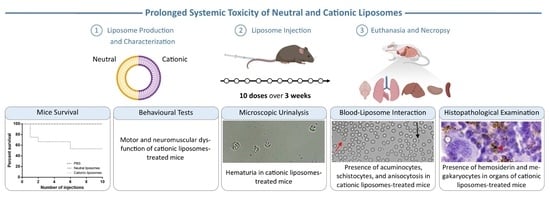
Multi-Dose Intravenous Administration of Neutral and Cationic Liposomes in Mice: An Extensive Toxicity Study
Abstract
:1. Introduction
2. Results and Discussion
2.1. Physicochemical Characterization of Liposomes
2.2. Mice Survival and Clinical Observations
2.3. Bodyweight
2.4. Glucose Levels
2.5. Behavioral Tests
2.6. Microscopic Urinalysis
2.7. Liposomes–Blood Interaction
2.8. Organ Weight and Morphology
2.9. Histopathological Examination
2.10. Serum Biochemistry
3. Materials and Methods
3.1. Chemicals
3.2. Preparation of Liposomes
3.3. Experimental Animals, Grouping, and Dosing Regime
3.4. Animal Survival, Clinical Observation, Body Weight, and Glucose Levels
3.5. Behavioral Tests
3.5.1. Rotarod Test
3.5.2. Forelimb Grip Strength Test
3.6. Microscopic Urinalysis
3.7. In Vitro Interaction between Blood and Liposomes
3.8. Acute In Vivo Interaction between Blood and Liposomes
3.9. Prolonged In Vivo Study to Assess the Interaction between Blood and Liposomes
3.10. Necropsy, Organ Weight, and Morphology
3.11. Histopathological Examinations
3.12. Quantification of Liver Injury Markers in Blood
3.13. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ramalho, M.J.; Andrade, S.; Loureiro, J.A.; Pereira, M.C. Nanotechnology to improve the Alzheimer’s disease therapy with natural compounds. Drug Deliv. Transl. Res. 2019, 10, 380–402. [Google Scholar] [CrossRef] [PubMed]
- Andrade, S.; Ramalho, M.J.; Loureiro, J.A.; Pereira, M.C. Liposomes as biomembrane models: Biophysical techniques for drug-membrane interaction studies. J. Mol. Liq. 2021, 334, 116141. [Google Scholar] [CrossRef]
- Guimarães, D.; Cavaco-Paulo, A.; Nogueira, E. Design of liposomes as drug delivery system for therapeutic applications. Int. J. Pharm. 2021, 601, 120571. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Chen, G.; Zhang, J. A Review of Liposomes as a Drug Delivery System: Current Status of Approved Products, Regulatory Environments, and Future Perspectives. Molecules 2022, 27, 1372. [Google Scholar] [CrossRef]
- Ewert, K.K.; Scodeller, P.; Simón-Gracia, L.; Steffes, V.M.; Wonder, E.A.; Teesalu, T.; Safinya, C.R. Cationic Liposomes as Vectors for Nucleic Acid and Hydrophobic Drug Therapeutics. Pharmaceutics 2021, 13, 1365. [Google Scholar] [CrossRef]
- Li, Y.; Cui, X.-L.; Chen, Q.-S.; Yu, J.; Zhang, H.; Gao, J.; Sun, D.-X.; Zhang, G.-Q. Cationic liposomes induce cytotoxicity in HepG2 via regulation of lipid metabolism based on whole-transcriptome sequencing analysis. BMC Pharmacol. Toxicol. 2018, 19, 43. [Google Scholar] [CrossRef] [Green Version]
- Dokka, S.; Toledo, D.; Shi, X.; Castranova, V.; Rojanasakul, Y. Oxygen Radical-Mediated Pulmonary Toxicity Induced by Some Cationic Liposomes. Pharm. Res. 2000, 17, 521–525. [Google Scholar] [CrossRef]
- Yu, J.; Zhang, H.; Li, Y.; Sun, S.; Gao, J.; Zhong, Y.; Sun, D.; Zhang, G. Metabolomics revealed the toxicity of cationic liposomes in HepG2 cells using UHPLC-Q-TOF/MS and multivariate data analysis. Biomed. Chromatogr. 2017, 31, e4036. [Google Scholar] [CrossRef]
- Singh, A.P.; Biswas, A.; Shukla, A.W.; Maiti, P. Targeted therapy in chronic diseases using nanomaterial-based drug delivery vehicles. Signal Transduct. Target. Ther. 2019, 4, 33. [Google Scholar] [CrossRef] [Green Version]
- Garbuzenko, O.; Barenholz, Y.; Priev, A. Effect of grafted PEG on liposome size and on compressibility and packing of lipid bilayer. Chem. Phys. Lipids 2005, 135, 117–129. [Google Scholar] [CrossRef]
- Hoshyar, N.; Gray, S.; Han, H.; Bao, G. The effect of nanoparticle size on in vivo pharmacokinetics and cellular interaction. Nanomedicine 2016, 11, 673–692. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Danaei, M.; Dehghankhold, M.; Ataei, S.; Hasanzadeh Davarani, F.; Javanmard, R.; Dokhani, A.; Khorasani, S.; Mozafari, M.R. Impact of Particle Size and Polydispersity Index on the Clinical Applications of Lipidic Nanocarrier Systems. Pharmaceutics 2018, 10, 57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ong, S.G.M.; Chitneni, M.; Lee, K.S.; Ming, L.C.; Yuen, K.H. Evaluation of Extrusion Technique for Nanosizing Liposomes. Pharmaceutics 2016, 8, 36. [Google Scholar] [CrossRef] [PubMed]
- Ramalho, M.J.; Loureiro, J.A.; Coelho, M.A.N.; Pereira, M.C. Factorial Design as a Tool for the Optimization of PLGA Nanoparticles for the Co-Delivery of Temozolomide and O6-Benzylguanine. Pharmaceutics 2019, 11, 401. [Google Scholar] [CrossRef] [Green Version]
- Ramalho, M.J.; Loureiro, J.A.; Gomes, B.; Frasco, M.F.; Coelho, M.A.N.; Pereira, M.C. PLGA nanoparticles for calcitriol delivery. In Proceedings of the 2015 IEEE 4th Portuguese Meeting on Bioengineering (ENBENG), Porto, Portugal, 26–28 February 2015; IEEE: Porto, Portugal. [Google Scholar]
- Chien, P.-Y.; Wang, J.; Carbonaro, D.; Lei, S.; Miller, B.; Sheikh, S.; Ali, S.M.; Ahmad, M.U.; Ahmad, I. Novel cationic cardiolipin analogue-based liposome for efficient DNA and small interfering RNA delivery in vitro and in vivo. Cancer Gene Ther. 2004, 12, 321–328. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lim, H.-S.; Seo, Y.S.; Ryu, S.M.; Moon, B.C.; Choi, G.; Kim, J.-S. Two-Week Repeated Oral Dose Toxicity Study of Mantidis Ootheca Water Extract in C57BL/6 Mice. Evid.-Based Complement. Altern. Med. 2019, 2019, 6180236. [Google Scholar] [CrossRef]
- Knudsen, K.B.; Northeved, H.; Kumar, P.E.K.; Permin, A.; Gjetting, T.; Andresen, T.L.; Larsen, S.; Wegener, K.M.; Lykkesfeldt, J.; Jantzen, K.; et al. In vivo toxicity of cationic micelles and liposomes. Nanomed. Nanotechnol. Biol. Med. 2015, 11, 467–477. [Google Scholar] [CrossRef] [Green Version]
- Quincozes-Santos, A.; Bobermin, L.D.; de Assis, A.M.; Gonçalves, C.-A.; Souza, D.O. Fluctuations in glucose levels induce glial toxicity with glutamatergic, oxidative and inflammatory implications. Biochim. Biophys. Acta BBA Mol. Basis Dis. 2017, 1863, 1–14. [Google Scholar] [CrossRef]
- National Research Council. Toxicity Testing: Strategies to Determine Needs and Priorities; National Academies Press: Washington, DC, USA, 1984. [Google Scholar]
- Dunham, W.N.; Miya, T.S. A Note on a Simple Apparatus for Detecting Neurological Deficit in Rats and Mice. J. Am. Pharm. Assoc. (Sci. Ed.) 1957, 46, 208–209. [Google Scholar] [CrossRef]
- Sills, G.J.; Brodie, M.J. Antiepileptic Drugs|Preclinical Drug Development in Epilepsy. In Encyclopedia of Basic Epilepsy Research; Schwartzkroin, P.A., Ed.; Academic Press: Oxford, UK, 2009; pp. 97–103. [Google Scholar]
- Nevins, M.E.; Nash, S.A.; Beardsley, P.M. Quantitative grip strength assessment as a means of evaluating muscle relaxation in mice. Psychopharmacology 1993, 110, 92–96. [Google Scholar] [CrossRef]
- Heine, G.H.; Sester, U.; Girndt, M.; Köhler, H. Acanthocytes in the urine: Useful tool to differentiate diabetic nephropathy from glomerulonephritis? Diabetes Care 2004, 27, 190–194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harashima, H.; Kiwada, H. Interactions of Liposomes with Cells In Vitro and In Vivo: Opsonins and Receptors. Curr. Drug Metab. 2001, 2, 397–409. [Google Scholar] [CrossRef]
- Senior, J.H.; Trimble, K.R.; Maskiewicz, R. Interaction of positively-charged liposomes with blood: Implications for their application in vivo. Biochim. Biophys. Acta (BBA)—Biomembr. 1991, 1070, 173–179. [Google Scholar] [CrossRef]
- Lippi, G.; Cervellin, G.; Sanchis-Gomar, F. Red blood cell distribution width: A marker of anisocytosis potentially associated with atrial fibrillation. World J. Cardiol. 2019, 11, 292–304. [Google Scholar] [CrossRef] [PubMed]
- Nishiguchi, E.; Okubo, K.; Nakamura, S. Adhesion of Human Red Blood Cells and Surface Charge of the Membrane. Cell Struct. Funct. 1998, 23, 143–152. [Google Scholar] [CrossRef] [Green Version]
- O’Connell, K.E.; Mikkola, A.M.; Stepanek, A.M.; Vernet, A.; Hall, C.D.; Sun, C.C.; Yildirim, E.; Staropoli, J.F.; Lee, J.T.; Brown, D.E. Practical murine hematopathology: A comparative review and implications for research. Comp. Med. 2015, 65, 96–113. [Google Scholar]
- de la Harpe, K.M.; Kondiah, P.P.; Choonara, Y.E.; Marimuthu, T.; du Toit, L.C.; Pillay, V. The hemocompatibility of nanoparticles: A review of cell–nanoparticle interactions and hemostasis. Cells 2019, 8, 1209. [Google Scholar] [CrossRef] [Green Version]
- Han, Y.; Wang, X.; Dai, H.; Li, S. Nanosize and Surface Charge Effects of Hydroxyapatite Nanoparticles on Red Blood Cell Suspensions. ACS Appl. Mater. Interfaces 2012, 4, 4616–4622. [Google Scholar] [CrossRef]
- Qi, X.; Zhao, W.; Zhuang, S. Comparative study of the in vitro and in vivo characteristics of cationic and neutral liposomes. Int. J. Nanomed. 2011, 6, 3087–3098. [Google Scholar] [CrossRef] [Green Version]
- Michael, B.; Yano, B.; Sellers, R.S.; Perry, R.; Morton, D.; Roome, N.; Johnson, J.K.; Schafer, K. Evaluation of Organ Weights for Rodent and Non-Rodent Toxicity Studies: A Review of Regulatory Guidelines and a Survey of Current Practices. Toxicol. Pathol. 2007, 35, 742–750. [Google Scholar] [CrossRef]
- Piao, Y.; Liu, Y.; Xie, X. Change Trends of Organ Weight Background Data in Sprague Dawley Rats at Different Ages. J. Toxicol. Pathol. 2013, 26, 29–34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suttie, A.W. Histopathology of the Spleen. Toxicol. Pathol. 2006, 34, 466–503. [Google Scholar] [CrossRef] [PubMed]
- Mendonça, M.C.P.; Radaic, A.; Garcia-Fossa, F.; da Cruz-Höfling, M.A.; Vinolo, M.A.R.; de Jesus, M.B. The in vivo toxicological profile of cationic solid lipid nanoparticles. Drug Deliv. Transl. Res. 2019, 10, 34–42. [Google Scholar] [CrossRef] [PubMed]
- Andrade, S.; Ramalho, M.J.; Loureiro, J.A.; Pereira, M.C. Interaction of natural compounds with biomembrane models: A biophysical approach for the Alzheimer’s disease therapy. Colloids Surf. B Biointerfaces 2019, 180, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Andrade, S.; Loureiro, J.A.; Pereira, M.C. Vitamin B12 Inhibits Aβ Fibrillation and Disaggregates Preformed Fibrils in the Presence of Synthetic Neuronal Membranes. ACS Chem. Neurosci. 2021, 12, 2491–2502. [Google Scholar] [CrossRef] [PubMed]
- Duran-Aniotz, C.; Moreno-Gonzalez, I.; Gamez, N.; Perez-Urrutia, N.; Vegas-Gomez, L.; Soto, C.; Morales, R. Amyloid pathology arrangements in Alzheimer’s disease brains modulate in vivo seeding capability. Acta Neuropathol. Commun. 2021, 9, 56. [Google Scholar] [CrossRef]








| Liposomes | Composition | Hydrodynamic Diameter (nm) | PDI | Zeta Potential (mV) |
|---|---|---|---|---|
| Neutral | DSPC:CHOL:DSPE-PEG(2000) amine | 139 ± 13 | 0.22 ± 0.02 | −1 ± 1 |
| Cationic | DOTAP:CHOL | 112 ± 3 | 0.13 ± 0.01 | 33 ± 2 |
| PBS Group | Neutral Group | Cationic Group | |
|---|---|---|---|
| Mean width of RBCs (pixel) | 44.7 ± 2.6 | 44.6 ± 2.6 | 43.4 ± 3.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Andrade, S.; Loureiro, J.A.; Ramirez, S.; Catumbela, C.S.G.; Soto, C.; Morales, R.; Pereira, M.C. Multi-Dose Intravenous Administration of Neutral and Cationic Liposomes in Mice: An Extensive Toxicity Study. Pharmaceuticals 2022, 15, 761. https://doi.org/10.3390/ph15060761
Andrade S, Loureiro JA, Ramirez S, Catumbela CSG, Soto C, Morales R, Pereira MC. Multi-Dose Intravenous Administration of Neutral and Cationic Liposomes in Mice: An Extensive Toxicity Study. Pharmaceuticals. 2022; 15(6):761. https://doi.org/10.3390/ph15060761
Chicago/Turabian StyleAndrade, Stéphanie, Joana A. Loureiro, Santiago Ramirez, Celso S. G. Catumbela, Claudio Soto, Rodrigo Morales, and Maria Carmo Pereira. 2022. "Multi-Dose Intravenous Administration of Neutral and Cationic Liposomes in Mice: An Extensive Toxicity Study" Pharmaceuticals 15, no. 6: 761. https://doi.org/10.3390/ph15060761
APA StyleAndrade, S., Loureiro, J. A., Ramirez, S., Catumbela, C. S. G., Soto, C., Morales, R., & Pereira, M. C. (2022). Multi-Dose Intravenous Administration of Neutral and Cationic Liposomes in Mice: An Extensive Toxicity Study. Pharmaceuticals, 15(6), 761. https://doi.org/10.3390/ph15060761