Phytosomes as a Plausible Nano-Delivery System for Enhanced Oral Bioavailability and Improved Hepatoprotective Activity of Silymarin
Abstract
:1. Introduction
2. Results and Discussion
2.1. Preparation of Silymarin Phytosomal Complex
2.2. Optimization of Silymarin Phytosomal Complex
2.3. Evaluation of Silymarin Phytosomal Complex
2.3.1. Average Particle Size, Polydispersity Index and Zeta Potential
2.3.2. Surface Morphology
2.4. Structural Characterization Silymarin Phytosomal Complex
2.4.1. Fourier Transform Infrared Spectroscopy (FTIR)
2.4.2. Differential Scanning Calorimetry (DSC)
2.4.3. X-ray Powder Diffractometry (XRD)
2.5. Solubility Study
2.6. Dissolution Study
2.7. In Vivo Hepatoprotective Effect of Silymarin-Phospholipid Phytosomal Complex
2.8. In Vivo Antioxidant Activity of Silymarin-Phospholipid Phytosomal Complex
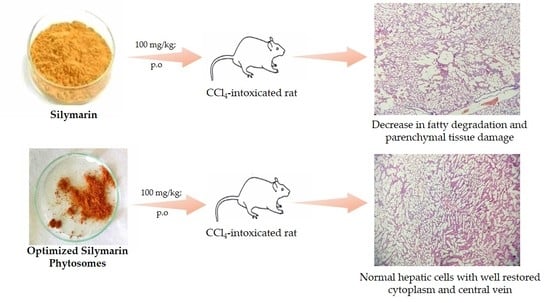
2.9. Histopathological Studies
2.10. Pharmacokinetics Study
3. Materials and Methods
3.1. Materials
3.2. Preparation of Silymarin Phytosomal Complex
3.3. Design of Experiments
3.4. Evaluation of Silymarin Phytosomes
3.4.1. Average Particle Size, Polydispersity Index and Zeta Potential
3.4.2. Surface Morphology
3.4.3. Estimation of Drug Content
3.4.4. Fourier Transform Infrared Spectroscopy (FTIR)
3.4.5. Differential Scanning Calorimetry (DSC)
3.4.6. X-ray Powder Diffractometry (XRD)
3.5. Solubility Study
3.6. In Vitro Dissolution Study
3.7. In Vivo Studies
3.7.1. Animals
3.7.2. In Vivo Hepatoprotective and Antioxidant Activity Studies
3.7.3. Histopathological Studies
3.7.4. In Vivo Pharmacokinetic Studies
3.8. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bhatt, A. Phytopharmaceuticals: A new drug class regulated in India. Perspect. Clin. Res. 2016, 7, 59–61. [Google Scholar] [CrossRef] [PubMed]
- Ekor, M. The growing use of herbal medicines: Issues relating to adverse reactions and challenges in monitoring safety. Front. Pharmacol. 2014, 4, 177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, M.; Qiu, Q.; Luo, X.; Liu, X.; Sun, J.; Wang, C.; Lin, X.; Deng, Y.; Song, Y. Phyto-phospholipid complexes (phytosomes): A novel strategy to improve the bioavailability of active constituents. Asian J. Pharm. Sci. 2019, 14, 265–274. [Google Scholar] [CrossRef] [PubMed]
- Aqil, F.; Munagala, R.; Jeyabalan, J.; Vadhanam, M.V. Bioavailability of phytochemicals and its enhancement by drug delivery systems. Cancer Lett. 2013, 334, 133–141. [Google Scholar] [CrossRef] [Green Version]
- Abdallah, M.H.; Abu Lila, A.S.; Shawky, S.M.; Almansour, K.; Alshammari, F.; Khafagy, E.-S.; Makram, T.S. Experimental Design and Optimization of Nano-Transfersomal Gel to Enhance the Hypoglycemic Activity of Silymarin. Polymers 2022, 14, 508. [Google Scholar] [CrossRef]
- Federico, A.; Dallio, M.; Loguercio, C. Silymarin/Silybin and Chronic Liver Disease: A Marriage of Many Years. Molecules 2017, 22, 191. [Google Scholar] [CrossRef] [Green Version]
- Ramasamy, K.; Agarwal, R. Multitargeted therapy of cancer by silymarin. Cancer Lett. 2008, 269, 352–362. [Google Scholar] [CrossRef] [Green Version]
- Liu, C.-H.; Jassey, A.; Hsu, H.-Y.; Lin, L.-T. Antiviral Activities of Silymarin and Derivatives. Molecules 2019, 24, 1552. [Google Scholar] [CrossRef] [Green Version]
- Borah, A.; Paul, R.; Choudhury, S.; Choudhury, A.; Bhuyan, B.; Das Talukdar, A.; Dutta Choudhury, M.; Mohanakumar, K.P. Neuroprotective potential of silymarin against CNS disorders: Insight into the pathways and molecular mechanisms of action. CNS Neurosci. Ther. 2013, 19, 847–853. [Google Scholar] [CrossRef]
- Pérez, H.J.; Carrillo, S.C.; García, E.; Ruiz-Mar, G.; Pérez-Tamayo, R.; Chavarría, A. Neuroprotective effect of silymarin in a MPTP mouse model of Parkinson’s disease. Toxicology 2014, 319, 38–43. [Google Scholar] [CrossRef]
- Di Costanzo, A.; Angelico, R. Formulation Strategies for Enhancing the Bioavailability of Silymarin: The State of the Art. Molecules 2019, 24, 2155. [Google Scholar] [CrossRef] [Green Version]
- Martínez-Ballesta, M.; Gil-Izquierdo, Á.; García-Viguera, C.; Domínguez-Perles, R. Nanoparticles and Controlled Delivery for Bioactive Compounds: Outlining Challenges for New "Smart-Foods" for Health. Foods 2018, 7, 72. [Google Scholar] [CrossRef] [Green Version]
- Yang, B.; Dong, Y.; Wang, F.; Zhang, Y. Nanoformulations to Enhance the Bioavailability and Physiological Functions of Polyphenols. Molecules 2020, 25, 4613. [Google Scholar] [CrossRef]
- Kotta, S.; Khan, A.W.; Pramod, K.; Ansari, S.H.; Sharma, R.K.; Ali, J. Exploring oral nanoemulsions for bioavailability enhancement of poorly water-soluble drugs. Expert Opin. Drug Deliv. 2012, 9, 585–598. [Google Scholar] [CrossRef]
- Alharbi, W.S.; Almughem, F.A.; Almehmady, A.M.; Jarallah, S.J.; Alsharif, W.K.; Alzahrani, N.M.; Alshehri, A.A. Phytosomes as an Emerging Nanotechnology Platform for the Topical Delivery of Bioactive Phytochemicals. Pharmaceutics 2021, 13, 1475. [Google Scholar] [CrossRef]
- Barani, M.; Sangiovanni, E.; Angarano, M.; Rajizadeh, M.A.; Mehrabani, M.; Piazza, S.; Gangadharappa, H.V.; Pardakhty, A.; Mehrbani, M.; Dell’Agli, M.; et al. Phytosomes as Innovative Delivery Systems for Phytochemicals: A Comprehensive Review of Literature. Int. J. Nanomed. 2021, 16, 6983–7022. [Google Scholar] [CrossRef]
- Telange, D.R.; Patil, A.T.; Pethe, A.M.; Fegade, H.; Anand, S.; Dave, V.S. Formulation and characterization of an apigenin-phospholipid phytosome (APLC) for improved solubility, in vivo bioavailability, and antioxidant potential. Eur. J. Pharm. Sci. 2017, 108, 36–49. [Google Scholar] [CrossRef] [Green Version]
- Rathee, S.; Kamboj, A. Optimization and development of antidiabetic phytosomes by the Box-Behnken design. J. Liposome Res. 2018, 28, 161–172. [Google Scholar] [CrossRef]
- Bannunah, A.M.; Vllasaliu, D.; Lord, J.; Stolnik, S. Mechanisms of Nanoparticle Internalization and Transport Across an Intestinal Epithelial Cell Model: Effect of Size and Surface Charge. Mol. Pharm. 2014, 11, 4363–4373. [Google Scholar] [CrossRef]
- Al Saqr, A.; Khafagy, E.S.; Alalaiwe, A.; Aldawsari, M.F.; Alshahrani, S.M.; Anwer, M.K.; Khan, S.; Lila, A.S.A.; Arab, H.H.; Hegazy, W.A.H. Synthesis of Gold Nanoparticles by Using Green Machinery: Characterization and In Vitro Toxicity. Nanomaterials 2021, 11, 808. [Google Scholar] [CrossRef]
- Hou, Z.; Li, Y.; Huang, Y.; Zhou, C.; Lin, J.; Wang, Y.; Cui, F.; Zhou, S.; Jia, M.; Ye, S.; et al. Phytosomes loaded with mitomycin C-soybean phosphatidylcholine complex developed for drug delivery. Mol. Pharm. 2013, 10, 90–101. [Google Scholar] [CrossRef]
- Hooresfand, Z.; Ghanbarzadeh, S.; Hamishehkar, H. Preparation and Characterization of Rutin-loaded Nanophytosomes. Pharm. Sci. 2015, 21, 145–151. [Google Scholar] [CrossRef] [Green Version]
- Ohtake, S.; Schebor, C.; Palecek, S.P.; de Pablo, J.J. Phase behavior of freeze-dried phospholipid–cholesterol mixtures stabilized with trehalose. Biochim. Biophys. Acta-Biomembr. 2005, 1713, 57–64. [Google Scholar] [CrossRef] [Green Version]
- Yanyu, X.; Yunmei, S.; Zhipeng, C.; Qineng, P. The preparation of silybin-phospholipid complex and the study on its pharmacokinetics in rats. Int. J. Pharm. 2006, 307, 77–82. [Google Scholar] [CrossRef]
- Cai, X.; Luan, Y.; Jiang, Y.; Song, A.; Shao, W.; Li, Z.; Zhao, Z. Huperzine A-phospholipid complex-loaded biodegradable thermosensitive polymer gel for controlled drug release. Int. J. Pharm. 2012, 433, 102–111. [Google Scholar] [CrossRef]
- Sherikar, A.; Siddique, M.U.M.; More, M.; Goyal, S.N.; Milivojevic, M.; Alkahtani, S.; Alarifi, S.; Hasnain, M.S.; Nayak, A.K. Preparation and Evaluation of Silymarin-Loaded Solid Eutectic for Enhanced Anti-Inflammatory, Hepatoprotective Effect: In Vitro-In Vivo Prospect. Oxidative Med. Cell. Longev. 2021, 2021, 1818538. [Google Scholar] [CrossRef]
- Zeng, Q.P.; Liu, Z.H.; Huang, A.W.; Zhang, J.; Song, H.T. Preparation and characterization of silymarin synchronized-release microporous osmotic pump tablets. Drug Des. Devel. Ther. 2016, 10, 519–531. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khan, R.A.; Khan, M.R.; Sahreen, S.; Shah, N.A. Hepatoprotective activity of Sonchus asper against carbon tetrachloride-induced injuries in male rats: A randomized controlled trial. BMC Complement. Altern. Med. 2012, 12, 90. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vargas-Mendoza, N.; Madrigal-Santillán, E.; Morales-González, A.; Esquivel-Soto, J.; Esquivel-Chirino, C.; García-Luna, Y.; González-Rubio, M.; Gayosso-de-Lucio, J.A.; Morales-González, J.A. Hepatoprotective effect of silymarin. World J. Hepatol. 2014, 6, 144–149. [Google Scholar] [CrossRef] [PubMed]
- Ohkawa, H.; Ohishi, N.; Yagi, K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 1979, 95, 351–358. [Google Scholar] [CrossRef]
- Li, X.-X.; Zheng, Q.-C.; Wang, Y.; Zhang, H.-X. Theoretical insights into the reductive metabolism of CCl4 by cytochrome P450 enzymes and the CCl4-dependent suicidal inactivation of P450. Dalton Trans. 2014, 43, 14833–14840. [Google Scholar] [CrossRef]
- Kim, H.J.; Bruckner, J.V.; Dallas, C.E.; Gallo, J.M. Effect of dosing vehicles on the pharmacokinetics of orally administered carbon tetrachloride in rats. Toxicol. Appl. Pharmacol. 1990, 102, 50–60. [Google Scholar] [CrossRef]
- Glende, E.A., Jr.; Hruszkewycz, A.M.; Recknagel, R.O. Critical role of lipid peroxidation in carbon tetrachloride-induced loss of aminopyrine demethylase, cytochrome P-450 and glucose 6-phosphatase. Biochem. Pharmacol. 1976, 25, 2163–2170. [Google Scholar] [CrossRef]
- Gnananath, K.; Sri Nataraj, K.; Ganga Rao, B. Phospholipid Complex Technique for Superior Bioavailability of Phytoconstituents. Adv. Pharm. Bull. 2017, 7, 35–42. [Google Scholar] [CrossRef] [Green Version]
- Soliman, W.E.; Khan, S.; Rizvi, S.M.D.; Moin, A.; Elsewedy, H.S.; Abulila, A.S.; Shehata, T.M. Therapeutic Applications of Biostable Silver Nanoparticles Synthesized Using Peel Extract of Benincasa hispida: Antibacterial and Anticancer Activities. Nanomaterials 2020, 10, 1954. [Google Scholar] [CrossRef]
- Bhattacharyya, S.; Ahammed, S.M.; Saha, B.P.; Mukherjee, P.K. The gallic acid-phospholipid complex improved the antioxidant potential of gallic acid by enhancing its bioavailability. AAPS PharmSciTech 2013, 14, 1025–1033. [Google Scholar] [CrossRef] [Green Version]
- Vasanth, S.; Dubey, A.; Ravi, G.S.; Lewis, S.A.; Ghate, V.M.; El-Zahaby, S.A.; Hebbar, S. Development and Investigation of Vitamin C-Enriched Adapalene-Loaded Transfersome Gel: A Collegial Approach for the Treatment of Acne Vulgaris. AAPS PharmSciTech 2020, 21, 61. [Google Scholar] [CrossRef]
- Tan, Q.; Liu, S.; Chen, X.; Wu, M.; Wang, H.; Yin, H.; He, D.; Xiong, H.; Zhang, J. Design and evaluation of a novel evodiamine-phospholipid complex for improved oral bioavailability. AAPS PharmSciTech 2012, 13, 534–547. [Google Scholar] [CrossRef] [Green Version]
- Singh, E.; Osmani, R.A.M.; Banerjee, R.; Abu Lila, A.S.; Moin, A.; Almansour, K.; Arab, H.H.; Alotaibi, H.F.; Khafagy, E.S. Poly ε-Caprolactone Nanoparticles for Sustained Intra-Articular Immune Modulation in Adjuvant-Induced Arthritis Rodent Model. Pharmaceutics 2022, 14, 519. [Google Scholar] [CrossRef]
- Al Saqr, A.; Wani, S.U.D.; Gangadharappa, H.V.; Aldawsari, M.F.; Khafagy, E.S.; Lila, A.S.A. Enhanced Cytotoxic Activity of Docetaxel-Loaded Silk Fibroin Nanoparticles against Breast Cancer Cells. Polymers 2021, 13, 1416. [Google Scholar] [CrossRef]
- Moin, A.; Wani, S.U.D.; Osmani, R.A.; Abu Lila, A.S.; Khafagy, E.S.; Arab, H.H.; Gangadharappa, H.V.; Allam, A.N. Formulation, characterization, and cellular toxicity assessment of tamoxifen-loaded silk fibroin nanoparticles in breast cancer. Drug Deliv. 2021, 28, 1626–1636. [Google Scholar] [CrossRef]
- Wu, J.W.; Lin, L.C.; Hung, S.C.; Chi, C.W.; Tsai, T.H. Analysis of silibinin in rat plasma and bile for hepatobiliary excretion and oral bioavailability application. J. Pharm. Biomed. Anal. 2007, 45, 635–641. [Google Scholar] [CrossRef]









| X1 (w:w) | X2 (°C) | Y1 (nm) | Y2 (w/w%) |
|---|---|---|---|
| +1 | −1 | 494.2 ± 6.64 | 67.3 ± 2.64 |
| −1 | +1 | 329.6 ± 4.21 | 81.7 ± 3.47 |
| 0 | −1 | 402.8 ± 3.87 | 75.6 ± 2.92 |
| +1 | +1 | 395.8 ± 2.45 | 78.7 ± 2.43 |
| 0 | +1 | 313.1 ± 4.69 | 83.5 ± 3.38 |
| −1 | −1 | 436.1 ± 5.92 | 70.3 ± 2.79 |
| 0 | 0 | 220.2 ± 1.27 | 92.4 ± 3.51 |
| −1 | 0 | 255.3 ± 1.52 | 89.2 ± 3.68 |
| +1 | 0 | 307.8 ± 3.95 | 85.4 ± 3.74 |
| Medium | Solubility (µg/mL) | |
|---|---|---|
| Pure Silymarin | Optimized Silymarin Phytosome | |
| Water | 45.73 ± 2.4 | 358.79 ± 9.4 |
| n-octanol | 129.29 ± 1.5 | 568.54 ± 8.5 |
| Hepatic Antioxidant Enzyme | Group—I (Normal Control) | Group—II (CCl4-Intoxicated Rats) | Group—III (Plain Silymarin) | Group—IV (Optimized Silymarin Phytosomes) |
|---|---|---|---|---|
| SGPT (U/L) | 42.77 ± 1.82 ** | 134.37 ± 3.61 | 95.68 ± 3.56 ** | 57.35 ± 2.73 ** |
| SGOT (U/L) | 38.22 ± 2.71 ** | 97.76 ± 3.38 | 75.19 ± 3.22 * | 46.88 ± 2.25 ** |
| SALP (U/L) | 141.53 ± 2.26 ** | 267.64 ± 3.29 | 221.77 ± 3.41 * | 159.43 ± 3.55 ** |
| Total bilirubin (mg/dL) | 0.66 ± 0.03 ** | 1.41 ± 0.02 | 0.95 ± 0.02 ** | 0.72 ± 0.01 ** |
| Hepatic Antioxidant Enzyme | Group—I (Normal Control) | Group—II (CCl4-Intoxicated Rats) | Group—III (Plain Silymarin) | Group—IV (Optimized Silymarin Phytosomes) |
|---|---|---|---|---|
| GSH (nmol/mg protein) | 49.16 ± 3.99 ** | 18.86 ± 1.28 | 29.37 ± 2.34 * | 41.22 ± 2.15 ** |
| GPx (nmol/mg protein) | 332.23 ± 4.91 ** | 193.76 ± 3.71 | 244.35 ± 4.27 * | 302.43 ± 3.33 ** |
| GST (nmol/mg protein) | 296.43 ± 4.73 ** | 165.28 ± 3.45 | 210.55 ± 4.28 | 264.88 ± 4.23 ** |
| GRD (nmol/mg protein) | 21.16 ± 1.54 ** | 7.89 ± 0.95 | 13.26 ± 1.54 | 18.47 ± 1.21 ** |
| SOD (U/mg protein) | 7.41 ± 0.11 ** | 4.21 ± 0.14 | 5.03 ± 0.22 | 6.21 ± 0.03 ** |
| CAT (U/mg protein) | 212.85 ± 2.87 ** | 95.46 ± 3.42 | 140.15 ± 3.76 * | 187.29 ± 2.58 ** |
| Pharmacokinetic Parameters | Pure Silymarin | Optimized Silymarin Phytosomes |
|---|---|---|
| Cmax (µg mL−1) | 0.40 ± 0.10 | 1.10 ± 0.21 ** |
| Tmax (h) | 4.0 | 6.0 ** |
| AUC0-t (µg mL−1 h) | 2.80 ± 0.71 | 45.76 ± 1.41 ** |
| AUC0-∞ (mL−1 h) | 3.84 ± 0.91 | 22.33 ± 2.13 |
| Elimination half-life (t1/2) (h) | 6.01 ± 0.70 | 12.31 ± 0.96 ** |
| Elimination rate constant (Kel) (h−1) | 0.12 ± 0.02 | 0.06 ± 0.01 ** |
| Mean residance time (MRT) (h) | 9.44 ± 1.10 | 20.43 ± 1.76 ** |
| Clearance (Cl) (mL·h−1) | 26.03 ± 1.93 | 4.48 ± 0.77 ** |
| Volume of distribution (Vd) (mL−1) | 255.48 ± 12.33 | 79.53 ± 8.11 ** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shriram, R.G.; Moin, A.; Alotaibi, H.F.; Khafagy, E.-S.; Al Saqr, A.; Abu Lila, A.S.; Charyulu, R.N. Phytosomes as a Plausible Nano-Delivery System for Enhanced Oral Bioavailability and Improved Hepatoprotective Activity of Silymarin. Pharmaceuticals 2022, 15, 790. https://doi.org/10.3390/ph15070790
Shriram RG, Moin A, Alotaibi HF, Khafagy E-S, Al Saqr A, Abu Lila AS, Charyulu RN. Phytosomes as a Plausible Nano-Delivery System for Enhanced Oral Bioavailability and Improved Hepatoprotective Activity of Silymarin. Pharmaceuticals. 2022; 15(7):790. https://doi.org/10.3390/ph15070790
Chicago/Turabian StyleShriram, Ravi Gundadka, Afrasim Moin, Hadil Faris Alotaibi, El-Sayed Khafagy, Ahmed Al Saqr, Amr Selim Abu Lila, and Rompicherla Narayana Charyulu. 2022. "Phytosomes as a Plausible Nano-Delivery System for Enhanced Oral Bioavailability and Improved Hepatoprotective Activity of Silymarin" Pharmaceuticals 15, no. 7: 790. https://doi.org/10.3390/ph15070790
APA StyleShriram, R. G., Moin, A., Alotaibi, H. F., Khafagy, E.-S., Al Saqr, A., Abu Lila, A. S., & Charyulu, R. N. (2022). Phytosomes as a Plausible Nano-Delivery System for Enhanced Oral Bioavailability and Improved Hepatoprotective Activity of Silymarin. Pharmaceuticals, 15(7), 790. https://doi.org/10.3390/ph15070790