Persimmon Leaves (Diospyros kaki) Extract Enhances the Viability of Human Corneal Endothelial Cells by Improving Na+-K+-ATPase Activity
Abstract
:1. Introduction
2. Results
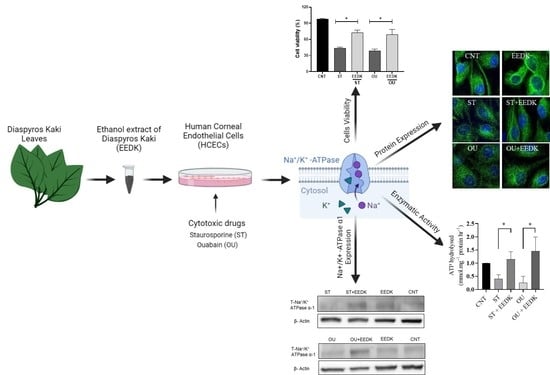
2.1. EEDK Increased HCECs Viability
2.2. Fluorescence Microscopy for Live/Dead Cells
2.3. Enhanced Na+/K+-ATPase Enzymatic Activity by EEDK
2.4. EEDK Co-Treatment Increased the Na+/K+-ATPase Expression in HCECs
2.5. Localization of Na+/K+-ATPase in Basolateral Membrane
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Cell Culture
4.3. Concentrations Optimization
4.4. Cell Viability (MTT) Assay
4.5. EZ-Live/Dead Imaging
4.6. Measurement of Na+/K+-ATPase Activity
4.7. Western Blot Analysis of the Na+/K+-ATPase α1 Subunit
4.8. Confocal Microscopy
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schmedt, T.; Silva, M.M.; Ziaei, A.; Jurkunas, U. Molecular bases of corneal endothelial dystrophies. Exp. Eye Res. 2012, 95, 24–34. [Google Scholar] [CrossRef] [Green Version]
- Joyce, N.C. Proliferative capacity of corneal endothelial cells. Exp. Eye Res. 2012, 95, 16–23. [Google Scholar] [CrossRef] [Green Version]
- Edelhauser, H.F. The balance between corneal transparency and edema: The Proctor Lecture. Investig. Ophthalmol. Vis. Sci. 2006, 47, 1754–1767. [Google Scholar] [CrossRef]
- Van den Bogerd, B.; Dhubhghaill, S.N.; Koppen, C.; Tassignon, M.-J.; Zakaria, N. A review of the evidence for in vivo corneal endothelial regeneration. Surv. Ophthalmol. 2018, 63, 149–165. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barry, P.A.; Petroll, W.M.; Andrews, P.M.; Cavanagh, H.D.; Jester, J.V. The spatial organization of corneal endothelial cytoskeletal proteins and their relationship to the apical junctional complex. Investig. Ophthalmol. Vis. Sci. 1995, 36, 1115–1124. [Google Scholar]
- Hoppenreijs, V.P.; Pels, E.; Vrensen, G.F.; Oosting, J.; Treffers, W.F. Effects of human epidermal growth factor on endothelial wound healing of human corneas. Investig. Ophthalmol. Vis. Sci. 1992, 33, 1946–1957. [Google Scholar]
- Matsubara, M.; Tanishima, T. Wound-healing of corneal endothelium in monkey: An autoradiographic study. Jpn. J. Ophthalmol. 1983, 27, 444–450. [Google Scholar] [PubMed]
- Krachmer, J.H.; Mannis, M.J.; Holland, E.J. Cornea, 3rd ed.; Elsevier: Amsterdam, The Netherlands, 2011. [Google Scholar]
- Lingrel, J.M.A.; Dostanic, I.; Cougnon, M.; He, S.; James, P.; Woo, A.; O’Connor, K.; Neumann, J. Functional roles of the α isoforms of Na K ATPase. N. Y. Acad. Sci. 2003, 986, 354–359. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.; Blanco, G.; Mercer, R.W.; Fleming, T.; Pepose, J.S. Human corneal endothelial cell expression of Na+/K+-Adenosine triphosphate isoforms. Arch Opthalmol. 2003, 121, 840–845. [Google Scholar] [CrossRef] [Green Version]
- de Carvalho Aguiar, P.; Sweadner, K.J.; Penniston, J.T.; Zaremba, J.; Liu, L.; Caton, M.; Linazasoro, G.; Borg, M.; Tijssen, M.A.; Bressman, S.B.; et al. Mutations in the Na+/K+-ATPase α3 Gene ATP1A3 are associated with Rapid-Onset Dystonia Parkinsonism. Neuron 2004, 43, 169–175. [Google Scholar] [CrossRef] [Green Version]
- Macdonald, J.M.; Geroski, D.H.; Edelhauser, H.F. Effect of inflammation on the corneal endothelial pump and barrier. Curr. Eye Res. 1987, 6, 1125–1132. [Google Scholar] [CrossRef]
- O’Brien, W.J.; Palmer, M.L.; Guy, J.; Taylor, J.L. Endothelial barrier function and Na+/K(+)-ATPase pump density in herpetic stromal disease. Investig. Ophthalmol. Vis. Sci. 1996, 37, 29–36. [Google Scholar]
- Mikkelsen, L.H.; Hamoudi, H.; Gül, C.A.; Heegaard, S. Corneal Toxicity Following Exposure to Asclepias Tuberosa. Open Ophthalmol. J. 2017, 11, 1–4. [Google Scholar] [CrossRef] [Green Version]
- Sato, A.; Yamada, M. Persimmon breeding in Japan for pollination-constant non-astringent (PCNA) type with marker-assisted selection. Breed. Sci. 2016, 66, 60–68. [Google Scholar] [CrossRef] [Green Version]
- Zhou, C.; Zhao, D.; Sheng, Y.; Tao, J.; Yang, Y. Carotenoids in fruits of different persimmon cultivars. Molecules 2011, 16, 624–636. [Google Scholar] [CrossRef] [Green Version]
- Sun, L.; Zhang, J.; Lu, X.; Zhang, L.; Zhang, Y. Evaluation to the antioxidant activity of total flavonoids extract from persimmon (Diospyros kaki L.) leaves. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2011, 49, 2689–2696. [Google Scholar] [CrossRef] [PubMed]
- Kawakami, K.; Aketa, S.; Sakai, H.; Watanabe, Y.; Nishida, H.; Hirayama, M. Antihypertensive and vasorelaxant effects of water-soluble proanthocyanidins from persimmon leaf tea in spontaneously hypertensive rats. Biosci. Biotechnol. Biochem. 2011, 75, 1435–1439. [Google Scholar] [CrossRef] [Green Version]
- Xie, C.; Xie, Z.; Xu, X.; Yang, D. Persimmon (Diospyros kaki L.) leaves: A review on traditional uses, phytochemistry and pharmacological properties. J. Ethnopharmacol. 2015, 163, 229–240. [Google Scholar] [CrossRef] [PubMed]
- Pan, M.H.; Lai, C.S.; Ho, C.T. Anti-inflammatory activity of natural dietary flavonoids. Food Funct. 2010, 1, 15–31. [Google Scholar] [CrossRef] [PubMed]
- Yoon, J.H.; Baek, S.J. Molecular targets of dietary polyphenols with anti-inflammatory properties. Yonsei Med. J. 2005, 46, 585–596. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, K.-A.; Lee, C.H.; Kang, T.K.; Yang, S.J.; Lee, C.Y.; Lee, W.-B.; Jung, S.H. Effect of persimmon leaves (Diospyros kaki) on goblet cell density and inflammation in experimental dry eye model. Appl. Biol. Chem. 2020, 63, 45. [Google Scholar] [CrossRef]
- Kim, K.-A.; Yang, S.J.; Kim, T.-J.; Kang, S.W.; Jung, S.H. Anti-inflammatory effects of Diospyros kaki in Mouse Dry Eye Model. Investig. Ophthalmol. Vis. Sci. 2016, 57, 408. [Google Scholar]
- Kim, K.A.; Hyun, L.C.; Jung, S.H.; Yang, S.J. The leaves of Diospyros kaki exert beneficial effects on a benzalkonium chloride-induced murine dry eye model. Mol. Vis. 2016, 22, 284–293. [Google Scholar] [PubMed]
- Meyer, T.; Regenass, U.; Fabbro, D.; Alteri, E.; Röusel, J.; Möller, M.; Caravatti, G.; Matter, A. A derivative of staurosporine (CGP 41 251) shows selectivity for protein kinase C inhibition and in vztro anti-proliferative as well as in vivo anti-tumor activity. Int. J. Cancer 1989, 43, 851–856. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.Q.; Xiao, A.Y.; Sheline, C.; Hyrc, K.; Yang, A.; Goldberg, M.P.; Choi, D.W.; Ping Yu, S. Apoptotic insults impair Na+, K+-ATPase activity as a mechanism of neuronal death mediated by concurrent ATP deficiency and oxidant stress. J. Cell Sci. 2003, 116, 2099–2110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klimanova, E.A.; Petrushanko, I.Y.; Mitkevich, V.A.; Anashkina, A.A.; Orlov, S.N.; Makarov, A.A.; Lopina, O.D. Binding of ouabain and marinobufagenin leads to different structural changes in Na, K-ATPase and depends on the enzyme conformation. FEBS Lett. 2015, 589, 2668–2674. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hatou, S.; Yamada, M.; Akune, Y.; Mochizuki, H.; Shiraishi, A.; Joko, T.; Nishida, T.; Tsubota, K. Role of Insulin in Regulation of Na+-/K+-Dependent ATPase Activity and Pump Function in Corneal Endothelial Cells. Investig. Ophthalmol. Vis. Sci. 2010, 51, 3935–3942. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wakamatsu, T.H.; Dogru, M.; Tsubota, K. Tearful relations: Oxidative stress, inflammation and eye diseases. Arq. Bras. De Oftalmol. 2008, 71, 72–79. [Google Scholar] [CrossRef] [Green Version]
- Ward, N.E.; O’Brian, C.A. Kinetic analysis of protein kinase C inhibition by staurosporine: Evidence that inhibition entails inhibitor binding at a conserved region of the catalytic domain but not competition with substrates. Mol. Pharmacol. 1992, 41, 387–392. [Google Scholar]
- Hatou, S.; Yamada, M.; Mochizuki, H.; Nishida, T. Role of protein kinase C in regulation of Na+- and K+-dependent ATPase activity and pump function in corneal endothelial cells. Jpn. J. Ophthalmol. 2009, 53, 235–242. [Google Scholar] [CrossRef]
- Qiu, J.; Gao, H.Q.; Zhou, R.H.; Liang, Y.; Zhang, X.H.; Wang, X.P.; You, B.A.; Cheng, M. Proteomics analysis of the proliferative effect of low-dose ouabain on human endothelial cells. Biol. Pharm. Bull. 2007, 30, 247–253. [Google Scholar] [CrossRef] [Green Version]
- Yeh, J.Y.; Huang, W.J.; Kan, S.F.; Wang, P.S. Inhibitory effects of digitalis on the proliferation of androgen dependent and independent prostate cancer cells. J. Urol. 2001, 166, 1937–1942. [Google Scholar] [CrossRef]
- Kometiani, P.; Liu, L.; Askari, A. Digitalis-induced signaling by Na+/K+-ATPase in human breast cancer cells. Mol. Pharmacol. 2005, 67, 929–936. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harris, J.E. Symposium on the Cornea: Introduction: Factors Influencing Corneal Hydration. Investig. Ophthalmol. Vis. Sci. 1962, 1, 151–157. [Google Scholar]
- Harris, J.E.; Nordquist, L.T. The hydration of the cornea. I. The transport of water from the cornea. Am. J. Ophthalmol. 1955, 40, 100–110. [Google Scholar] [CrossRef]
- Leuenberger, P.M.; Novikoff, A.B. Localization of transport adenosine triphosphatase in rat cornea. J. Cell Biol. 1974, 60, 721–731. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaye, G.I.; Tice, L.W. Studies on the Cornea. V. Electron Microscopic Localization of Adenosine Triphosphatase Activity in the Rabbit Cornea in Relation to Transport. Investig. Ophthalmol. Vis. Sci. 1966, 5, 22–32. [Google Scholar]
- Lichtenfels, R.; Biddison, W.E.; Schulz, H.; Vogt, A.B.; Martin, R. CARE-LASS (calcein-release-assay), an improved fluorescence-based test system to measure cytotoxic T lymphocyte activity. J. Immunol. Methods 1994, 172, 227–239. [Google Scholar] [CrossRef]
- Davey, H.M.; Hexley, P. Red but not dead? Membranes of stressed Saccharomyces cerevisiae are permeable to propidium iodide. Environ. Microbiol. 2011, 13, 163–171. [Google Scholar] [CrossRef] [PubMed]
- Delamere, N.A.; Parkerson, J.; Hou, Y. Indomethacin alters the Na,K-ATPase response to protein kinase C activation in cultured rabbit nonpigmented ciliary epithelium. Investig. Ophthalmol. Vis. Sci. 1997, 38, 866–875. [Google Scholar]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Afzal, R.; Hwang, H.B. Persimmon Leaves (Diospyros kaki) Extract Enhances the Viability of Human Corneal Endothelial Cells by Improving Na+-K+-ATPase Activity. Pharmaceuticals 2022, 15, 72. https://doi.org/10.3390/ph15010072
Afzal R, Hwang HB. Persimmon Leaves (Diospyros kaki) Extract Enhances the Viability of Human Corneal Endothelial Cells by Improving Na+-K+-ATPase Activity. Pharmaceuticals. 2022; 15(1):72. https://doi.org/10.3390/ph15010072
Chicago/Turabian StyleAfzal, Ramsha, and Hyung Bin Hwang. 2022. "Persimmon Leaves (Diospyros kaki) Extract Enhances the Viability of Human Corneal Endothelial Cells by Improving Na+-K+-ATPase Activity" Pharmaceuticals 15, no. 1: 72. https://doi.org/10.3390/ph15010072
APA StyleAfzal, R., & Hwang, H. B. (2022). Persimmon Leaves (Diospyros kaki) Extract Enhances the Viability of Human Corneal Endothelial Cells by Improving Na+-K+-ATPase Activity. Pharmaceuticals, 15(1), 72. https://doi.org/10.3390/ph15010072