New Olive Pomace Functional Ingredient. Pharmaceuticals, 14(9), 913. https://doi.org/10.3390/ph14090913