Molecular Signaling Mechanisms for the Antidepressant Effects of NLX-101, a Selective Cortical 5-HT1A Receptor Biased Agonist
Abstract
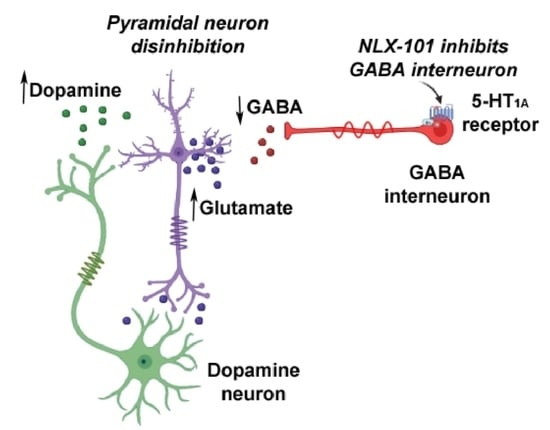
:1. Introduction
2. Results
2.1. Behavioral Effects of NLX-101
2.2. Biochemical Effects of NLX-101
2.3. Effects of NLX-101 on Prefrontal Protein Expression
3. Discussion
3.1. Effects of NLX-101 on FST and Cortical Neurotransmitter Levels
3.2. Effects of NLX-101 on Intracellular Signaling Biomarkers
4. Materials and Methods
4.1. Animals
4.2. Drugs and Reagents
4.3. Forced Swim Test (FST)
4.4. Open Field Test (OFT)
4.5. Microdialysis Procedure
4.6. Protein Extraction and Western Blotting
4.7. Statistics
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rush, A.J.; Trivedi, M.H.; Wisniewski, S.R.; Nierenberg, A.A.; Stewart, J.W.; Warden, D.; Niederehe, G.; Thase, M.E.; Lavori, P.W.; Lebowitz, B.D.; et al. Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: A STAR*D report. Am. J. Psychiatry 2006, 163, 1905–1917. [Google Scholar] [CrossRef] [PubMed]
- McIntyre, R.S.; Filteau, M.J.; Martin, L.; Patry, S.; Carvalho, A.; Cha, D.S.; Barakat, M.; Miguelez, M. Treatment-resistant depression: Definitions, review of the evidence, and algorithmic approach. J. Affect. Disord. 2014, 156, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Johnston, K.M.; Powell, L.C.; Anderson, I.M.; Szabo, S.; Cline, S. The burden of treatment-resistant depression: A systematic review of the economic and quality of life literature. J. Affect. Disord. 2019, 242, 195–210. [Google Scholar] [CrossRef]
- Berman, R.M.; Cappiello, A.; Anand, A.; Oren, D.A.; Heninger, G.R.; Charney, D.S.; Krystal, J.H. Antidepressant effects of ketamine in depressed patients. Biol. Psychiatry 2000, 47, 351–354. [Google Scholar] [CrossRef]
- Zarate, C.A., Jr.; Singh, J.B.; Carlson, P.J.; Brutsche, N.E.; Ameli, R.; Luckenbaugh, D.A.; Charney, D.S.; Manji, H.K. A randomized trial of an N-methyl-D-aspartate antagonist in treatment-resistant major depression. Arch. Gen. Psychiatry 2006, 63, 856–864. [Google Scholar] [CrossRef]
- Gigliucci, V.; O’Dowd, G.; Casey, S.; Egan, D.; Gibney, S.; Harkin, A. Ketamine elicits sustained antidepressant-like activity via a serotonin-dependent mechanism. Psychopharmacology 2013, 228, 157–166. [Google Scholar] [CrossRef] [PubMed]
- Fukumoto, K.; Iijima, M.; Chaki, S. The antidepressant effects of an mGlu2/3 receptor antagonist and ketamine require AMPA receptor stimulation in the mPFC and subsequent activation of the 5-HT neurons in the DRN. Neuropsychopharmacology 2016, 41, 1046–1056. [Google Scholar] [CrossRef]
- Du Jardin, K.G.; Liebenberg, N.; Müller, H.K.; Elfving, B.; Sanchez, C.; Wegener, G. Differential interaction with the serotonin system by S-ketamine, vortioxetine, and fluoxetine in a genetic rat model of depression. Psychopharmacology 2016, 233, 2813–2825. [Google Scholar] [CrossRef] [PubMed]
- Fukumoto, K.; Iijima, M.; Funakoshi, T.; Chaki, S. Role of 5-HT1A receptor stimulation in the medial prefrontal cortex in the sustained antidepressant effects of ketamine. Int. J. Neuropsychopharmacol. 2018, 21, 371–381. [Google Scholar] [CrossRef] [PubMed]
- Blier, P.; Bergeron, R.; de Montigny, C. Selective activation of postsynaptic 5-HT1A receptors induces rapid antidepressant response. Neuropsychopharmacology 1997, 16, 333–338. [Google Scholar] [CrossRef] [Green Version]
- Haddjeri, N.; Blier, P.; de Montigny, C. Long-term antidepressant treatments result in a tonic activation of forebrain 5-HT1A receptors. J. Neurosci. 1998, 18, 10150–10156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Assie, M.B.; Bardin, L.; Auclair, A.L.; Carilla-Durand, E.; Depoortere, R.; Koek, W.; Newman-Tancredi, A. F15599, a highly selective post-synaptic 5-HT1A receptor agonist: In-vivo profile in behavioural models of antidepressant and serotonergic activity. Int. J. Neuropsychopharmacol. 2010, 13, 1285–1298. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jastrzębska-Więsek, M.; Partyka, A.; Rychtyk, J.; Śniecikowska, J.; Kołaczkowski, M.; Wesołowska, A.; Varney, M.A.; Newman-Tancredi, A. Activity of serotonin 5-HT1A receptor biased agonists in rat: Anxiolytic and antidepressant-like properties. ACS Chem. Neurosci. 2018, 9, 1040–1050. [Google Scholar] [CrossRef]
- Depoortère, R.; Papp, M.; Gruca, P.; Lason-Tyburkiewicz, M.; Niemczyk, M.; Varney, M.A.; Newman-Tancredi, A. Cortical 5-hydroxytryptamine 1A receptor biased agonist, NLX-101, displays rapid-acting antidepressant-like properties in the rat chronic mild stress model. J. Psychopharmacol. 2019, 33, 1456–1466. [Google Scholar] [CrossRef] [PubMed]
- Głuch-Lutwin, M.; Sałaciak, K.; Gawalska, A.; Jamrozik, M.; Śniecikowska, J.; Newman-Tancredi, A.; Pytka, K. The selective 5-HT1A receptor biased agonists, F15599 and F13714, show antidepressant-like properties after a single administration in the mouse model of unpredictable chronic mild stress. Psychopharmacology 2021, 238, 2249–2260. [Google Scholar] [CrossRef] [PubMed]
- Depoortère, R.; Auclair, A.L.; Newman-Tancredi, A. NLX-101, a highly selective 5-HT1A receptor biased agonist, mediates antidepressant-like activity in rats via prefrontal cortex 5-HT1A receptors. Behav. Brain Res. 2021, 401, 113082. [Google Scholar] [CrossRef] [PubMed]
- Lladó-Pelfort, L.; Assié, M.B.; Newman-Tancredi, A.; Artigas, F.; Celada, P. Preferential in vivo action of F15599, a novel 5-HT1A receptor agonist, at postsynaptic 5-HT1A receptors. Br. J. Pharmacol. 2010, 160, 1929–1940. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aguiar, R.P.; Soares, L.M.; Meyer, E.; da Silveira, F.C.; Milani, H.; Newman-Tancredi, A.; Oliveira, R.M.W. Activation of 5-HT1A postsynaptic receptors by NLX-101 results in functional recovery and an increase in neuroplasticity in mice with brain ischemia. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2019, 99, 109832. [Google Scholar] [CrossRef] [PubMed]
- Buritova, J.; Berrichon, G.; Cathala, C.; Colpaert, F.; Cussac, D. Region-specific changes in 5-HT1A agonist-induced extracellular signal-regulated kinases 1/2 phosphorylation in rat brain: A quantitative ELISA study. Neuropharmacology 2009, 56, 350–361. [Google Scholar] [CrossRef]
- Detke, M.J.; Rickels, M.; Lucki, I. Active behaviors in the rat forced swimming test differentially produced by serotonergic and noradrenergic antidepressants. Psychopharmacology 1995, 121, 66–72. [Google Scholar] [CrossRef]
- Lucki, I. The forced swimming test as a model for core and component behavioral effects of antidepressant drugs. Behav. Pharmacol. 1997, 8, 523–532. [Google Scholar] [CrossRef] [PubMed]
- Sequeira-Cordero, A.; Mora-Gallegos, A.; Cuenca-Berger, P.; Fornaguera-Trías, J. Individual differences in the forced swimming test and neurochemical kinetics in the rat brain. Physiol. Behav. 2014, 128, 60–69. [Google Scholar] [CrossRef] [PubMed]
- Tanda, G.; Carboni, E.; Frau, R.; Di Chiara, G. Increase of extracellular dopamine in the prefrontal cortex: A trait of drugs with antidepressant potential? Psychopharmacology 1994, 115, 285–288. [Google Scholar] [CrossRef]
- Díaz-Mataix, L.; Scorza, M.C.; Bortolozzi, A.; Toth, M.; Celada, P.; Artigas, F. Involvement of 5-HT1A receptors in prefrontal cortex in the modulation of dopaminergic activity: Role in atypical antipsychotic action. J. Neurosci. 2005, 25, 10831–10843. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lladó-Pelfort, L.; Santana, N.; Ghisi, V.; Artigas, F.; Celada, P. 5-HT1A receptor agonists enhance pyramidal cell firing in prefrontal cortex through a preferential action on GABA interneurons. Cereb. Cortex 2012, 22, 1487–1497. [Google Scholar] [CrossRef] [Green Version]
- Floresco, S.B.; West, A.R.; Ash, B.; Moore, H.; Grace, A.A. Afferent modulation of dopamine neuron firing differentially regulates tonic and phasic dopamine transmission. Nat. Neurosci. 2003, 6, 968–973. [Google Scholar] [CrossRef] [PubMed]
- Carr, D.B.; Sesack, S.R. Projections from the rat prefrontal cortex to the ventral tegmental area: Target specificity in the synaptic associations with mesoaccumbens and mesocortical neurons. J. Neurosci. 2000, 20, 3864–3873. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Di Chiara, G.; Loddo, P.; Tanda, G. Reciprocal changes in prefrontal and limbic dopamine responsiveness to aversive and rewarding stimuli after chronic mild stress: Implications for the psychobiology of depression. Biol. Psychiatry 1999, 46, 1624–1633. [Google Scholar] [CrossRef]
- Dunlop, B.W.; Nemeroff, C.B. The role of dopamine in the pathophysiology of depression. Arch. Gen. Psychiatry 2007, 64, 327–337. [Google Scholar] [CrossRef]
- Witkin, J.M.; Monn, J.A.; Schoepp, D.D.; Li, X.; Overshiner, C.; Mitchell, S.N.; Carter, G.; Johnson, B.; Rasmussen, K.; Rorick-Kehn, L.M. The rapidly acting antidepressant ketamine and the mGlu2/3 receptor antagonist LY341495 rapidly engage dopaminergic mood circuits. J. Pharmacol. Exp. Ther. 2016, 358, 71–82. [Google Scholar] [CrossRef] [PubMed]
- Papp, M.; Gruca, P.; Lason-Tyburkiewicz, M.; Litwa, E.; Niemczyk, M.; Tota-Glowczyk, K.; Willner, P. Dopaminergic mechanisms in memory consolidation and antidepressant reversal of a chronic mild stress-induced cognitive impairment. Psychopharmacology 2017, 234, 2571–2585. [Google Scholar] [CrossRef] [Green Version]
- Tye, K.M.; Mirzabekov, J.J.; Warden, M.R.; Ferenczi, E.A.; Tsai, H.C.; Finkelstein, J.; Kim, S.Y.; Adhikari, A.; Thompson, K.R.; Andalman, A.S.; et al. Dopamine neurons modulate neural encoding and expression of depression-related behaviour. Nature 2013, 493, 537–541. [Google Scholar] [CrossRef] [Green Version]
- Moghaddam, B.; Adams, B.; Verma, A.; Daly, D. Activation of glutamatergic neurotransmission by ketamine: A novel step in the pathway from NMDA receptor blockade to dopaminergic and cognitive disruptions associated with the prefrontal cortex. J. Neurosci. 1997, 17, 2921–2927. [Google Scholar] [CrossRef]
- López-Gil, X.; Jiménez-Sánchez, L.; Romón, T.; Campa, L.; Artigas, F.; Adell, A. Importance of inter-hemispheric prefrontal connection in the effects of non-competitive NMDA receptor antagonists. Int. J. Neuropsychopharmacol. 2012, 15, 945–956. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- López-Gil, X.; Jiménez-Sánchez, L.; Campa, L.; Castro, E.; Frago, C.; Adell, A. Role of serotonin and noradrenaline in the rapid antidepressant action of ketamine. ACS Chem. Neurosci. 2019, 10, 3318–3326. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maeng, S.; Zarate, C.A., Jr.; Du, J.; Schloesser, R.J.; McCammon, J.; Chen, G.; Manji, H.K. Cellular mechanisms underlying the antidepressant effects of ketamine: Role of α-amino-3-hydroxy-5-methylisoxazole-4-propionic acid receptors. Biol. Psychiatry 2008, 63, 349–352. [Google Scholar] [CrossRef] [PubMed]
- Aleksandrova, L.R.; Phillips, A.G.; Wang, Y.T. Antidepressant effects of ketamine and the roles of AMPA glutamate receptors and other mechanisms beyond NMDA receptor antagonism. J. Psychiatry Neurosci. 2017, 42, 222–2229. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pham, T.H.; Defaix, C.; Nguyen, T.M.L.; Mendez-David, I.; Tritschler, L.; David, D.J.; Gardier, A.M. Cortical and raphe GABAA, AMPA receptors and glial GLT-1 glutamate transporter contribute to the sustained antidepressant activity of ketamine. Pharmacol. Biochem. Behav. 2020, 192, 172913. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Lee, B.; Liu, R.J.; Banasr, M.; Dwyer, J.M.; Iwata, M.; Li, X.Y.; Aghajanian, G.; Duman, R.S. mTOR-dependent synapse formation underlies the rapid antidepressant effects of NMDA antagonists. Science 2010, 329, 959–964. [Google Scholar] [CrossRef] [Green Version]
- Duman, R.S.; Aghajanian, G.K. Synaptic dysfunction in depression: Potential therapeutic targets. Science 2012, 338, 68–72. [Google Scholar] [CrossRef] [Green Version]
- Duman, R.S.; Aghajanian, G.K.; Sanacora, G.; Krystal, J.H. Synaptic plasticity and depression: New insights from stress and rapid-acting antidepressants. Nat. Med. 2016, 22, 238–249. [Google Scholar] [CrossRef] [Green Version]
- Aleksandrova, L.R.; Phillips, A.G. Neuroplasticity as a convergent mechanism of ketamine and classical psychedelics. Trends Pharmacol. Sci. 2021, 42, 929–942. [Google Scholar] [CrossRef]
- Newman-Tancredi, A.; Martel, J.C.; Assié, M.B.; Buritova, J.; Lauressergues, E.; Cosi, C.; Heusler, P.; Bruins Slot, L.; Colpaert, F.C.; Vacher, B.; et al. Signal transduction and functional selectivity of F15599, a preferential post-synaptic 5-HT1A receptor agonist. Br. J. Pharmacol. 2009, 156, 338–353. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Réus, G.Z.; Vieira, F.G.; Abelaira, H.M.; Michels, M.; Tomaz, D.B.; dos Santos, M.A.; Carlessi, A.S.; Neotti, M.V.; Matias, B.I.; Luz, J.R.; et al. MAPK signaling correlates with the antidepressant effects of ketamine. J. Psychiatr. Res. 2014, 55, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Seo, J.S.; Svenningsson, P. Modulation of ion channels and receptors by p11 (S100A10). Trends Pharmacol. Sci. 2020, 41, 487–497. [Google Scholar] [CrossRef] [PubMed]
- Svenningsson, P.; Chergui, K.; Rachleff, I.; Flajolet, M.; Zhang, X.; El Yacoubi, M.; Vaugeois, J.M.; Nomikos, G.G.; Greengard, P. Alterations in 5-HT1B receptor function by p11 in depression-like states. Science 2006, 311, 77–80. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seo, J.S.; Wei, J.; Qin, L.; Kim, Y.; Yan, Z.; Greengard, P. Cellular and molecular basis for stress-induced depression. Mol. Psychiatry 2017, 22, 1440–1447. [Google Scholar] [CrossRef] [Green Version]
- Svenningsson, P.; Kim, Y.; Warner-Schmidt, J.; Oh, Y.S.; Greengard, P. p11 and its role in depression and therapeutic responses to antidepressants. Nat. Rev. Neurosci. 2013, 14, 673–680. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duman, R.S.; Shinohara, R.; Fogaça, M.V.; Hare, B. Neurobiology of rapid-acting antidepressants: Convergent effects on GluA1-synaptic function. Mol. Psychiatry 2019, 24, 1816–1832. [Google Scholar] [CrossRef]
- Dwivedi, Y.; Rizavi, H.S.; Zhang, H.; Roberts, R.C.; Conley, R.R.; Pandey, G.N. Aberrant extracellular signal-regulated kinase (ERK)1/2 signalling in suicide brain: Role of ERK kinase 1 (MEK1). Int. J. Neuropsychopharmacol. 2009, 12, 1337–1354. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jernigan, C.S.; Goswami, D.B.; Austin, M.C.; Iyo, A.H.; Chandran, A.; Stockmeier, C.A.; Karolewicz, B. The mTOR signaling pathway in the prefrontal cortex is compromised in major depressive disorder. Prog. Neuropsychopharmacol. Biol. Psychiatry 2011, 35, 1774–1779. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duric, V.; Banasr, M.; Stockmeier, C.A.; Simen, A.A.; Newton, S.S.; Overholser, J.C.; Jurjus, G.J.; Dieter, L.; Duman, R.S. Altered expression of synapse and glutamate related genes in post-mortem hippocampus of depressed subjects. Int. J. Neuropsychopharmacol. 2013, 16, 69–82. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rosa, M.L.N.M.; Guimarães, F.S.; Pearson, R.C.A.; Del Bel, E.A. Effects of single or repeated restraint stress on GluR1 and GluR2 flip and flop mRNA expression in the hippocampal formation. Brain Res. Bull. 2002, 59, 117–124. [Google Scholar] [CrossRef]
- Toth, E.; Gersner, R.; Wilf-Yarkoni, A.; Raizel, H.; Dar, D.E.; Richter-Levin, G.; Levit, O.; Zangen, A. Age-dependent effects of chronic stress on brain plasticity and depressive behavior. J. Neurochem. 2008, 107, 522–532. [Google Scholar] [CrossRef] [PubMed]
- Autry, A.E.; Adachi, M.; Nosyreva, E.; Na, E.S.; Los, M.F.; Cheng, P.F.; Kavalali, E.T.; Monteggia, L.M. NMDA receptor blockade at rest triggers rapid behavioural antidepressant responses. Nature 2011, 475, 91–95. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abdallah, C.G.; Adams, T.G.; Kelmendi, B.; Esterlis, I.; Sanacora, G.; Krystal, J.H. Ketamine’s mechanism of action: A path to rapid-acting antidepressants. Depress. Anxiety 2016, 33, 689–697. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wohleb, E.S.; Gerhard, D.; Thomas, A.; Duman, R.S. Molecular and cellular mechanisms of rapid-acting antidepressants ketamine and scopolamine. Curr. Neuropharmacol. 2017, 15, 11–20. [Google Scholar] [CrossRef] [Green Version]
- Cryan, J.F.; Markou, A.; Lucki, I. Assessing antidepressant activity in rodents: Recent developments and future needs. Trends Pharmacol. Sci. 2002, 23, 238–245. [Google Scholar] [CrossRef]
- Paxinos, G.; Watson, C. The Rat Brain in Stereotaxic Coordinates; Academic Press: San Diego, CA, USA, 2008. [Google Scholar]








Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cabanu, S.; Pilar-Cuéllar, F.; Zubakina, P.; Florensa-Zanuy, E.; Senserrich, J.; Newman-Tancredi, A.; Adell, A. Molecular Signaling Mechanisms for the Antidepressant Effects of NLX-101, a Selective Cortical 5-HT1A Receptor Biased Agonist. Pharmaceuticals 2022, 15, 337. https://doi.org/10.3390/ph15030337
Cabanu S, Pilar-Cuéllar F, Zubakina P, Florensa-Zanuy E, Senserrich J, Newman-Tancredi A, Adell A. Molecular Signaling Mechanisms for the Antidepressant Effects of NLX-101, a Selective Cortical 5-HT1A Receptor Biased Agonist. Pharmaceuticals. 2022; 15(3):337. https://doi.org/10.3390/ph15030337
Chicago/Turabian StyleCabanu, Sharon, Fuencisla Pilar-Cuéllar, Paula Zubakina, Eva Florensa-Zanuy, Júlia Senserrich, Adrian Newman-Tancredi, and Albert Adell. 2022. "Molecular Signaling Mechanisms for the Antidepressant Effects of NLX-101, a Selective Cortical 5-HT1A Receptor Biased Agonist" Pharmaceuticals 15, no. 3: 337. https://doi.org/10.3390/ph15030337
APA StyleCabanu, S., Pilar-Cuéllar, F., Zubakina, P., Florensa-Zanuy, E., Senserrich, J., Newman-Tancredi, A., & Adell, A. (2022). Molecular Signaling Mechanisms for the Antidepressant Effects of NLX-101, a Selective Cortical 5-HT1A Receptor Biased Agonist. Pharmaceuticals, 15(3), 337. https://doi.org/10.3390/ph15030337