Expanding Theranostic Radiopharmaceuticals for Tumor Diagnosis and Therapy
Abstract
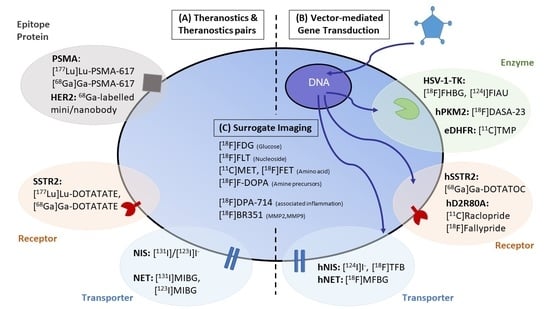
:1. Introduction
2. Direct Visualization of Target Expression and Therapy Response
3. Theranostic Pairs
3.1. Thyroid Cancer
3.2. Neuroendocrine Tumors
3.2.1. Norepinephrine Transporter (NET)
3.2.2. Somatostatin Receptors
3.3. Prostate Cancer
3.4. Alternative Approaches following the Classical Theranostic Approach
3.4.1. Human Epidermal Growth Factor Receptor Type 2
3.4.2. Fibroblast Activating Protein
3.4.3. Chemokine Receptor C-X-CR-4
4. Theranostics Using Gene and Cell-Based Therapy
4.1. Tools for Gene and Cell-Based Therapy
4.2. Gene and Cell-Based Therapy for Theranostic Applications
4.2.1. Enzyme Gene Reporter: HSV-1-tk Imaging Using [18F]FHBG or [124I]FIAU

4.2.2. Receptor Gene Reporter: HSSTR2 Imaging Using [68Ga]Ga-DOTATOC, [90Y]Y-DOTATATE or [177Lu]Lu-DOTATATE
4.2.3. Transporter Gene Reporter: Human Sodium/Iodide Symporter (hNIS): [124I]Iodide and [18F]TFB
4.2.4. Transporter Gene Reporter: Human Norepinephrine Transporter (hNET): [18F]MFBG
4.3. Surrogate Imaging
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Drude, N.; Tienken, L.; Mottaghy, F.M. Theranostic and nanotheranostic probes in nuclear medicine. Methods 2017, 130, 14–22. [Google Scholar] [CrossRef] [PubMed]
- Navalkissoor, S.; Grossman, A. Targeted Alpha particle therapy for Neuroendocrine tumours: The next generation of peptide receptor radionuclide therapy. Neuroendocrinology 2018, 108, 256–264. [Google Scholar] [CrossRef]
- Ballal, S.; Yadav, M.P.; Bal, C.; Sahoo, R.K.; Tripathi, M. Broadening horizons with 225Ac-DOTATATE targeted alpha therapy for gastroenteropancreatic neuroendocrine tumour patients stable or refractory to 177Lu-DOTATATE PRRT: First clinical experience on the efficacy and safety. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 934–946. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Kulkarni, H.R.; Baum, R.P. Peptide receptor radionuclide therapy using 225Ac-DOTATOC achieves partial remission in a patient with progressive neuroendocrine liver metastases after repeated β-emitter peptide receptor radionuclide therapy. Clin. Nucl. Med. 2020, 45, 241–243. [Google Scholar] [CrossRef]
- Kratochwil, C.; Apostolidis, L.; Rathke, H.; Apostolidis, C.; Bicu, F.; Bruchertseifer, F.; Choyke, P.L.; Haberkorn, U.; Giesel, F.L.; Morgenstern, A. Dosing 225Ac-DOTATOC in patients with somatostatin-receptor-positive solid tumors: 5-year follow-up of hematological and renal toxicity. Eur. J. Nucl. Med. Mol. Imaging 2021. [Google Scholar] [CrossRef]
- Jacobs, A.H.; Schelhaas, S.; Viel, T.; Waerzeggers, Y.; Winkeler, A.; Zinnhardt, B.; Gelovani, J. Imaging of Gene and Cell-Based Therapies: Basis and Clinical Trials; Elsevier: Amsterdam, The Netherlands, 2021; pp. 1539–1587. [Google Scholar]
- Lecocq, Q.; De Vlaeminck, Y.; Hanssens, H.; D’Huyvetter, M.; Raes, G.; Goyvaerts, C.; Keyaerts, M.; Devoogdt, N.; Breckpot, K. Theranostics in immuno-oncology using nanobody derivatives. Theranostics 2019, 9, 7772–7791. [Google Scholar] [CrossRef] [PubMed]
- Luster, M.; Clarke, S.E.; Dietlein, M.; Lassmann, M.; Lind, P.; Oyen, W.J.G.; Tennvall, J.; Bombardieri, E. Guidelines for radioiodine therapy of differentiated thyroid cancer. Eur. J. Nucl. Med. Mol. Imaging 2008, 35, 1941–1959. [Google Scholar] [CrossRef]
- Ballinger, J.R. Theranostic radiopharmaceuticals: Established agents in current use. Br. J. Radiol. 2018, 91, 20170969. [Google Scholar] [CrossRef]
- Marin, J.F.G.; Nunes, R.F.; Coutinho, A.M.; Zaniboni, E.C.; Costa, L.B.; Barbosa, F.G.; Queiroz, M.A.; Cerri, G.G.; Buchpiguel, C.A. Theranostics in nuclear medicine: Emerging and re-emerging integrated imaging and therapies in the era of precision oncology. Radiographics 2020, 40, 1715–1740. [Google Scholar] [CrossRef]
- Reiners, C.; Hänscheid, H.; Luster, M.; Lassmann, M.; Verburg, F.A. Radioiodine for remnant ablation and therapy of metastatic disease. Nat. Rev. Endocrinol. 2011, 7, 589–595. [Google Scholar] [CrossRef] [PubMed]
- Zilioli, V.; Peli, A.; Panarotto, M.B.; Magri, G.; Alkraisheh, A.; Wiefels, C.; Rodella, C.; Giubbini, R. Differentiated thyroid carcinoma: Incremental diagnostic value of 131I SPECT/CT over planar whole body scan after radioiodine therapy. Endocrine 2016, 56, 551–559. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qaim, S.M.; Scholten, B.; Neumaier, B. New developments in the production of theranostic pairs of radionuclides. J. Radioanal. Nucl. Chem. 2018, 318, 1493–1509. [Google Scholar] [CrossRef] [Green Version]
- Rösch, F.; Herzog, H.; Qaim, S.M. The beginning and development of the theranostic approach in nuclear medicine, as exemplified by the radionuclide pair 86Y and 90Y. Pharmaceuticals 2017, 10, 56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Herzog, H.; Rösch, F.; Stöcklin, G.; Lueders, C.; Qaim, S.M.; Feinendegen, E.L. Measurement of pharmacokinetics of yttrium-86 radiopharmaceuticals with PET and radiation dose calculation of analogous yttrium-90 radiotherapeutics. J. Nucl. Med. 1993, 34, 2222–2226. [Google Scholar]
- Sgouros, G.; Bodei, L.; McDevitt, M.R.; Nedrow, J.R. Radiopharmaceutical therapy in cancer: Clinical advances and challenges. Nat. Rev. Drug Discov. 2020, 19, 589–608. [Google Scholar] [CrossRef]
- Kratochwil, C.; Giesel, F.L.; Stefanova, M.; Benešová, M.; Bronzel, M.; Afshar-Oromieh, A.; Mier, W.; Eder, M.; Kopka, K.; Haberkorn, U. PSMA-targeted radionuclide therapy of metastatic castration-resistant prostate cancer with 177Lu-labeled PSMA-617. J. Nucl. Med. 2016, 57, 1170–1176. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tworowska, I.; Stallons, T.; Saidi, A.; Wagh, N.; Rojas-Quijano, F.; Jurek, P.; Kiefer, G.; Torgue, J.; Delpassand, E. Pb 203-AR-RMX conjugates for image-guided TAT of neuroendocrine tumors (NETs). In Proceedings of the Endocrinology; American Association for Cancer Research, Washington, DC, USA, 1–5 April 2017; Volume 77, p. LB-259. [Google Scholar]
- Dos Santos, J.C.; Schäfer, M.; Bauder-Wüst, U.; Lehnert, W.; Leotta, K.; Morgenstern, A.; Kopka, K.; Haberkorn, U.; Mier, W.; Kratochwil, C. Development and dosimetry of 203Pb/212Pb-labelled PSMA ligands: Bringing “the lead” into PSMA-targeted alpha therapy? Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 1081–1091. [Google Scholar] [CrossRef] [Green Version]
- Sheikh, A.; Polack, B.; Rodriguez, Y.; Kuker, R. Nuclear molecular and theranostic imaging for differentiated thyroid cancer. Mol. Imaging Radionucl. Ther. 2017, 26, 50–65. [Google Scholar] [CrossRef] [PubMed]
- Bombardieri, E.; Giammarile, F.; Aktolun, C.; Baum, R.P.; Delaloye, A.B.; Maffioli, L.; Moncayo, R.; Mortelmans, L.; Pepe, G.; Reske, S.N.; et al. 131I/123I-Metaiodobenzylguanidine (mIBG) scintigraphy: Procedure guidelines for tumour imaging. Eur. J. Nucl. Med. Mol. Imaging 2010, 37, 2436–2446. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, J. Targeting uptake transporters for cancer imaging and treatment. Acta Pharm. Sin. B 2019, 10, 79–90. [Google Scholar] [CrossRef]
- Pandit-Taskar, N.; Modak, S. Norepinephrine transporter as a target for imaging and therapy. J. Nucl. Med. 2017, 58, 39S–53S. [Google Scholar] [CrossRef] [Green Version]
- Pryma, D.A.; Chin, B.B.; Noto, R.B.; Dillon, J.S.; Perkins, S.; Solnes, L.; Kostakoglu, L.; Serafini, A.N.; Pampaloni, M.H.; Jensen, J.; et al. Efficacy and safety of high-specific-activity 131I-MIBG therapy in patients with advanced pheochromocytoma or paraganglioma. J. Nucl. Med. 2019, 60, 623–630. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Noto, R.B.; Pryma, A.D.; Jensen, J.; Lin, T.; Stambler, N.; Strack, T.; Wong, V.; Goldsmith, S.J. Phase 1 study of high-specific-activity I-131 MIBG for metastatic and/or recurrent pheochromocytoma or paraganglioma. J. Clin. Endocrinol. Metab. 2018, 103, 213–220. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kotecka-Blicharz, A.; Hasse-Lazar, K.; Handkiewicz-Junak, D.; Gawlik, T.; Pawlaczek, A.; Oczko-Wojciechowska, M.; Michalik, B.; Szpak, S.; Krajewska, J.; Jurecka-Lubieniecka, B.; et al. Terapia radioizotopowa 131-MIBG złośliwych guzów chromochłonnych i przyzwojaków—Badanie jednoośrodkowe. Endokrynol. Pol. 2018, 69, 246–251. [Google Scholar] [CrossRef]
- Schmidt, M.; Simon, T.; Hero, B.; Schicha, H.; Berthold, F. The prognostic impact of functional imaging with 123I-mIBG in patients with stage 4 neuroblastoma >1 year of age on a high-risk treatment protocol: Results of the German neuroblastoma trial NB97. Eur. J. Cancer 2008, 44, 1552–1558. [Google Scholar] [CrossRef]
- Vik, T.; Pfluger, T.; Kadota, R.; Castel, V.; Tulchinsky, M.; Farto, J.A.; Heiba, S.; Serafini, A.; Tumeh, S.; Khutoryansky, N.; et al. 123I-mIBG scintigraphy in patients with known or suspected neuroblastoma: Results from a prospective multicenter trial. Pediatr. Blood Cancer 2009, 52, 784–790. [Google Scholar] [CrossRef]
- Wiseman, G.A.; Pacak, K.; O’Dorisio, M.S.; Neumann, D.R.; Waxman, A.D.; Mankoff, D.A.; Heiba, S.I.; Serafini, A.N.; Tumeh, S.S.; Khutoryansky, N.; et al. Usefulness of 123I-MIBG scintigraphy in the evaluation of patients with known or suspected primary or metastatic pheochromocytoma or paraganglioma: Results from a prospective multicenter trial. J. Nucl. Med. 2009, 50, 1448–1454. [Google Scholar] [CrossRef] [Green Version]
- Pandit-Taskar, N.; Zanzonico, P.B.; Staton, K.D.; Carrasquillo, J.; Reidy-Lagunes, D.; Lyashchenko, S.K.; Burnazi, E.; Zhang, H.; Lewis, J.S.; Blasberg, R.; et al. Biodistribution and dosimetry of 18F-Meta-Fluorobenzylguanidine: A first-in-human PET/CT imaging study of patients with neuroendocrine malignancies. J. Nucl. Med. 2018, 59, 147–153. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poot, A.J.; Lam, M.G.E.H.; Van Noesel, M.M. The current status and future potential of theranostics to diagnose and treat childhood cancer. Front. Oncol. 2020, 10, 578286. [Google Scholar] [CrossRef]
- Bodei, L.; Weber, W.A. Somatostatin receptor imaging of neuroendocrine tumors: From agonists to antagonists. J. Nucl. Med. 2018, 59, 907–908. [Google Scholar] [CrossRef] [Green Version]
- Eychenne, R.; Bouvry, C.; Bourgeois, M.; Loyer, P.; Benoist, E.; Lepareur, N. Overview of radiolabeled somatostatin analogs for cancer imaging and therapy. Molecules 2020, 25, 4012. [Google Scholar] [CrossRef]
- Xu, C.; Zhang, H. Somatostatin receptor based imaging and radionuclide therapy. BioMed Res. Int. 2015, 2015, 917968. [Google Scholar] [CrossRef] [PubMed]
- Yordanova, A.; Eppard, E.; Kürpig, S.; Bundschuh, R.A.; Schönberger, S.; Gonzalez-Carmona, M.; Feldmann, G.; Ahmadzadehfar, H.; Essler, M. Theranostics in nuclear medicine practice. Onco. Targets Ther. 2017, 10, 4821–4828. [Google Scholar] [CrossRef] [Green Version]
- Deppen, S.A.; Liu, E.; Blume, J.D.; Clanton, J.; Shi, C.; Jones-Jackson, L.B.; Lakhani, V.; Baum, R.P.; Berlin, J.; Smith, G.T.; et al. Safety and efficacy of 68Ga-DOTATATE PET/CT for diagnosis, staging, and treatment management of neuroendocrine tumors. J. Nucl. Med. 2016, 57, 708–714. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Desai, H.; Borges-Neto, S.; Wong, T.Z. Molecular imaging and therapy for neuroendocrine tumors. Curr. Treat. Options Oncol. 2019, 20, 78. [Google Scholar] [CrossRef] [PubMed]
- Poeppel, T.D.; Binse, I.; Petersenn, S.; Lahner, H.; Schott, M.; Antoch, G.; Brandau, W.; Bockisch, A.; Boy, C. 68Ga-DOTATOC versus 68Ga-DOTATATE PET/CT in functional imaging of neuroendocrine tumors. J. Nucl. Med. 2011, 52, 1864–1870. [Google Scholar] [CrossRef] [Green Version]
- Hennrich, U.; Kopka, K. Lutathera®: The first FDA- and EMA-approved radiopharmaceutical for peptide receptor radionuclide therapy. Pharmaceuticals 2019, 12, 114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pencharz, D.; Gnanasegaran, G.; Navalkissoor, S. Theranostics in neuroendocrine tumours: Somatostatin receptor imaging and therapy. Br. J. Radiol. 2018, 91, 20180108. [Google Scholar] [CrossRef]
- Strosberg, J.; El-Haddad, G.; Wolin, E.; Hendifar, A.; Yao, J.; Chasen, B.; Mittra, E.; Kunz, P.L.; Kulke, M.H.; Jacene, H.; et al. Phase 3 trial of 177Lu-dotatate for midgut neuroendocrine tumors. N. Engl. J. Med. 2017, 376, 125–135. [Google Scholar] [CrossRef]
- Strosberg, J.; Wolin, E.; Chasen, B.; Kulke, M.; Bushnell, D.; Caplin, M.; Baum, R.P.; Kunz, P.; Hobday, T.; Hendifar, A.; et al. Health-related quality of life in patients with progressive midgut neuroendocrine tumors treated with 177Lu-dotatate in the phase III NETTER-1 trial. J. Clin. Oncol. 2018, 36, 2578–2584. [Google Scholar] [CrossRef]
- Strosberg, J.R.; Caplin, E.M.; Kunz, P.L.; Ruszniewski, P.B.; Bodei, L.; Hendifar, A.E.; Mittra, E.; Wolin, E.M.; Yao, J.C.; Pavel, E.M.; et al. Final overall survival in the phase 3 NETTER-1 study of lutetium-177-DOTATATE in patients with midgut neuroendocrine tumors. J. Clin. Oncol. 2021, 39, 4112. [Google Scholar] [CrossRef]
- Delpassand, E.; Tworowska, I.; Esfandiari, R.; Torgue, J.; Hurt, J.D.; Nunez, R. Phase I dose-escalation study of AlphaMedix for targeted-alpha-emitter therapy of PRRT-naive neuroendocrine patients. J. Clin. Oncol. 2021, 39, 4117. [Google Scholar] [CrossRef]
- Zechmann, C.M.; Afshar-Oromieh, A.; Armor, T.; Stubbs, J.B.; Mier, W.; Hadaschik, B.; Joyal, J.; Kopka, K.; Debus, J.; Babich, J.W.; et al. Radiation dosimetry and first therapy results with a 124I/131I-labeled small molecule (MIP-1095) targeting PSMA for prostate cancer therapy. Eur. J. Nucl. Med. Mol. Imaging 2014, 41, 1280–1292. [Google Scholar] [CrossRef] [Green Version]
- Afshar-Oromieh, A.; Haberkorn, U.; Zechmann, C.; Armor, T.; Mier, W.; Spohn, F.; Debus, N.; Holland-Letz, T.; Babich, J.; Kratochwil, C. Repeated PSMA-targeting radioligand therapy of metastatic prostate cancer with 131I-MIP-1095. Eur. J. Nucl. Med. Mol. Imaging 2017, 44, 950–959. [Google Scholar] [CrossRef] [Green Version]
- Afshar-Oromieh, A.; Holland-Letz, T.; Giesel, F.L.; Kratochwil, C.; Mier, W.; Haufe, S.; Debus, N.; Eder, M.; Eisenhut, M.; Schäfer, M.; et al. Diagnostic performance of 68Ga-PSMA-11 (HBED-CC) PET/CT in patients with recurrent prostate cancer: Evaluation in 1007 patients. Eur. J. Nucl. Med. Mol. Imaging 2017, 44, 1258–1268. [Google Scholar] [CrossRef] [Green Version]
- Kratochwil, C.; Giesel, F.L.; Eder, M.; Afshar-Oromieh, A.; Benešová, M.; Mier, W.; Kopka, K.; Haberkorn, U. [177Lu] Lutetium-labelled PSMA ligand-induced remission in a patient with metastatic prostate cancer. Eur. J. Nucl. Med. Mol. Imaging 2015, 42, 987–988. [Google Scholar] [CrossRef]
- Ahmadzadehfar, H.; Eppard, E.; Kürpig, S.; Fimmers, R.; Yordanova, A.; Schlenkhoff, C.D.; Gärtner, F.; Rogenhofer, S.; Essler, M. Therapeutic response and side effects of repeated radioligand therapy with 177Lu-PSMA-DKFZ-617 of castrate-resistant metastatic prostate cancer. Oncotarget 2016, 7, 12477–12488. [Google Scholar] [CrossRef] [PubMed]
- Hofman, M.S.; Violet, J.; Hicks, R.J.; Ferdinandus, J.; Thang, S.P.; Akhurst, T.; Iravani, A.; Kong, G.; Kumar, A.R.; Murphy, D.G.; et al. [177 Lu]-PSMA-617 radionuclide treatment in patients with metastatic castration-resistant prostate cancer (LuPSMA trial): A single-centre, single-arm, phase 2 study. Lancet Oncol. 2018, 19, 825–833. [Google Scholar] [CrossRef]
- Maffey-Steffan, J.; Scarpa, L.; Svirydenka, A.; Nilica, B.; Mair, C.; Buxbaum, S.; Bektic, J.; von Guggenberg, E.; Uprimny, C.; Horninger, W.; et al. The 68Ga/177Lu-theragnostic concept in PSMA-targeting of metastatic castration–resistant prostate cancer: Impact of post-therapeutic whole-body scintigraphy in the follow-up. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 695–712. [Google Scholar] [CrossRef] [Green Version]
- Yadav, M.P.; Ballal, S.; Bal, C.; Sahoo, R.K.; Damle, N.A.; Tripathi, M.; Seth, A. Efficacy and safety of 177Lu-PSMA-617 radioligand therapy in metastatic castration-resistant prostate cancer patients. Clin. Nucl. Med. 2020, 45, 19–31. [Google Scholar] [CrossRef]
- Kratochwil, C.; Bruchertseifer, F.; Giesel, F.L.; Weis, M.; Verburg, F.A.; Mottaghy, F.; Kopka, K.; Apostolidis, C.; Haberkorn, U.; Morgenstern, A. 225Ac-PSMA-617 for PSMA-targeted α-radiation therapy of metastatic castration-resistant prostate cancer. J. Nucl. Med. 2016, 57, 1941–1944. [Google Scholar] [CrossRef] [Green Version]
- Kratochwil, C.; Bruchertseifer, F.; Rathke, H.; Hohenfellner, M.; Giesel, F.L.; Haberkorn, U.; Morgenstern, A. Targeted α-therapy of metastatic castration-resistant prostate cancer with 225Ac-PSMA-617: Swimmer-plot analysis suggests efficacy regarding duration of tumor control. J. Nucl. Med. 2018, 59, 795–802. [Google Scholar] [CrossRef] [Green Version]
- Bräuer, A.; Grubert, L.S.; Roll, W.; Schrader, A.J.; Schäfers, M.; Bögemann, M.; Rahbar, K. 177Lu-PSMA-617 radioligand therapy and outcome in patients with metastasized castration-resistant prostate cancer. Eur. J. Nucl. Med. Mol. Imaging 2017, 44, 1663–1670. [Google Scholar] [CrossRef]
- Sathekge, M.; Bruchertseifer, F.; Knoesen, O.; Reyneke, F.; Lawal, I.; Lengana, T.; Davis, C.; Mahapane, J.; Corbett, C.; Vorster, M.; et al. 225Ac-PSMA-617 in chemotherapy-naive patients with advanced prostate cancer: A pilot study. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 129–138. [Google Scholar] [CrossRef] [Green Version]
- Nonnekens, J.; Chatalic, K.L.; Molkenboer-Kuenen, J.D.; Beerens, C.E.; Bruchertseifer, F.; Morgenstern, A.; Veldhoven-Zweistra, J.; Schottelius, M.; Wester, H.-J.; Van Gent, D.C.; et al. 213Bi-labeled prostate-specific membrane antigen-targeting agents induce DNA double-strand breaks in prostate cancer xenografts. Cancer Biother. Radiopharm. 2017, 32, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Chatalic, K.L.; Veldhoven-Zweistra, J.; Bolkestein, M.; Hoeben, S.; Koning, G.A.; Boerman, O.C.; de Jong, M.; van Weerden, W.M. A novel 111In-labeled anti–prostate-specific membrane antigen nanobody for targeted SPECT/CT imaging of prostate cancer. J. Nucl. Med. 2015, 56, 1094–1099. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Evazalipour, M.; D’Huyvetter, M.; Tehrani, B.S.; Abolhassani, M.; Omidfar, K.; Abdoli, S.; Arezumand, R.; Morovvati, H.; Lahoutte, T.; Muyldermans, S.; et al. Generation and characterization of nanobodies targeting PSMA for molecular imaging of prostate cancer. Contrast Media Mol. Imaging 2014, 9, 211–220. [Google Scholar] [CrossRef] [PubMed]
- Dehdashti, F.; Wu, N.; Bose, R.; Naughton, M.J.; Ma, C.X.; Marquez-Nostra, B.; Diebolder, P.; Mpoy, C.; Rogers, B.E.; Lapi, S.E.; et al. Evaluation of [89Zr] trastuzumab-PET/CT in differentiating HER2-positive from HER2-negative breast cancer. Breast Cancer Res. Treat. 2018, 169, 523–530. [Google Scholar] [CrossRef]
- Laforest, R.; Lapi, S.; Oyama, R.; Bose, R.; Tabchy, A.; Marquez-Nostra, B.; Burkemper, J.; Wright, B.D.; Frye, J.; Frye, S.; et al. [89Zr] Trastuzumab: Evaluation of radiation dosimetry, safety, and optimal imaging parameters in women with HER2-positive breast cancer. Mol. Imaging Biol. 2016, 18, 952–959. [Google Scholar] [CrossRef] [Green Version]
- Ulaner, G.A.; Hyman, D.M.; Ross, D.S.; Corben, A.; Chandarlapaty, S.; Goldfarb, S.; McArthur, H.; Erinjeri, J.P.; Solomon, S.B.; Kolb, H.; et al. Detection of HER2-positive metastases in patients with HER2-negative primary breast cancer using 89Zr-Trastuzumab PET/CT. J. Nucl. Med. 2016, 57, 1523–1528. [Google Scholar] [CrossRef] [Green Version]
- Gebhart, G.; Lamberts, L.E.; Wimana, Z.; Garcia, C.; Emonts, P.; Ameye, L.; Stroobants, S.; Huizing, M.; Aftimos, P.; Tol, J.; et al. Molecular imaging as a tool to investigate heterogeneity of advanced HER2-positive breast cancer and to predict patient outcome under trastuzumab emtansine (T-DM1): The ZEPHIR trial. Ann. Oncol. 2016, 27, 619–624. [Google Scholar] [CrossRef]
- Bhusari, P.; Vatsa, R.; Singh, G.; Parmar, M.; Bal, A.; Dhawan, D.K.; Mittal, B.R.; Shukla, J. Development of Lu-177-trastuzumab for radioimmunotherapy of HER2 expressing breast cancer and its feasibility assessment in breast cancer patients. Int. J. Cancer 2017, 140, 938–947. [Google Scholar] [CrossRef]
- D’Huyvetter, M.; De Vos, J.; Caveliers, V.; Vaneycken, I.; Heemskerk, J.; Duhoux, F.P.; Fontaine, C.; Vanhoeij, M.; Windhorst, A.D.; van der Aa, F.; et al. Phase I trial of 131I-GMIB-Anti-HER2-VHH1, a new promising candidate for HER2-targeted radionuclide therapy in breast cancer patients. J. Nucl. Med. 2021, 62, 1097–1105. [Google Scholar] [CrossRef] [PubMed]
- Keyaerts, M.; Xavier, C.; Heemskerk, J.; Devoogdt, N.; Everaert, H.; Ackaert, C.; Vanhoeij, M.; Duhoux, F.P.; Gevaert, T.; Simon, P.; et al. Phase I study of 68Ga-HER2-nanobody for PET/CT assessment of HER2 expression in breast carcinoma. J. Nucl. Med. 2016, 57, 27–33. [Google Scholar] [CrossRef] [Green Version]
- Keyaerts, M.; Xavier, C.; Everaert, H.; Vaneycken, I.; Fontaine, C.; DeCoster, L.; Vanhoeij, M.; Caveliers, V.; Lahoutte, T. Phase II trial of HER2-PET/CT using 68Ga-anti-HER2 VHH1 for characterization of HER2 presence in brain metastases of breast cancer patients. Ann. Oncol. 2019, 30, iii25–iii26. [Google Scholar] [CrossRef]
- Xavier, C.; Vaneycken, I.; D’Huyvetter, M.; Heemskerk, J.; Keyaerts, M.; Vincke, C.; Devoogdt, N.; Muyldermans, S.; Lahoutte, T.; Caveliers, V. Synthesis, preclinical validation, dosimetry, and toxicity of 68Ga-NOTA-Anti-HER2 nanobodies for iPET imaging of HER2 receptor expression in cancer. J. Nucl. Med. 2013, 54, 776–784. [Google Scholar] [CrossRef] [Green Version]
- Lindner, T.; Loktev, A.; Altmann, A.; Giesel, F.; Kratochwil, C.; Debus, J.; Jäger, D.; Mier, W.; Haberkorn, U. Development of quinoline-based theranostic ligands for the targeting of fibroblast activation protein. J. Nucl. Med. 2018, 59, 1415–1422. [Google Scholar] [CrossRef] [Green Version]
- Kratochwil, C.; Flechsig, P.; Lindner, T.; Abderrahim, L.; Altmann, A.; Mier, W.; Adeberg, S.; Rathke, H.; Röhrich, M.; Winter, H.; et al. 68Ga-FAPI PET/CT: Tracer uptake in 28 different kinds of cancer. J. Nucl. Med. 2019, 60, 801–805. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ballal, S.; Yadav, M.P.; Kramer, V.; Moon, E.S.; Roesch, F.; Tripathi, M.; Mallick, S.; ArunRaj, S.T.; Bal, C. A theranostic approach of [68Ga]Ga-DOTA.SA.FAPi PET/CT-guided [177Lu]Lu-DOTA.SA.FAPi radionuclide therapy in an end-stage breast cancer patient: New frontier in targeted radionuclide therapy. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 942–944. [Google Scholar] [CrossRef]
- Ferdinandus, J.; Costa, P.F.; Kessler, L.; Weber, M.; Hirmas, N.; Kostbade, K.; Bauer, S.; Schuler, M.; Ahrens, M.; Schildhaus, H.-U.; et al. Initial clinical experience with 90Y-FAPI-46 radioligand therapy for advanced stage solid tumors: A case series of nine patients. J. Nucl. Med. 2021, 62, 262468. [Google Scholar] [CrossRef] [PubMed]
- Kratochwil, C.; Giesel, F.L.; Rathke, H.; Fink, R.; Dendl, K.; Debus, J.; Mier, W.; Jäger, D.; Lindner, T.; Haberkorn, U. [153Sm]Samarium-labeled FAPI-46 radioligand therapy in a patient with lung metastases of a sarcoma. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 3011–3013. [Google Scholar] [CrossRef]
- Rathke, H.; Fuxius, S.; Giesel, F.L.; Lindner, T.; Debus, J.; Haberkorn, U.; Kratochwil, C. Two tumors, one target: Preliminary experience with 90Y-FAPI therapy in a patient with metastasized breast and colorectal cancer. Clin. Nucl. Med. 2021, 10, 842–844. [Google Scholar] [CrossRef]
- Herrmann, K.; Schottelius, M.; Lapa, C.; Osl, T.; Poschenrieder, A.; Hänscheid, H.; Lückerath, K.; Schreder, M.; Bluemel, C.; Knott, M.; et al. First-in-human experience of CXCR4-directed endoradiotherapy with 177Lu- and 90Y-labeled pentixather in advanced-stage multiple myeloma with extensive intra- and extramedullary disease. J. Nucl. Med. 2016, 57, 248–251. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lapa, C.; Herrmann, K.; Schirbel, A.; Hänscheid, H.; Lückerath, K.; Schottelius, M.; Kircher, M.; Werner, R.A.; Schreder, M.; Samnick, S.; et al. CXCR4-directed endoradiotherapy induces high response rates in extramedullary relapsed Multiple Myeloma. Theranostics 2017, 7, 1589–1597. [Google Scholar] [CrossRef] [Green Version]
- Solnes, L.B.; Shokeen, M.; Pandit-Taskar, N. Novel agents and future perspectives on theranostics. Semin. Radiat. Oncol. 2021, 31, 83–92. [Google Scholar] [CrossRef]
- Naldini, L. Gene therapy returns to centre stage. Nature 2015, 526, 351–360. [Google Scholar] [CrossRef]
- High, K.A.; Roncarolo, M.G. Gene therapy. N. Engl. J. Med. 2019, 381, 455–464. [Google Scholar] [CrossRef]
- Serganova, I.; Blasberg, R.G. Molecular imaging with reporter genes: Has its promise been delivered? J. Nucl. Med. 2019, 60, 1665–1681. [Google Scholar] [CrossRef]
- Niu, G.; Chen, X. Molecular Imaging with Activatable Reporter Systems. Theranostics 2012, 2, 413–423. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sena-Esteves, M.; Gao, G. Introducing genes into mammalian cells: Viral vectors. Cold Spring Harb. Protoc. 2020, 2020, 297–329. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, A.; Dubrovin, M.; Hewett, J.; Sena-Esteves, M.; Tan, C.-W.; Slack, M.; Sadelain, M.; Breakefield, X.O.; Tjuvajev, J.G. Functional coexpression of HSV-1 thymidine kinase and green fluorescent protein: Implications for noninvasive imaging of transgene expression. Neoplasia 1999, 1, 154–161. [Google Scholar] [CrossRef] [Green Version]
- Jacobs, A.H.; Winkeler, A.; Hartung, M.; Slack, M.; Dittmar, C.; Kummer, C.; Knoess, C.; Galldiks, N.; Vollmar, S.; Wienhard, K.; et al. Improved herpes simplex virus type 1 amplicon vectors for proportional coexpression of positron emission tomography marker and therapeutic genes. Hum. Gene Ther. 2003, 14, 277–297. [Google Scholar] [CrossRef] [PubMed]
- Ray, P.; Tsien, R.; Gambhir, S.S. Construction and validation of improved triple fusion reporter gene vectors for molecular imaging of living subjects. Cancer Res. 2007, 67, 3085–3093. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ray, P.; De, A.; Min, J.-J.; Tsien, R.Y.; Gambhir, S.S. Imaging tri-fusion multimodality reporter gene expression in living subjects. Cancer Res. 2004, 64, 1323–1330. [Google Scholar] [CrossRef] [Green Version]
- Gambhir, S.S.; Bauer, E.; Black, M.E.; Liang, Q.; Kokoris, M.S.; Barrio, J.R.; Iyer, M.; Namavari, M.; Phelps, M.E.; Herschman, H.R. A mutant herpes simplex virus type 1 thymidine kinase reporter gene shows improved sensitivity for imaging reporter gene expression with positron emission tomography. Proc. Natl. Acad. Sci. USA 2000, 97, 2785–2790. [Google Scholar] [CrossRef] [Green Version]
- Tjuvajev, J.G.; Joshi, A.; Callegari, J.; Lindsley, L.; Joshi, R.; Balaton, J.; Find, R.; Larso, S.M.; Sadelain, M.; Blasberg, R.G. A general approach to the non-invasive imaging of transgenes using cis-linked herpes simplex virus thymidine kinase. Neoplasia 1999, 1, 315–320. [Google Scholar] [CrossRef] [Green Version]
- Hossain, A.J.; Marchini, A.; Fehse, B.; Bjerkvig, R.; Miletic, H. Suicide gene therapy for the treatment of high-grade glioma: Past lessons, present trends, and future prospects. Neuro-Oncol. Adv. 2020, 2, vdaa013. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, A.H.; Rueger, M.A.; Winkeler, A.; Li, H.; Vollmar, S.; Waerzeggers, Y.; Rueckriem, B.; Kummer, C.; Dittmar, C.; Klein, M.; et al. Imaging-guided gene therapy of experimental gliomas. Cancer Res. 2007, 67, 1706–1715. [Google Scholar] [CrossRef] [Green Version]
- Hossain, A.J.; Latif, A.; Ystaas, L.A.R.; Ninzima, S.; Riecken, K.; Muller, A.; Azuaje, F.; Joseph, J.V.; Talasila, K.M.; Ghimire, J.; et al. Long-term treatment with valganciclovir improves lentiviral suicide gene therapy of glioblastoma. Neuro-Oncol. 2019, 21, 890–900. [Google Scholar] [CrossRef]
- Jacobs, A.; Voges, J.; Reszka, R.; Lercher, M.; Gossmann, A.; Kracht, L.; Kaestle, C.; Wagner, R.; Wienhard, K.; Heiss, W. Positron-emission tomography of vector-mediated gene expression in gene therapy for gliomas. Lancet 2001, 358, 727–729. [Google Scholar] [CrossRef]
- Johnson, M.; Sato, M.; Jeremy, B.; Gambhir, S.S.; Carey, M.; Wu, L.; Byun, J.; Lee, J.-Y.; Lee, Y.-S.; Kim, J.-M.; et al. Prostate cancer-targeted suicide gene therapy achieved effective tumor destruction while safeguarding against systemic toxicity. Proc. Mol. Ther. 2005, 11, S406. [Google Scholar]
- Monfared, P.; Winkeler, A.; Klein, M.; Li, H.; Klose, A.; Hoesel, M.; Waerzeggers, Y.; Korsching, S.; Jacobs, A.H. Noninvasive assessment of E2F-1–mediated transcriptional regulation in vivo. Cancer Res. 2008, 68, 5932–5940. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thunemann, M.; Schörg, B.F.; Feil, S.; Lin, Y.; Voelkl, J.; Golla, M.; Vachaviolos, A.; Kohlhofer, U.; Quintanilla-Martinez, L.; Olbrich, M.; et al. Cre/lox-assisted non-invasive in vivo tracking of specific cell populations by positron emission tomography. Nat. Commun. 2017, 8, 444. [Google Scholar] [CrossRef] [Green Version]
- Keu, K.V.; Witney, T.H.; Yaghoubi, S.; Rosenberg, J.; Kurien, A.; Magnusson, R.; Williams, J.; Habte, F.; Wagner, J.R.; Forman, S.; et al. Reporter gene imaging of targeted T cell immunotherapy in recurrent glioma. Sci. Transl. Med. 2017, 9, eaag2196. [Google Scholar] [CrossRef] [Green Version]
- Rajendran, R.L.; Jogalekar, M.P.; Gangadaran, P.; Ahn, B.-C. Noninvasive in vivo cell tracking using molecular imaging: A useful tool for developing mesenchymal stem cell-based cancer treatment. World J. Stem Cells 2020, 12, 1492–1510. [Google Scholar] [CrossRef]
- Ashmore-Harris, C.; Iafrate, M.; Saleem, A.; Fruhwirth, G.O. Non-invasive reporter gene imaging of cell therapies, including T cells and stem cells. Mol. Ther. 2020, 28, 1392–1416. [Google Scholar] [CrossRef] [PubMed]
- Miletic, H.; Fischer, Y.; Litwak, S.; Giroglou, T.; Waerzeggers, Y.; Winkeler, A.; Li, H.; Himmelreich, U.; Lange, C.; Stenzel, W.; et al. Bystander killing of malignant glioma by bone marrow–derived tumor-infiltrating progenitor cells expressing a suicide gene. Mol. Ther. 2007, 15, 1373–1381. [Google Scholar] [CrossRef]
- Waerzeggers, Y.; Klein, M.; Miletic, H.; Himmelreich, U.; Li, H.; Monfared, P.; Herrlinger, U.; Hoehn, M.; Coenen, H.H.; Weller, M.; et al. Multimodal imaging of neural progenitor cell fate in rodents. Mol. Imaging 2008, 7, 77–91. [Google Scholar] [CrossRef] [Green Version]
- Deroose, C.; De, A.; Loening, A.; Chow, P.L.; Ray, P.; Chatziioannou, A.F.; Gambhir, S.S. Multimodality imaging of tumor xenografts and metastases in mice with combined small-animal PET, small-animal CT, and bioluminescence imaging. J. Nucl. Med. 2007, 48, 295–303. [Google Scholar]
- Shu, C.J.; Guo, S.; Kim, Y.J.; Shelly, S.M.; Nijagal, A.; Ray, P.; Gambhir, S.S.; Radu, C.; Witte, O.N. Visualization of a primary anti-tumor immune response by positron emission tomography. Proc. Natl. Acad. Sci. USA 2005, 102, 17412–17417. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hung, S.-C.; Deng, W.-P.; Yang, W.K.; Liu, R.-S.; Lee, C.-C.; Su, T.-C.; Lin, R.-J.; Yang, D.-M.; Chang, C.-W.; Chen, W.-H.; et al. Mesenchymal stem cell targeting of microscopic tumors and tumor stroma development monitored by noninvasive in vivo positron emission tomography imaging. Clin. Cancer Res. 2005, 11, 7749–7756. [Google Scholar] [CrossRef] [Green Version]
- Davidson, B.L.; Breakefield, X.O. Viral vectors for gene delivery to the nervous system. Nat. Rev. Neurosci. 2003, 4, 353–364. [Google Scholar] [CrossRef] [PubMed]
- Yaghoubi, S.; Barrio, J.R.; Dahlbom, M.; Iyer, M.; Namavari, M.; Satyamurthy, N.; Goldman, R.; Herschman, H.R.; Phelps, E.M.; Gambhir, S.S. Human pharmacokinetic and dosimetry studies of [(18)F]FHBG: A reporter probe for imaging herpes simplex virus type-1 thymidine kinase reporter gene expression. J. Nucl. Med. 2001, 42, 1225–1234. [Google Scholar] [PubMed]
- Zinn, K.R.; Chaudhuri, T.R. The type 2 human somatostatin receptor as a platform for reporter gene imaging. Eur. J. Nucl. Med. Mol. Imaging 2002, 29, 388–399. [Google Scholar] [CrossRef] [PubMed]
- Rogers, B.E.; Parry, J.J.; Andrews, R.; Cordopatis, P.; Nock, B.A.; Maina, T. MicroPET imaging of gene transfer with a somatostatin receptor–based reporter gene and 94mTc-demotate 1. J. Nucl. Med. 2005, 46, 1889–1897. [Google Scholar]
- Lears, K.A.; Parry, J.J.; Andrews, R.; Nguyen, K.; Wadas, T.; Rogers, B.E. Adenoviral-mediated imaging of gene transfer using a somatostatin receptor-cytosine deaminase fusion protein. Cancer Gene Ther. 2015, 22, 215–221. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cotugno, G.; Aurilio, M.; Annunziata, P.; Capalbo, A.; Faella, A.; Rinaldi, V.; Strisciuglio, C.; Di Tommaso, M.; Aloj, L.; Auricchio, A. Noninvasive repetitive imaging of somatostatin receptor 2 gene transfer with positron emission tomography. Hum. Gene Ther. 2011, 22, 189–196. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Moroz, M.A.; Serganova, I.; Ku, T.; Huang, R.; Vider, J.; Maecke, H.R.; Larson, S.M.; Blasberg, R.; Smith-Jones, P.M. Imaging expression of the human somatostatin receptor Subtype-2 reporter gene with 68Ga-DOTATOC. J. Nucl. Med. 2011, 52, 123–131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heidari, P.; Kunawudhi, A.; Martinez-Quintanilla, J.; Szretter, A.; Shah, K.; Mahmood, U. Somatostatin receptor type 2 as a radiotheranostic PET reporter gene for oncologic interventions. Theranostics 2018, 8, 3380–3391. [Google Scholar] [CrossRef]
- Wang, J.; Arulanandam, R.; Wassenaar, R.; Falls, T.; Petryk, J.; Paget, J.; Garson, K.; Cemeus, C.; Vanderhyden, B.C.; Wells, R.G.; et al. Enhancing expression of functional human sodium iodide symporter and somatostatin receptor in recombinant oncolytic vaccinia virus for in vivo imaging of tumors. J. Nucl. Med. 2017, 58, 221–227. [Google Scholar] [CrossRef] [Green Version]
- McCart, J.A.; Mehta, N.; Scollard, D.; Reilly, R.M.; Carrasquillo, J.A.; Tang, N.; Deng, H.; Miller, M.; Xu, H.; Libutti, S.K.; et al. Oncolytic vaccinia virus expressing the human somatostatin receptor SSTR2: Molecular imaging after systemic delivery using 111In-pentetreotide. Mol. Ther. 2004, 10, 553–561. [Google Scholar] [CrossRef]
- Vedvyas, Y.; Shevlin, E.; Zaman, M.; Min, I.M.; Amor-Coarasa, A.; Park, S.; Park, S.; Kwon, K.-W.; Smith, T.; Luo, Y.; et al. Longitudinal PET imaging demonstrates biphasic CAR T cell responses in survivors. JCI Insight 2016, 1, e90064. [Google Scholar] [CrossRef] [PubMed]
- Shi, S.; Zhang, M.; Guo, R.; Miao, Y.; Li, B. Bone marrow–derived mesenchymal stem cell–mediated dual-gene therapy for glioblastoma. Hum. Gene Ther. 2019, 30, 106–117. [Google Scholar] [CrossRef] [PubMed]
- Spitzweg, C.; Dietz, A.; O’Connor, M.K.; Bergert, E.R.; Tindall, D.J.; Young, C.; Morris, J.C. In vivo sodium iodide symporter gene therapy of prostate cancer. Gene Ther. 2001, 8, 1524–1531. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boland, A.; Ricard, M.; Opolon, P.; Bidart, J.M.; Yeh, P.; Filetti, S.; Schlumberger, M.; Perricaudet, M. Adenovirus-mediated transfer of the thyroid sodium/iodide symporter gene into tumors for a targeted radiotherapy. Cancer Res. 2000, 60, 3484–3492. [Google Scholar]
- Huang, M.; Batra, R.K.; Kogai, T.; Lin, Y.Q.; Hershman, J.M.; Lichtenstein, A.; Sharma, S.; Zhu, L.X.; Brent, A.G.; Dubinett, S.M. Ectopic expression of the thyroperoxidase gene augments radioiodide uptake and retention mediated by the sodium iodide symporter in non–small cell lung cancer. Cancer Gene Ther. 2001, 8, 612–618. [Google Scholar] [CrossRef] [Green Version]
- Schug, C.; Sievert, W.; Urnauer, S.; Müller, A.M.; Schmohl, K.A.; Wechselberger, A.; Schwenk, N.; Lauber, K.; Schwaiger, M.; Multhoff, G.; et al. External beam radiation therapy enhances mesenchymal stem cell–mediated sodium–iodide symporter gene delivery. Hum. Gene Ther. 2018, 29, 1287–1300. [Google Scholar] [CrossRef]
- Tutter, M.; Schug, C.; Schmohl, K.A.; Urnauer, S.; Schwenk, N.; Petrini, M.; Lokerse, W.J.M.; Zach, C.; Ziegler, S.; Bartenstein, P.; et al. Effective control of tumor growth through spatial and temporal control of theranostic sodium iodide symporter (NIS) gene expression using a heat-inducible gene promoter in engineered mesenchymal stem cells. Theranostics 2020, 10, 4490–4506. [Google Scholar] [CrossRef]
- Dispenzieri, A.; Tong, C.; LaPlant, B.; Lacy, M.Q.; Laumann, K.; Dingli, D.; Zhou, Y.; Federspiel, M.J.; Gertz, M.A.; Hayman, S.; et al. Phase I trial of systemic administration of Edmonston strain of measles virus genetically engineered to express the sodium iodide symporter in patients with recurrent or refractory multiple myeloma. Leukemia 2017, 31, 2791–2798. [Google Scholar] [CrossRef]
- Galanis, E.; Atherton, P.J.; Maurer, M.J.; Knutson, K.L.; Dowdy, S.C.; Cliby, W.A.; Haluska, P.; Long, H.J.; Oberg, A.; Aderca, I.; et al. Oncolytic measles virus expressing the sodium iodide symporter to treat drug-resistant ovarian cancer. Cancer Res. 2015, 75, 22–30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, H.; DeGrado, T.R. [18F]Tetrafluoroborate ([18F]TFB) and its analogs for PET imaging of the sodium/iodide symporter. Theranostics 2018, 8, 3918–3931. [Google Scholar] [CrossRef]
- Brader, P.; Kelly, K.J.; Chen, N.; Yu, Y.A.; Zhang, Q.; Zanzonico, P.; Burnazi, E.M.; Ghani, R.E.; Serganova, I.; Hricak, H.; et al. Imaging a genetically engineered oncolytic vaccinia virus (GLV-1h99) using a human norepinephrine transporter reporter gene. Clin. Cancer Res. 2009, 15, 3791–3801. [Google Scholar] [CrossRef] [Green Version]
- Moroz, M.A.; Zhang, H.; Lee, J.; Moroz, E.; Zurita, J.; Shenker, L.; Serganova, I.; Blasberg, R.; Ponomarev, V. Comparative analysis of T cell imaging with human nuclear reporter genes. J. Nucl. Med. 2015, 56, 1055–1060. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Viel, T.; Schelhaas, S.; Wagner, S.; Wachsmuth, L.; Schwegmann, K.; Kuhlmann, M.; Faber, C.; Kopka, K.; Schafers, M.; Jacobs, A.H. Early assessment of the efficacy of temozolomide chemotherapy in experimental glioblastoma using [18F]FLT-PET imaging. PLoS ONE 2013, 8, e67911. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Viel, T.; Monfared, P.; Schelhaas, S.; Fricke, I.B.; Kuhlmann, M.T.; Fraefel, C.; Jacobs, A.H. Optimizing glioblastoma temozolomide chemotherapy employing lentiviral-based Anti-MGMT shRNA technology. Mol. Ther. 2013, 21, 570–579. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Viel, T.; Talasila, K.M.; Monfared, P.; Wang, J.; Jikeli, J.F.; Waerzeggers, Y.; Neumaier, B.; Backes, H.; Brekka, N.; Thorsen, F.; et al. Analysis of the growth dynamics of angiogenesis-dependent and -independent experimental glioblastomas by multimodal small-animal PET and MRI. J. Nucl. Med. 2012, 53, 1135–1145. [Google Scholar] [CrossRef] [Green Version]
- Zinnhardt, B.; Pigeon, H.; Thézé, B.; Viel, T.; Wachstmuth, L.; Fricke, I.; Schelhaas, S.; Honold, L.; Schwegmann, K.; Wagner, S.; et al. Combined PET Imaging of the inflammatory tumor microenvironment identifies margins of unique radiotracer uptake. Cancer Res. 2017, 77, 1831–1841. [Google Scholar] [CrossRef] [Green Version]
- Foray, C.; Valtorta, S.; Barca, C.; Winkeler, A.; Roll, W.; Müther, M.; Wagner, S.; Gardner, M.L.; Hermann, S.; Schäfers, M.; et al. Imaging temozolomide-induced changes in the myeloid glioma microenvironment. Theranostics 2021, 11, 2020–2033. [Google Scholar] [CrossRef] [PubMed]


| Class | Diagnostic Agent | Therapeutic Agent | Target | Disease |
|---|---|---|---|---|
| Transporter | [123I]I− | [131I]I− | NIS (SLC5A5) | Differentiated thyroid cancer, hyperthyroidism |
| [123I]MIBG, [124I]MIBG, [18F]MFBG | [131I]I-MIBG | NET (SLC6A2) | Neuroendocrine tumors, including neuroblastoma, pheochromocytomas, paragangliomas, medullary thyroid carcinoma | |
| Cell-surface receptor | [68Ga]Ga-DOTATATE, [68Ga]Ga-DOTATOC, [68Ga]Ga-DOTANOC | [111In]In-pentetreotide, [177Lu]Lu-DOTATATE (LUTATHERA®), [90Y]Y-DOTATATE, [225Ac]Ac-DOTATATE, [177Lu]Lu-DOTATOC, [90Y]Y-DOTATOC, [225Ac]Ac-DOTATOC, [212Pb]Pb-DOTAMTATE | SSTRs | Neuroendocrine tumors, mostly gastroenteropancreatic tumor (GEP-NET) |
| Cell-surface protein | [123I]MIP-1072, [123I]MIP-1095, [68Ga]Ga-PSMA-11, [68Ga]Ga-PSMA-I&T, [68Ga]Ga-PSMA-617 | [131I]I-MIP-1095, [177Lu]Lu-PSMA-I&T, [177Lu]Lu-PSMA-617, [225Ac]Ac-PSMA-617 | PSMA | Metastatic prostate cancer |
| [89Zr]Zr-trastuzumab, [68Ga]Ga-NOTA-HER2 | [177Lu]Lu-trastuzumab and derivatives, [131I]GMIB-anti-HER2-VHH1 | HER2 | Breast, ovarian and gastric cancer |
| Transgene | Imaging Probe | Clinical Trials/Applications |
|---|---|---|
| HSV-1-tk HSV-1-sr39tk | [123I]/[124I]/[131I]FIAU [18F]FHBG | Yes |
| hSSTR2 | [111In]In-pentetreotide [68Ga]Ga-DOTATATE [68Ga]Ga-DOTATOC | No |
| hNIS | [211At]At−, [188Re]ReO4−, [124I]/[131I]I−, [18F]TFB | Yes |
| hNET | [123I]MIBG [18F]MFBG | No |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barca, C.; Griessinger, C.M.; Faust, A.; Depke, D.; Essler, M.; Windhorst, A.D.; Devoogdt, N.; Brindle, K.M.; Schäfers, M.; Zinnhardt, B.; et al. Expanding Theranostic Radiopharmaceuticals for Tumor Diagnosis and Therapy. Pharmaceuticals 2022, 15, 13. https://doi.org/10.3390/ph15010013
Barca C, Griessinger CM, Faust A, Depke D, Essler M, Windhorst AD, Devoogdt N, Brindle KM, Schäfers M, Zinnhardt B, et al. Expanding Theranostic Radiopharmaceuticals for Tumor Diagnosis and Therapy. Pharmaceuticals. 2022; 15(1):13. https://doi.org/10.3390/ph15010013
Chicago/Turabian StyleBarca, Cristina, Christoph M. Griessinger, Andreas Faust, Dominic Depke, Markus Essler, Albert D. Windhorst, Nick Devoogdt, Kevin M. Brindle, Michael Schäfers, Bastian Zinnhardt, and et al. 2022. "Expanding Theranostic Radiopharmaceuticals for Tumor Diagnosis and Therapy" Pharmaceuticals 15, no. 1: 13. https://doi.org/10.3390/ph15010013
APA StyleBarca, C., Griessinger, C. M., Faust, A., Depke, D., Essler, M., Windhorst, A. D., Devoogdt, N., Brindle, K. M., Schäfers, M., Zinnhardt, B., & Jacobs, A. H. (2022). Expanding Theranostic Radiopharmaceuticals for Tumor Diagnosis and Therapy. Pharmaceuticals, 15(1), 13. https://doi.org/10.3390/ph15010013