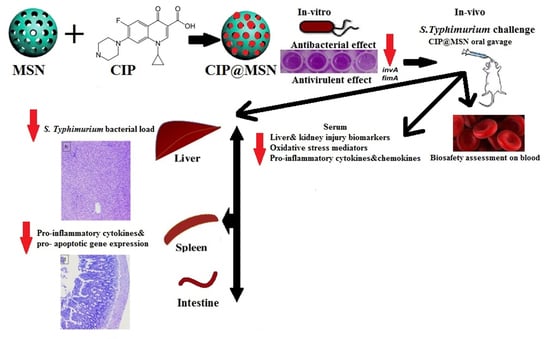
Novel In Vivo Assessment of Antimicrobial Efficacy of Ciprofloxacin Loaded Mesoporous Silica Nanoparticles against Salmonella typhimurium Infection
Abstract
:1. Introduction
2. Results
2.1. Characterization of Mesoporous Silica Nanoparticles (MSNs), Ciprofloxacin Loading, and Release
2.2. In Vitro Antibacterial Activity of CIP–MSN against Decreased CIP Susceptible Salmonella typhimurium
2.3. Biofilm Inhibition and Transcriptional Modulatory Effect of CIP–MSN
2.4. Hematological, Biochemical, Antioxidant, and Immunological Effect of CIP–MSN on Blood, and Serum Constituents
2.5. Inhibitory Effect of CIP–MSN on Hepatic Salmonella typhimurium Load
2.6. Pro-Inflammatory Cytokines Transcriptional Modulatory Effect of CIP–MSN
2.7. Modulation of Pro-Apoptoticgenes Expression
2.8. Histopathological Evaluation
3. Discussion
4. Materials and Methods
4.1. Synthesis and Characterization of Mesoporous Silica Nanoparticles (MSNs) and Ciprofloxacin Loading
4.2. Antibacterial Effect of Ciprofloxacin—Loaded Mesoporous Silica Nanoparticles
4.2.1. Agar Well Diffusion Assay
4.2.2. Minimum Inhibitory Concentration
4.3. Ciprofloxacin Loaded Mesoporous Silica Nanoparticles Effect on Biofilm Formation
4.4. Expression of Genes Associated Virulence in Biofilm Culture
4.5. Experimental Design, and Oral Challenging with Salmonella typhimurium
4.6. Hematological, Biochemical, Oxidative Stress Mediators, and Immunological Measurements
4.7. Quantification of S. typhimurium DNA Copies
4.8. Pro-Inflammatory Cytokines, and Pro-Apoptotic Gene Expression Analysis by Real-Time PCR
4.9. Histopathologic Evaluation
4.10. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Scallan, E.; Hoekstra, R.M.; Angulo, F.J.; Tauxe, R.V.; Widdowson, M.-A.; Roy, S.L.; Jones, J.L.; Griffin, P.M. Foodborne illness acquired in the United States—Major pathogens. Emerg. Infect. Dis. 2011, 17, 7. [Google Scholar] [CrossRef] [PubMed]
- Monack, D.M.; Mueller, A.; Falkow, S. Persistent bacterial infections: The interface of the pathogen and the host immune system. Nat. Rev. Microbiol. 2004, 2, 747–765. [Google Scholar] [CrossRef] [PubMed]
- Vazquez-Torres, A.; Jones-Carson, J.; Bäumler, A.J.; Falkow, S.; Valdivia, R.; Brown, W.; Le, M.; Berggren, R.; Parks, W.T.; Fang, F.C. Extraintestinal dissemination of Salmonella by CD18-expressing phagocytes. Nature 1999, 401, 804–808. [Google Scholar] [CrossRef] [PubMed]
- Melzak, K.A.; Melzak, S.A.; Gizeli, E.; Toca-Herrera, J.L. Cholesterol organization in phosphatidylcholine liposomes: A surface plasmon resonance study. Materials 2012, 5, 2306–2325. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, A.M.; Younis, E.E.; Ishida, Y.; Shimamoto, T. Genetic basis of multidrug resistance in Salmonella enterica serovars Enteritidis and Typhimurium isolated from diarrheic calves in Egypt. Acta Trop. 2009, 111, 144–149. [Google Scholar] [CrossRef]
- Foley, S.; Lynne, A.; Nayak, R. Salmonella challenges: Prevalence in swine and poultry and potential pathogenicity of such isolates. J. Anim. Sci. 2008, 86, E149–E162. [Google Scholar] [CrossRef]
- Hassing, R.-J.; Goessens, W.; Mevius, D.J.; van Pelt, W.; Mouton, J.W.; Verbon, A.; Van Genderen, P. Decreased ciprofloxacin susceptibility in Salmonella Typhi and Paratyphi infections in ill-returned travellers: The impact on clinical outcome and future treatment options. Eur. J. Clin. Microbiol. Infect. Dis. 2013, 32, 1295–1301. [Google Scholar] [CrossRef]
- Ahmed, A.I.; van der Heijden, F.M.; van den Berkmortel, H.; Kramers, K. A man who wanted to commit suicide by hanging himself: An adverse effect of ciprofloxacin. Gen. Hosp. Psychiatry 2011, 33, 82.e85–82.e87. [Google Scholar] [CrossRef]
- Barnhart, M.M.; Chapman, M.R. Curli biogenesis and function. Annu. Rev. Microbiol. 2006, 60, 131–147. [Google Scholar] [CrossRef] [Green Version]
- World Health Organization. Antimicrobial Resistance: Global Report on Surveillance; World Health Organization: Geneva, Switzerland, 2014. [Google Scholar]
- Slowing, I.I.; Vivero-Escoto, J.L.; Wu, C.-W.; Lin, V.S.-Y. Mesoporous silica nanoparticles as controlled release drug delivery and gene transfection carriers. Adv. Drug Deliv. Rev. 2008, 60, 1278–1288. [Google Scholar] [CrossRef]
- Egger, S.; Lehmann, R.P.; Height, M.J.; Loessner, M.J.; Schuppler, M. Antimicrobial properties of a novel silver-silica nanocomposite material. Appl. Environ. Microbiol. 2009, 75, 2973–2976. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sulaiman, G.M.; Mohammed, W.H.; Marzoog, T.R.; Al-Amiery, A.A.A.; Kadhum, A.A.H.; Mohamad, A.B. Green synthesis, antimicrobial and cytotoxic effects of silver nanoparticles using Eucalyptus chapmaniana leaves extract. Asian Pac. J. Trop. Biomed. 2013, 3, 58–63. [Google Scholar] [CrossRef] [Green Version]
- Seleem, M.N.; Munusamy, P.; Ranjan, A.; Alqublan, H.; Pickrell, G.; Sriranganathan, N. Silica-antibiotic hybrid nanoparticles for targeting intracellular pathogens. Antimicrob. Agents Chemother. 2009, 53, 4270–4274. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiao, Y.; Sun, Y.; Chang, B.; Lu, D.; Yang, W. Redox-and temperature-controlled drug release from hollow mesoporous silica nanoparticles. Chem.—Eur. J. 2013, 19, 15410–15420. [Google Scholar] [CrossRef] [PubMed]
- Mudakavi, R.J.; Raichur, A.M.; Chakravortty, D. Lipid coated mesoporous silica nanoparticles as an oral delivery system for targeting and treatment of intravacuolar Salmonella infections. RSC Adv. 2014, 4, 61160–61166. [Google Scholar] [CrossRef]
- de Juan Mora, B.; Filipe, L.; Forte, A.; Santos, M.M.; Alves, C.; Teodoro, F.; Pedrosa, R.; Ribeiro Carrott, M.; Branco, L.C.; Gago, S. Boosting Antimicrobial Activity of Ciprofloxacin by Functionalization of Mesoporous Silica Nanoparticles. Pharmaceutics 2021, 13, 218. [Google Scholar] [CrossRef]
- E.C.o.A.S. Testing., Breakpoint Tables for Interpretation of Mics and Zone Diameters. Version 7.1, Valid from 2017-03-10. Available online: http://www.eucast.org/clinical_breakpoints/ (accessed on 20 August 2019).
- Borges, O.; Cordeiro-da-Silva, A.; Romeijn, S.G.; Amidi, M.; de Sousa, A.; Borchard, G.; Junginger, H.E. Uptake studies in rat Peyer’s patches, cytotoxicity and release studies of alginate coated chitosan nanoparticles for mucosal vaccination. J. Control. Release 2006, 114, 348–358. [Google Scholar] [CrossRef] [Green Version]
- Rivero, P.J.; Urrutia, A.; Goicoechea, J.; Zamarreño, C.R.; Arregui, F.J.; Matías, I.R. An antibacterial coating based on a polymer/sol-gel hybrid matrix loaded with silver nanoparticles. Nanoscale Res. Lett. 2011, 6, 305. [Google Scholar] [CrossRef] [Green Version]
- Camporotondi, D.; Foglia, M.; Alvarez, G.; Mebert, A.; Diaz, L.; Coradin, T.; Desimone, M. Antimicrobial properties of silica modified nanoparticles. Microb. Pathog. Strateg. Combat. Sci. Technol. Educ. 2013, 2, 283–290. [Google Scholar]
- Van Houdt, R.; Michiels, C. Biofilm formation and the food industry, a focus on the bacterial outer surface. J. Appl. Microbiol. 2010, 109, 1117–1131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, S.-H.; Wei, C.-i. Biofilm formation by multidrug-resistant Salmonella enterica serotype Typhimurium phage type DT104 and other pathogens. J. Food Prot. 2007, 70, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Morones, J.R.; Elechiguerra, J.L.; Camacho, A.; Holt, K.; Kouri, J.B.; Ramírez, J.T.; Yacaman, M.J. The bactericidal effect of silver nanoparticles. Nanotechnology 2005, 16, 2346. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Massa, M.A.; Covarrubias, C.; Bittner, M.; Fuentevilla, I.A.; Capetillo, P.; Von Marttens, A.; Carvajal, J.C. Synthesis of new antibacterial composite coating for titanium based on highly ordered nanoporous silica and silver nanoparticles. Mater. Sci. Eng. C 2014, 45, 146–153. [Google Scholar] [CrossRef] [PubMed]
- Naughton, P.J.; Grant, G.; Bardocz, S.; Allen-Vercoe, E.; Woodward, M.J.; Pusztai, A. Expression of type 1 fimbriae (SEF 21) of Salmonella enterica serotype enteritidis in the early colonisation of the rat intestine. J. Med. Microbiol. 2001, 50, 191–197. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boddicker, J.D.; Ledeboer, N.A.; Jagnow, J.; Jones, B.D.; Clegg, S. Differential binding to and biofilm formation on, HEp-2 cells by Salmonella enterica serovar Typhimurium is dependent upon allelic variation in the fimH gene of the fim gene cluster. Mol. Microbiol. 2002, 45, 1255–1265. [Google Scholar] [CrossRef] [Green Version]
- Ghosh, S.; Mittal, A.; Vohra, H.; Ganguly, N.K. Interaction of a rat intestinal brush border membrane glycoprotein with type-1 fimbriae of Salmonella typhimurium. Mol. Cell. Biochem. 1996, 158, 125–131. [Google Scholar] [CrossRef]
- Khan, T.A.; Zafar, F. Haematological study in response to varying doses of estrogen in broiler chicken. Int. J. Poult. Sci. 2005, 4, 748–751. [Google Scholar]
- McGill, M.R. The past and present of serum aminotransferases and the future of liver injury biomarkers. EXCLI J. 2016, 15, 817–828. [Google Scholar] [CrossRef]
- Kengni, F.; Fodouop, S.P.; Tala, D.S.; Djimeli, M.N.; Fokunang, C.; Gatsing, D. Antityphoid properties and toxicity evaluation of Harungana madagascariensis Lam (Hypericaceae) aqueous leaf extract. J. Ethnopharmacol. 2016, 179, 137–145. [Google Scholar] [CrossRef]
- Osman, K.M.; El-Enbaawy, M.I.; Ezzeldin, N.A.; Hussein, H.M. Nitric oxide and lysozyme production as an impact to Clostridium perfringens mastitis. Comp. Immunol. Microbiol. Infect. Dis. 2010, 33, 505–511. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Li, S.; Jia, H.; Si, X.; Lei, Y.; Lyu, J.; Dai, Z.; Wu, Z. Protective Effects of Cinnamaldehyde on the Inflammatory Response, Oxidative Stress, and Apoptosis in Liver of Salmonella typhimurium-Challenged Mice. Molecules 2021, 26, 2309. [Google Scholar] [CrossRef] [PubMed]
- Dai, C.; Xiao, X.; Li, D.; Tun, S.; Wang, Y.; Velkov, T.; Tang, S. Chloroquine ameliorates carbon tetrachloride-induced acute liver injury in mice via the concomitant inhibition of inflammation and induction of apoptosis. Cell Death Dis. 2018, 9, 1164. [Google Scholar] [CrossRef] [PubMed]
- Kostura, M.J.; Tocci, M.J.; Limjuco, G.; Chin, J.; Cameron, P.; Hillman, A.G.; Chartrain, N.A.; Schmidt, J.A. Identification of a monocyte specific pre-interleukin 1 beta convertase activity. Proc. Natl. Acad. Sci. USA 1989, 86, 5227–5231. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lara-Tejero, M.; Sutterwala, F.S.; Ogura, Y.; Grant, E.P.; Bertin, J.; Coyle, A.J.; Flavell, R.A.; Galán, J.E. Role of the caspase-1 inflammasome in Salmonella typhimurium pathogenesis. J. Exp. Med. 2006, 203, 1407–1412. [Google Scholar] [CrossRef]
- Martinon, F.; Tschopp, J. Inflammatory caspases: Linking an intracellular innate immune system to autoinflammatory diseases. Cell 2004, 117, 561–574. [Google Scholar] [CrossRef] [Green Version]
- Brennan, M.A.; Cookson, B.T. Salmonella induces macrophage death by caspase-1-dependent necrosis. Mol. Microbiol. 2000, 38, 31–40. [Google Scholar] [CrossRef] [Green Version]
- Sairanen, T.; Szepesi, R.; Karjalainen-Lindsberg, M.L.; Saksi, J.; Paetau, A.; Lindsberg, P.J. Neuronal caspase-3 and PARP-1 correlate differentially with apoptosis and necrosis in ischemic human stroke. Acta Neuropathol. 2009, 118, 541–552. [Google Scholar] [CrossRef]
- Tian, T.; Wang, Z. Pathomechanisms of Oxidative Stress in Inflammatory Bowel Disease and Potential Antioxidant Therapies. Oxidative Med. Cell. Longev. 2017, 2017, 4535194. [Google Scholar] [CrossRef]
- Allam, N.G.; Eldrieny, E.A.E.-A.; Mohamed, A.Z. Effect of combination therapy between thyme oil and ciprofloxacin on ulcer-forming Shigella flexneri. J. Infect. Dev. Ctries. 2015, 9, 486–495. [Google Scholar] [CrossRef] [Green Version]
- Nawaz, A.; Ali, S.M.; Rana, N.F.; Tanweer, T.; Batool, A.; Webster, T.J.; Menaa, F.; Riaz, S.; Rehman, Z.; Batool, F. Ciprofloxacin-Loaded Gold Nanoparticles against Antimicrobial Resistance: An In Vivo Assessment. Nanomaterials 2021, 11, 3152. [Google Scholar] [CrossRef] [PubMed]
- Bouchoucha, M.; Cote, M.-F.; C.-Gaudreault, R.; Fortin, M.-A.; Kleitz, F. Size-controlled functionalized mesoporous silica nanoparticles for tunable drug release and enhanced anti-tumoral activity. Chem. Mater. 2016, 28, 4243–4258. [Google Scholar] [CrossRef]
- Ibrahim, D.; Abdelfattah-Hassan, A.; Badawi, M.; Ismail, T.A.; Bendary, M.M.; Abdelaziz, A.M.; Mosbah, R.A.; Mohamed, D.I.; Arisha, A.H.; Abd El-Hamid, M.I. Thymol nanoemulsion promoted broiler chicken’s growth, gastrointestinal barrier and bacterial community and conferred protection against Salmonella Typhimurium. Sci. Rep. 2021, 11, 7742. [Google Scholar] [CrossRef] [PubMed]
- Selim, M.S.; Hamouda, H.; Hao, Z.; Shabana, S.; Chen, X. Design of γ-AlOOH, γ-MnOOH, and α-Mn2O3 nanorods as advanced antibacterial active agents. Dalton Trans. 2020, 49, 8601–8613. [Google Scholar] [CrossRef]
- Parvekar, P.; Palaskar, J.; Metgud, S.; Maria, R.; Dutta, S. The minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) of silver nanoparticles against Staphylococcus aureus. Biomater. Investig. Dent. 2020, 7, 105–109. [Google Scholar] [CrossRef]
- Lamas, A.; Fernandez-No, I.; Miranda, J.; Vázquez, B.; Cepeda, A.; Franco, C. Biofilm formation and morphotypes of Salmonella enterica subsp. arizonae differs from those of other Salmonella enterica subspecies in isolates from poultry houses. J. Food Prot. 2016, 79, 1127–1134. [Google Scholar] [CrossRef]
- Stepanović, S.; Ćirković, I.; Ranin, L.; S✓vabić-Vlahović, M. Biofilm formation by Salmonella spp. and Listeria monocytogenes on plastic surface. Lett. Appl. Microbiol. 2004, 38, 428–432. [Google Scholar] [CrossRef]
- Roehm, N.W.; Rodgers, G.H.; Hatfield, S.M.; Glasebrook, A.L. An improved colorimetric assay for cell proliferation and viability utilizing the tetrazolium salt XTT. J. Immunol. Methods 1991, 142, 257–265. [Google Scholar] [CrossRef]
- Xu, H.; Lee, H.-Y.; Ahn, J. Growth and virulence properties of biofilm-forming Salmonella enterica serovar Typhimurium under different acidic conditions. Appl. Environ. Microbiol. 2010, 76, 7910–7917. [Google Scholar] [CrossRef] [Green Version]
- EN ISO 6579:2002/A1:2007; Microbiology of Food and Animal Feeding Stuffs: Horizontal Method for the Detection of Salmonella spp.—Amendment 1: Annex D: Detection of Salmonella spp. in Animal Faeces and in Environmental Samples from the Primary Production Stage. ISO: Geneva, Switzerland, 2007.
- Hawk, P.B. Practical Physiological Chemistry; P. Blakiston’s Son & Company: Philadelphia, PA, USA, 1916. [Google Scholar]
- Motor, S.; Ozturk, S.; Ozcan, O.; Gurpinar, A.B.; Can, Y.; Yuksel, R.; Yenin, J.Z.; Seraslan, G.; Ozturk, O.H. Evaluation of total antioxidant status, total oxidant status and oxidative stress index in patients with alopecia areata. Int. J. Clin. Exp. Med. 2014, 7, 1089. [Google Scholar]
- Hutchinson, M.K. C-Reactive Protein in Serum by Nephelometry. University of Washington Medical Center, Department of Laboratory Medicine, Immunology Division. 2000. Available online: http://www.cdc.gov/nchs/data/nhanes/nhanes_01_02/l11_b_met_c_reactive_protein.pdf (accessed on 11 August 2014).
- Huyghebaert, G.; Ducatelle, R.; Van Immerseel, F. An update on alternatives to antimicrobial growth promoters for broilers. Vet. J. 2011, 187, 182–188. [Google Scholar] [CrossRef] [Green Version]
- Alandiyjany, M.N.; Kishawy, A.T.; Abdelfattah-Hassan, A.; Eldoumani, H.; Elazab, S.T.; El-Mandrawy, S.A.; Saleh, A.A.; ElSawy, N.A.; Attia, Y.A.; Arisha, A.H. Nano-silica and magnetized-silica mitigated lead toxicity: Their efficacy on bioaccumulation risk, performance, and apoptotic targeted genes in Nile tilapia (Oreochromis niloticus). Aquat. Toxicol. 2022, 242, 106054. [Google Scholar] [CrossRef] [PubMed]






| Zone of Inhibition (mm) | MIC (mg/L) | MBC (mg/L) | |
|---|---|---|---|
| CIP | 8.7 b ± 0.2 | 1.0 | 2.0 |
| MSN | 3.4 c ± 0.37 | ND | ND |
| CIP–MSN | 40.5 a ± 0.4 | 0.03125 | 0.0625 |
| At 7 Days Postinfection | At 14 Days Postinfection | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Groups | NC | PC | CIP | MSN | CIP@ MSN | p Value | SEM | NC | PC | CIP | MSN | CIP–MSN | p Value | SEM |
| RBCs | 12.5 a | 7.96 b | 9.17 ab | 8.23 b | 11.19 a | <0.001 | 0.09 | 12.63 a | 9.66 c | 10.30 b | 10.20 b | 11.60 ab | 0.09 | 12.63 a |
| Hb | 12.9 a | 6.3 b | 11.2 a | 7.10 b | 11.56 a | <0.001 | 0.07 | 12.80 a | 8.80 c | 11.98 a | 10.26 b | 12.00 a | 0.12 | 12.80 a |
| PCV | 41.4 a | 24.5 c | 37.43 b | 26.60 c | 39.5 ab | 0.03 | 0.14 | 42.30 a | 29.69 c | 38.6 ab | 37.75 b | 39.5 a | 0.13 | 42.30 a |
| Biochemical biomarkers for tissue injury analysis | ||||||||||||||
| ALT (U/L) | 48.3 c | 74.7 a | 58.7 b | 65.63 ab | 50.86 c | <0.001 | 0.21 | 46.7 d | 85.1 a | 52.36 c | 57.99 b | 48.36 cd | 0.25 | 48.3 c |
| AST (U/L) | 25.2 b | 42.3 a | 27.6 b | 26.30 b | 25.53 b | <0.001 | 0.19 | 24.7 c | 48.4 a | 25.8 c | 34.96 b | 23.46 c | 0.16 | 25.2 b |
| Urea (μmol/L) | 32.1 c | 52.3 a | 38.76 b | 50.90 a | 35.53 bc | <0.001 | 0.12 | 31.6 c | 59.8 a | 32.6 c | 41.90 b | 32.1 c | 0.14 | 32.1 c |
| Creatinine (mg/dL) | 1.33 b | 2.73 a | 1.45 b | 1.95 ab | 1.41 b | <0.001 | 0.09 | 1.34 c | 3.07 a | 1.38 c | 2.35 b | 1.33 c | 0.08 | 1.33 b |
| Oxidative stress mediators analysis | ||||||||||||||
| NO | 153.8 d | 532.60 a | 246.40 b | 480.60 | 185.36 c | 0.027 | 0.25 | 150.5 e | 559.1 a | 202.16 d | 140.22 b | 177.66 c | 0.31 | 153.8 d |
| MPO | 2.21 e | 10.80 a | 7.62 c | 8.65 b | 6.11 d | 0.03 | 0.09 | 2.16 c | 10.08 a | 6.76 b | 3.57 c | 0.10 | 2.21 c | |
| CRP | 1.12 e | 54.30 a | 24.4 c | 50.36 b | 16.26 d | 0.14 | 0.14 | 1.16 e | 53.3 a | 17.4 c | 44.30 b | 9.2 d | <0.001 | 0.08 |
| Chemokines and pro-inflammatory cytokines analysis | ||||||||||||||
| CXCL10 | 156.00 e | 393.70 a | 224.80 c | 385.64 b | 181.6 d | 0.03 | 0.24 | 152.6 d | 416.3 a | 197.9 b | 400.02 a | 161.83 c | 0.02 | 0.24 |
| CXCL11 | 108.86 e | 238.70 a | 161.30 c | 220.30 b | 130.53 d | 0.02 | 0.29 | 108.5 d | 243.4 a | 140.6 b | 239.23 a | 113.16 c | 0.01 | 0.31 |
| IFN- γ | 38.36 e | 99.50 a | 53.26 c | 82.23 b | 43.00 d | <0.001 | 0.11 | 38.7 c | 104.4 a | 46.4 b | 100.23 a | 38.36 c | 0.03 | 0.23 |
| IL-6 | 16.28 c | 47.36 a | 47.93 a | 45.636 a | 34.86 b | <0.001 | 0.13 | 15.86 c | 47.56 a | 34.56 b | 49.36 a | 16.59 c | <0.001 | 0.14 |
| TNF-α | 11.5 c | 23.61 a | 16.50 b | 20.36 a | 16.4 b | <0.001 | 0.10 | 11.7 c | 21.96 a | 15.1 b | 22.90 a | 13.2 c | <0.001 | 0.06 |
| Target Gene | Primer Sequence (5′-3′) | Accession No./Reference |
|---|---|---|
| iNOS | F-ACCTTCCGGGCAGCCTGTGA R-CAAGGAGGGTGGTGCGGCTG-3′ | NM_012611 |
| COX-2 | F-GCTCAGCC ATACAGCAAATCC R-GGGAGTCGGGCAAT CATCAG | NM_017232 |
| Caspase-3 | F-GCAGCTAACCTCAGAGAGACATTC R-ACGAGTAAGGTCATTTTTATTCCTGACTT | NM_012922 |
| Bcl-2 | F-TGCGCTCAGCCCTGTG R-GGTAGCGACGAGAGAAGTCATC | NM_016993 |
| BAX | F-CAAGAAGCTGAGCGAGTGTCT R-CAATCATCCTCTGCAGCTCCATATT | NM_017059 |
| Cytochrome C | F-TTTGAATTCCTCATTAGTAGCTTTTTTGG R-CCATCCCTACGCATCCTTTAC | NM_012839 |
| IL-1β | F-TGACAGACCCCAAAAGATTAAGG R-CTCATCTGGACAGCCCAAGTC | NM_031512.2 |
| IL-6 | F-CCACCAGGAACGAAAGTCAAC R-TTGCGGAGAGAAACTTCATAGCT | NM_012589.2 |
| TNF-α | F-CAGCCGATTTGCCATTTCA R-AGGGCTCTTGATGGCAGAGA | L19123.1 |
| β-actin | F-CGCAGTTGGTTGGAGCAAA R-ACAATCAAAGTCCTCAGCCACAT | V01217.1 |
| GAPDH | F-TGCTGGTGCTGAGTATGTCG-3′ R-TTGAGAGCAATGCCAGCC-3′ | NM_017008 |
| invA. | F-ACAGTGCTCGTTTACGACCTGAAT R-AGACGACTGGTACTGATCGATAAT | [55] |
| FimA | F-TTGCGAGTCTGATGTTTGTCG 62 R-CACGCTCACCGGAGTAGGAT | [55] |
| 16S rRNA. | F-AGGCCTTCGGGTTGTAAAGT R-GTTAGCCGGTGCTTCTTCTG | [55] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alandiyjany, M.N.; Abdelaziz, A.S.; Abdelfattah-Hassan, A.; Hegazy, W.A.H.; Hassan, A.A.; Elazab, S.T.; Mohamed, E.A.A.; El-Shetry, E.S.; Saleh, A.A.; ElSawy, N.A.; et al. Novel In Vivo Assessment of Antimicrobial Efficacy of Ciprofloxacin Loaded Mesoporous Silica Nanoparticles against Salmonella typhimurium Infection. Pharmaceuticals 2022, 15, 357. https://doi.org/10.3390/ph15030357
Alandiyjany MN, Abdelaziz AS, Abdelfattah-Hassan A, Hegazy WAH, Hassan AA, Elazab ST, Mohamed EAA, El-Shetry ES, Saleh AA, ElSawy NA, et al. Novel In Vivo Assessment of Antimicrobial Efficacy of Ciprofloxacin Loaded Mesoporous Silica Nanoparticles against Salmonella typhimurium Infection. Pharmaceuticals. 2022; 15(3):357. https://doi.org/10.3390/ph15030357
Chicago/Turabian StyleAlandiyjany, Maher N., Ahmed S. Abdelaziz, Ahmed Abdelfattah-Hassan, Wael A. H. Hegazy, Arwa A. Hassan, Sara T. Elazab, Eman A. A. Mohamed, Eman S. El-Shetry, Ayman A. Saleh, Naser A. ElSawy, and et al. 2022. "Novel In Vivo Assessment of Antimicrobial Efficacy of Ciprofloxacin Loaded Mesoporous Silica Nanoparticles against Salmonella typhimurium Infection" Pharmaceuticals 15, no. 3: 357. https://doi.org/10.3390/ph15030357
APA StyleAlandiyjany, M. N., Abdelaziz, A. S., Abdelfattah-Hassan, A., Hegazy, W. A. H., Hassan, A. A., Elazab, S. T., Mohamed, E. A. A., El-Shetry, E. S., Saleh, A. A., ElSawy, N. A., & Ibrahim, D. (2022). Novel In Vivo Assessment of Antimicrobial Efficacy of Ciprofloxacin Loaded Mesoporous Silica Nanoparticles against Salmonella typhimurium Infection. Pharmaceuticals, 15(3), 357. https://doi.org/10.3390/ph15030357