1. Introduction
Thymol is a phenolic compound present in the essential oil extracted from the culinary herb Thyme (Thymus vulgaris). It is a member of the Lamiaceae family and is one of the most common ingredients found in antiseptic mouthwashes [
1]. Its antifungal mechanism of action occurs through its ability to increase the fluidity and permeability of membrane lipids which interferes with hyphael production [
2]. The major reason for pathogenicity in
C. albicans is attributed to its ability to form a biofilm, which is more resistant to antifungals [
3]. In previous work, it was found that thymol significantly decreased the adhesion of
C. albicans to epithelial cells and such adhesion is considered the first step in pathogenesis [
4].
The biocompatibility of thymol has been reported by various researchers including the ability to induce chromatin condensation and DNA cleavage in human gastric carcinoma cells (AGS), leading to depolarisation of mitochondrial membrane potentials and apoptosis [
5]. Moreover, in Caco-2 cells, thymol caused lipid degeneration, mitochondrial damage, nucleolar segregation and apoptosis [
6].
The inhibition of biofilm formation is paramount to prevent chronic infection. Different studies have investigated the effectiveness of polymer-antimicrobial combinations to inhibit biofilm formation. In a previous study, polymethylmethacrylate was observed to improve the activity of nystatin, polymyxin B and chlorhexidine to inhibit
C. albicans biofilm formation [
7]. However, no improvement was found in the antimicrobial activity of gentamycin and vancomycin when combined with poloxamer against
Staphylococcus aureus and
S. epidermidis, although, the polymer inhibited the adhesion of both microorganisms [
8]. In another study, poloxamer 338 showed a 100% inhibition of
Escherichia coli biofilm formation on silicone urinary catheters [
9].
In the current study, the impact of hydroxypropyl methylcellulose (HPMC) and poloxamer 407 (P407) on the effect of thymol was investigated. HPMC has been included in commercially available products and is thoroughly investigated in buccal drug delivery [
10]. Furthermore, P407 is widely used in several pharmaceutical preparations and it is one of the ingredients in Listerine
® mouthwash (Johnson & Johnson, Louis, MO, USA) [
11]. In this present study, the antifungal activity and the biocompatibility of thymol were investigated in an attempt to evaluate the feasibility of formulating thymol for controlled buccal delivery in the treatment of oral candidiasis. The investigations were undertaken for two hours to evaluate both the efficacy and the safety of thymol in the presence of the carriers HPMC and P407.
3. Discussion
The antifungal effects of thymol on
C. albicans planktonic and biofilm cells are equally important. Although the pathogenicity of
C. albicans is primarily attributed to biofilm formation, the first stage of its pathogenesis starts with the adhesion of yeast cells to surfaces including the buccal mucosa [
3]. For planktonic cells, the MBC was 250 mg/L on
C. albicans (ATCC 10231, Manassas, VA, USA); whereas the MIC was 125 mg/L and it is identical with that found in a previous study when the effect of thymol was instigated on
C. albicans [
2]. Although the used polymers are bioadhesive, the HPMC did not impact the MBC of thymol, while thymol-P407 increased it 250 mg/L to ≥250 mg/L which may be explained by its surfactant activity [
11]. The P407 increased the solubility of thymol in the aqueous medium and interfered with its ability to be incorporated into the cell membrane and disrupt the phospholipid bilayer [
12].
In a recent study looking at the effect of flavours on gelling temperature and the viscosity of P407, the authors found an interaction between thymol and P407; the former changed the thermal behaviour and the gelling temperature of the latter [
13]. In another study, the authors found that the non-ionic surfactant Tween 80 counteracted the effect of thymol against the bacterium
Salmonella typhimurium and the inhibitory effect increased with an increasing concentration of Tween 80. The authors hypothesised that the solubility of thymol in an aqueous media was increased upon the addition of the surfactant, leading to a decrease in thymol solubility in the
S. typhimurium cell membrane [
14].
In the current study, the effect of thymol on
Candida biofilm was analysed using CV and NR assays. CV measure the biomass of both dead and live cells in biofilms. Consequently, it does not reflect the degree of inactivation of the cells in the biofilm, but its mass left after treatment [
15]. NR reflected the viability of the cells; due to its basic properties, it is accumulated in the viable vacuoles of fungi or lysosomes of cells. Due to their low pH compared to the cytoplasm, NR retention in these organelles stains them red [
16]. This assay is widely used to measure the biocompatibility of human cell lines, and it was used in this work for testing
Candida biofilms since the pH of the vacuoles is around 5.5 [
17]. The pH gradient is maintained in healthy living cells; however, the pH gradient will be lost when the cell dies and subsequently lysosomes and vacuoles are not able to retain the dye [
16].
The P407 and HPMC both improved the biofilm inhibitory effect of thymol and the thymol-P407 combination showed a 100% biofilm inhibitory formation at all investigated concentrations based on NR assay. The effect of P407 and thymol is additive, the biofilm inhibitory activity of poloxamer was recorded at the lower concentrations and thymol at the higher concentrations. The inhibitory effect of poloxamer is inversely proportional to its concentration, which might be attributed to the self-association of poloxamer resulting in decreasing the interaction of the polymer with the
Candida (
Figure 1 and
Figure 2) [
18]. P407 is a nonionic surfactant polymer and is known to inhibit bacterial aggregation by suppressing protein aggregation in the biofilm matrix. Thymol-HPMC and Thymol showed 25 and 50% biofilm formation at 3.9 mg/L (
Figure 1). The effect of HPMC on biofilm inhibitory formation is attributed to its properties as a suspending and thickening agent. Thymol interferes with the production of hyphae and it showed an inhibitory effect of 80% on
C. albicans biofilm development after treatment with 600 mg/L of thymol for 24 h [
2,
19].
The effect of Thymol on the vacuolar activity and biofilm mass was concentration dependent, and its effectiveness decreased with an increase in the maturation of the biofilm. The effect on the biomass was maximal with 250 mg/L, leaving the biofilm with 29% and 63% biomass for 4-h and 24-h biofilms, respectively (
Figure 3), the decrease in thymol concentrations led to a decrease in the reduction of biomass. Moreover, at a concentration of 250 mg/L the vacuolar activity was 10% and 47% for the 4-h and 24-h biofilms, respectively (
Figure 4). It was found that thymol interfered with hyphael production by interacting with
C. albicans and as a consequence, it changed the cell membrane morphology leading to an increase in fluidity and permeability [
20]
The activity of thymol was decreased when combined with HPMC at 250 mg/L and tested using neutral red assay, at lower concentrations this effect was diminished (
Figure 4). This effect was not observed on planktonic cells and when using CV analysis and the inconsistency of the results might be attributed to the mucoadhesive nature of HPMC. The P407 had no impact on the activity of thymol when tested by CV assay, which might be attributed to the measurement of the mass of both dead and living cells. However, when assessed by NR assay, thymol activity decreased at 250 mg/L of thymol for the 4-h and 24-h biofilms, a result similar to that for planktonic cells. Accordingly, HPMC had no effect on the activity of thymol whereas high concentrations of P407 decreased thymol activity.
Thymol is considered cytotoxic to HEK293 cells at 125 and 250 mg/L [
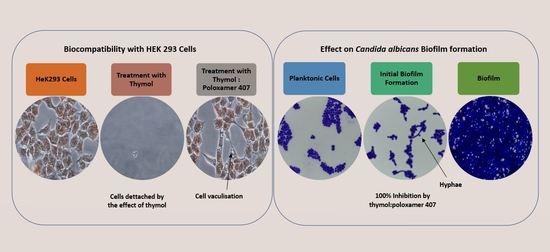
20], and it was highly antagonised by P407 (
Figure 6). This observation is supported by a previous investigation which showed no effect on the mitochondrial activity of neuronal cells when treated with thymol at a concentration of 150 mg/L for 30 min and 24 h using an MTT assay [
21]. However, there was a 40% reduction in the viability of human blood cells at concentrations of 150 and 200 mg/L and tested using MTT assay [
22].
In this study, the viability of HEK293 cells treated with thymol and investigated using NR showed a suppression of activity of nearly 100% at 250 mg/L with approximately 40% of activity remaining at 125 mg/L. P407 presented similar protective pattern with lower viability (
Figure 6). This observation agrees with a previous study, which found that the toxicity of tramadol has decreased approximately two-fold when combined with P407 and tested using MTT and NR uptake assays [
23]. In
Figure 7 and
Figure 10, the micrographs of cells treated with 250 mg/L of thymol show the disappearance of the cells due to their detachment by the effect of thymol, which might be explained by anoikis, which is a type of cell death induced upon their detachment from the neighbouring cells and extracellular matrix [
24].
Whereas when thymol at the same concentration combined with P407 resulted in the protection of the cells from being detached. Previously poloxamers have been found to promote wound healing by sealing the pores of the cellular membrane helping to maintain cellular integrity [
25]. Although, P407 protects the cells from detaching it did not inhibit their vacuolization (
Figure 8). The opposing effect of P407 to thymol on biofilm might be attributed to the same mechanism of healing in a human cell line. In this study cytoplasmic vacuolization confirms a previous investigation performed on Caco-2 cells which were treated with thymol for 48 h at a concentration of 37.5 mg/L. Cell detachment was observed with carvacrol but not with thymol in the same investigation, which might be due to the lower concentration of thymol used in that study (37.5 mg/L). Both thymol and carvacrol have very similar chemical structures, and both are constituents in thyme oil.
Furthermore, in a previous study, the effect of thymol on Caco-2 cells was investigated. The authors observed cellular necrosis when they were treated with 120 mg/L at approximately 10 and 15% after 1 and 24 h, respectively, with apoptosis of less than 1% [
26]. However, in the current investigation, cell death is attributed to apoptosis as a consequence of anoikis and vacuolization with no reported sign of necrosis. This toxic effect is concentration-dependent, and it was found after two hours treatment with 250 mg/L of thymol.
Biocompatibility was also tested using an SRB assay. SRB is used to measure the density of cells based on the cellular protein content of dead and live cells [
27]. Cells treated with thymol and thymol-HPMC showed a substantial loss of proteins at a concentration of 250 mg/L, and this is due to cells detachment. Thymol-P407 showed a potent biofilm inhibitory formation at a low concentration of 2 mg/L. This effect is considered a promising effect in the prophylaxis treatment of oral candidiasis by inhibiting biofilm formation.
4. Materials and Methods
4.1. Test Compounds
Thymol (Alfa Aesar, Heysham, UK), Poloxamer 407 (P407) (Sigma-Aldrich, Gillingham, UK) and MethocelTM F4M (HPMC, which was a kind gift from Colorcon, Dartford, UK). Thymol was serially diluted from 250 to 1.95 mg/L and Thymol-HPMC and Thymol-P407 were co-dissolved individually with thymol at a ratio of 5:1.
4.2. C. albicans Strain and Inoculum Preparation
C. albicans (ATCC 10321, Virginia Manassas, VA, USA) were grown on Sabauroud dextrose agar (SDA, Sigma-Aldrich, Gillingham, UK) and incubated at 30 °C for 18–24 h. Then, few colonies were transferred to Muller Hinton broth (MHB, Sigma-Aldrich, Gillingham, UK). The optical density was equivalent to 0.5 McFarland standard solution, and the concentration of the C. albicans overnight culture was 1–5 × 106 CFU/mL.
4.3. Minimum Inhibitory Concentration (MIC) and Minimum Biocidal Concentration (MBC)
The broth macro dilution method was used to test the MIC. A two-fold dilution of thymol with and without P407 and HPMC was performed in MHB to a final volume of 5 mL. Then, each tube was inoculated with 200 µL of the inoculum (giving a final cell density of 1 × 104–105 CFU/mL) then incubated at 30 °C for 24–48 h.
The MBC was tested on SDA plates by inoculating 10 µL of broth from the tubes which showed no visible growth (MIC). The plates were incubated at 30 °C for 24–48 h.
4.4. C. albicans Biofilm Formation
The pH of the RPMI-1640 medium (Sigma-Aldrich, Gillingham, UK) (RPMI) was adjusted to 7.2 with 0.165 M MOPS (3-morpholinopropane-1-sulfonic acid (Sigma-Aldrich, Gillingham, UK). RPMI-1640 medium was seeded with
C. albicans to a final concentration of 5×10
5 CFU/mL. The 96-well microtiter plate was inoculated with 200 µL/well and incubated at 37 °C for 4 h for initial biofilm formation and 24 h for a mature biofilm) [
28].
4.5. Inhibition of Biofilm Formation
Thymol with and without HPMC was serially double diluted in RPMI-1640 in a 96-well plate at a concentration range of 2–250 mg/L. Then Candida suspension was added to the wells and incubated at 37 °C for four hours. The inhibition was tested using NR and CV assays as explained below.
4.6. Thymol Antibiofilm Activity
The biofilms were washed twice with 200 µL of phosphate buffer saline (PBS) (10 mM, pH = 7.4) to remove non-adherent cells. The washed biofilms were cultured for two hours at 37 °C with pre-prepared serial double dilutions of the tested compounds. Which, then removed and the treated biofilm was washed with 200 µL of PBS and the antibiofilm activity was determined using neutral red uptake and crystal violet assays.
4.7. Neutral Red Uptake (NR) Assay (C. albicans Biofilm)
Neutral red (NR, 3-amino-7-dimethylamino-2-methyl-phenazine hydrochloride, Sigma-Aldrich, Gillingham, UK) (NR) assay has been described in detail in earlier studies [
16] Briefly, after washing biofilm with PBS, aliquots of 100 µL of NR (80 mg/L in PBS) was added to each well to determine the vacuole activity, and the plate was incubated at 37 °C for two hours. The stain was removed, and the biofilms were washed with 150 µL of PBS. Then was fixed for 2 min with 100 µL of 5% glutaraldehyde (Alfa Aeser, Heysham, UK). NR was extracted from the vacuoles with 150 µL of de-stain solution (50% absolute ethanol, 48% ultrapure water and 2% glacial acetic acid). Then the plate was placed on an orbital shaker for 30 min to extract the NR from the biofilm. Finally, a 100 µL aliquot was transferred from each well into a new microtiter plate and the absorbance was measured at λ
540nm (Multiskan Ascent, Thermo Labsystems, Vantaa, Finland). Biofilm viability was calculated as a percentage of untreated cells.
4.8. Crystal Violet (CV) Assay
Absolute ethanol was used to fix the washed biofilms, with a volume of 100 µL/well was added and left for 15 min, then the latter was removed, and the microtiter plate was left to air-dry. The CV stain (Chadwell Heath, London, UK) was prepared at a concentration of 0.1% (w/v) and aliquots of 100 µL were added to the wells for 20 min. Then the CV was removed, and the biofilms were washed under a gentle stream of running tap water. Acetic acid (Sigma-Aldrich, Gillingham, UK) was used to extract the CV from the biofilm by adding 150 µL/well. Then, from each well a 100 µL of the supernatant was moved to a new plate, and the absorbance was recorded at λ595nm using a plate reader (BioTec, EL800, Dorset, UK). The biomass was calculated as a percentage of untreated cells.
4.9. Cell Line and Culture Medium
The in vitro biocompatibility was tested on HEK293 cells (Human embryonic kidney cells). Dulbecco’s Modified Eagle’s Medium (DMEM) was supplemented with 10% (v/v) Foetal Bovine Serum (FBS), 1% (v/v) L-Glutamine solution (200 mM) and 1% (v/v) Antibiotic-Antimycotic Solution (10,000 U penicillin, 10 mg streptomycin and 25 μg amphotericin B per mL) (Sigma-Aldrich, Gillingham, UK).
4.10. Biocompatibility Assays
The biocompatibility assay was performed by inoculating a 96-well microtiter plate with HEK293 cells (4.0 × 104/well). The plate was incubated overnight at 37 °C in a humidified incubator with 5% CO2. Then DMEM was replaced with the test compounds, which were previously two-fold diluted in DMEM and the plate was incubated for two hours. The cell viability was evaluated using NR and Sulforhodamine B (SRB) assays.
4.11. Neutral Red Uptake (NR) Assay (HEK 293 Cells)
The NR was prepared in DMEM (40 mg/L) then aliquots of 100 µL of NR were added to each well of pretreated HEK 293 cells as explained in
Section 4.10 to determine the lysosomal activity, and the plate was incubated at 37 °C for two hours. The stain was removed, and the cells were washed with 150 µL of PBS. This was then fixed for 2 min with 100 µL of 5% glutaraldehyde. NR was extracted from the lysosomes with 150 µL of de-stain solution (50% absolute ethanol, 49% ultrapure water and 1% glacial acetic acid). Then the plate was placed on an orbital shaker for 30 min to extract the NR from the biofilm. Finally, a 100 µL aliquot was transferred from each well into a new microtiter plate and the absorbance was measured at λ
540nm (Multiskan Ascent, Thermo Labsystems, Finland). Biofilm viability was calculated as a percentage of untreated cells.
4.12. Sulforhodamine B (SRB) Assay
SRB assays have been described in detail elsewhere [
27]. SRB (Alfa-Aeser, Heysham, UK) stock solution was prepared in 1% (
v/
v) acetic acid at a concentration of 0.057% (
v/
v). A 100 µL volume of cold TCA 10% (
v/
v) (Trichloroacetic acid, Sigma-Aldrich, Gillingham, UK) was used to fix HEK 293 cells which were previously treated with thymol solutions and incubated at 4 °C for 1 h. The TCA was removed under a gentle stream of running tap water, then the plate was left to air dry at room temperature. The cells were stained with SRB solution (100 µL/well) for 30 min incubated at room temperature, then SRB was aspirated, and the cells were washed quickly with 1% (
v/
v) acetic acid to remove the excess dye. The SRB was extracted with 200 µL of 10 mM Tris base solution (Sigma-Aldrich, Gillingham, UK), having a pH of 10.5. The absorbance was measured at λ
540nm and finally, the biocompatibility was calculated as the percentage of the absorbance of treated to untreated cells.
4.13. Imaging of HEK293 Cells
HEK293 cells with a density of 2.0 × 105/2 mL were seeded in a 6-well plate and incubated for 24 h. The cells were treated with thymol and Thymol poloxamer at a concentration of 250 and 125 mg/L. Images are acquired for cells stained with NR and SRB using a phase contrast microscope (Nikon Eclipse TS100, Surbiton, UK).