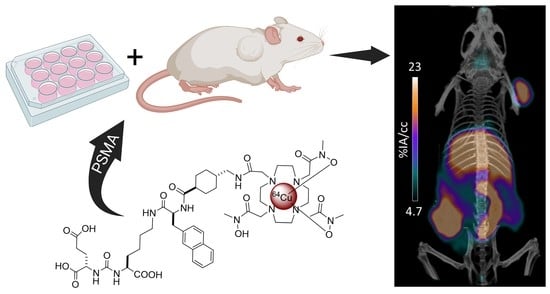
64Cu-DOTHA2-PSMA, a Novel PSMA PET Radiotracer for Prostate Cancer with a Long Imaging Time Window
Abstract
:1. Introduction
2. Results
2.1. Synthesis, Radiolabeling, and Characterization
2.1.1. Synthesis of DOTHA2(OtBu)3
2.1.2. Synthesis, Radiolabeling, and Characterization of 64Cu-DOTHA2-PSMA
2.1.3. Preparation of 68Ga-PSMA-617
2.2. Stability Studies
2.2.1. Plasma Stability (Ex Vivo)
2.2.2. In Vivo Stability
2.3. Cellular Assays
2.3.1. Competition Assays
2.3.2. Uptake, Internalization, and Efflux Assays
2.4. Animal Studies
2.4.1. Balb/c Mice Biodistribution
2.4.2. PET Imaging
2.4.3. Tumor-Bearing Mice Biodistribution
3. Discussion
3.1. Synthesis, Radiolabeling, and Characterization
3.2. Stability
3.3. Cellular Assays
3.4. Animal Assays
3.4.1. Biodistribution
3.4.2. PET Imaging
3.5. Implications and Future Research
4. Materials and Methods
4.1. Chemistry
4.1.1. General
4.1.2. Synthesis of DOTHA2 (OtBu)3
4.1.3. Synthesis of DOTHA2-PSMA
4.1.4. Preparation of natCu-DOTHA2-PSMA
4.1.5. Radiolabeling of DOTHA2-PSMA with 64Cu(OAc)2
4.1.6. Lipophilicity (log D) Measurements
4.1.7. Preparation of 68Ga-PSMA-617
4.2. Stability Studies
4.2.1. Plasma Stability (Ex Vivo)
4.2.2. In Vivo Stability
4.3. Cellular Assays
4.3.1. Cellular Model
4.3.2. Cellular Competition Assays
4.3.3. Cellular Uptake, Internalization and Efflux Assays
4.4. Animal Studies
4.4.1. Animal Models
4.4.2. Balb/c Mice Biodistribution
4.4.3. PET Imaging
4.4.4. Tumor-Bearing Mice Biodistribution
4.5. Statistics
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jones, W.; Griffiths, K.; Barata, P.C.; Paller, C.J. PSMA Theranostics: Review of the Current Status of PSMA-Targeted Imaging and Radioligand Therapy. Cancers 2020, 12, 1367. [Google Scholar] [CrossRef] [PubMed]
- Eapen, R.S.; Nzenza, T.C.; Murphy, D.G.; Hofman, M.S.; Cooperberg, M.; Lawrentschuk, N. PSMA PET Applications in the Prostate Cancer Journey: From Diagnosis to Theranostics. World J. Urol. 2019, 37, 1255–1261. [Google Scholar] [CrossRef] [PubMed]
- Witkowska-Patena, E.; Mazurek, A.; Dziuk, M. 68Ga-PSMA PET/CT Imaging in Recurrent Prostate Cancer: Where Are We Now? Cent. Eur. J. Urol. 2017, 70, 37–43. [Google Scholar] [CrossRef]
- Arsenault, F.; Beauregard, J.-M.; Pouliot, F. Prostate-Specific Membrane Antigen for Prostate Cancer Theranostics: From Imaging to Targeted Therapy. Curr. Opin. Support. Palliat. Care 2018, 12, 359–365. [Google Scholar] [CrossRef] [PubMed]
- Kratochwil, C.; Afshar-Oromieh, A.; Kopka, K.; Haberkorn, U.; Giesel, F.L. Current Status of Prostate-Specific Membrane Antigen Targeting in Nuclear Medicine: Clinical Translation of Chelator Containing Prostate-Specific Membrane Antigen Ligands into Diagnostics and Therapy for Prostate Cancer. Semin. Nucl. Med. 2016, 46, 405–418. [Google Scholar] [CrossRef] [PubMed]
- Alberts, I.; Seide, S.; Mingels, C.; Bohn, K.; Shi, K.; Zacho, H.; Arominger, A.; Afshar-Oromieh, A. Comparing the Diagnostic Performance of Radiotracers in Recurrent Prostate Cancer: A Systematic Review and Network Meta-Analysis. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 2978–2989. [Google Scholar] [CrossRef]
- Eppard, E.; De La Fuente, A.; Benešová, M.; Khawar, A.; Bundschuh, R.A.; Gärtner, F.C.; Kreppel, B.; Kopka, K.; Essler, M.; Rösch, F. Clinical Translation and First In-Human Use of [44Sc]Sc-PSMA-617 for PET Imaging of Metastasized Castrate-Resistant Prostate Cancer. Theranostics 2017, 7, 4359–4369. [Google Scholar] [CrossRef]
- Privé, B.M.; Derks, Y.H.W.; Rosar, F.; Franssen, G.M.; Peters, S.M.B.; Khreish, F.; Bartholomä, M.; Maus, S.; Gotthardt, M.; Laverman, P.; et al. 89 Zr-labeled PSMA Ligands for Pharmacokinetic PET Imaging and Dosimetry of PSMA-617 and PSMA-I&T: A Preclinical Evaluation and First in Man. Eur. J. Nucl. Med. Mol. Imaging 2022, 49, 2064–2076. [Google Scholar] [CrossRef]
- Benešová, M.; Schäfer, M.; Bauder-Wüst, U.; Afshar-Oromieh, A.; Kratochwil, C.; Mier, W.; Haberkorn, U.; Kopka, K.; Eder, M. Preclinical Evaluation of a Tailor-Made DOTA-Conjugated PSMA Inhibitor with Optimized Linker Moiety for Imaging and Endoradiotherapy of Prostate Cancer. J. Nucl. Med. 2015, 56, 914–920. [Google Scholar] [CrossRef]
- Ahmedova, A.; Todorov, B.; Burdzhiev, N.; Goze, C. Copper Radiopharmaceuticals for Theranostic Applications. Eur. J. Med. Chem. 2018, 157, 1406–1425. [Google Scholar] [CrossRef]
- Cai, Z.; Anderson, C.J. Chelators for Copper Radionuclides in Positron Emission Tomography Radiopharmaceuticals. J. Label. Compd. Radiopharm. 2014, 57, 224–230. [Google Scholar] [CrossRef] [PubMed]
- Brookhaven National Laboratory National Nuclear Data Center 3.0. Available online: https://www.nndc.bnl.gov/nudat3/ (accessed on 22 February 2022).
- Cui, C.; Hanyu, M.; Hatori, A.; Zhang, Y.; Xie, L.; Ohya, T.; Fukada, M.; Suzuki, H.; Nagatsu, K.; Jiang, C.; et al. Synthesis and Evaluation of [64Cu]PSMA-617 Targeted for Prostate-Specific Membrane Antigen in Prostate Cancer. Am. J. Nucl. Med. Mol. Imaging 2017, 7, 40–52. [Google Scholar]
- Han, X.; Liu, C.; Liu, F.; Xie, Q.; Liu, T.; Guo, X.; Xu, X.; Yang, X.; Zhu, H.; Yang, Z. 64 Cu-PSMA-617: A Novel PSMA-Targeted Radio-Tracer for PET Imaging in Gastric Adenocarcinoma Xenografted Mice Model. Oncotarget 2017, 8, 74159–74169. [Google Scholar] [CrossRef] [PubMed]
- dos Santos, J.C.; Beijer, B.; Bauder-Wüst, U.; Schäfer, M.; Leotta, K.; Eder, M.; Benešová, M.; Kleist, C.; Giesel, F.; Kratochwil, C.; et al. Development of Novel PSMA Ligands for Imaging and Therapy with Copper Isotopes. J. Nucl. Med. 2020, 61, 70–79. [Google Scholar] [CrossRef]
- Banerjee, S.R.; Pullambhatla, M.; Foss, C.A.; Nimmagadda, S.; Ferdani, R.; Anderson, C.J.; Mease, R.C.; Pomper, M.G. 64Cu-Labeled Inhibitors of Prostate-Specific Membrane Antigen for PET Imaging of Prostate Cancer. J. Med. Chem. 2014, 57, 2657–2669. [Google Scholar] [CrossRef]
- Gourni, E.; Canovas, C.; Goncalves, V.; Denat, F.; Meyer, P.T.; Maecke, H.R. (R)-NODAGA-PSMA: A Versatile Precursor for Radiometal Labeling and Nuclear Imaging of PSMA-Positive Tumors. PLoS ONE 2015, 10, e0145755. [Google Scholar] [CrossRef] [PubMed]
- Zia, N.A.; Cullinane, C.; Van Zuylekom, J.K.; Waldeck, K.; McInnes, L.E.; Buncic, G.; Haskali, M.B.; Roselt, P.D.; Hicks, R.J.; Donnelly, P.S. A Bivalent Inhibitor of Prostate Specific Membrane Antigen Radiolabeled with Copper-64 with High Tumor Uptake and Retention. Angew. Chemie Int. Ed. 2019, 58, 14991–14994. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Liu, C.; Zhang, Z.; Zhang, N.; Guo, X.; Xia, L.; Jiang, J.; Xie, Q.; Yan, K.; Rowe, S.P.; et al. 64Cu-PSMA-BCH: A New Radiotracer for Delayed PET Imaging of Prostate Cancer. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 4508–4516. [Google Scholar] [CrossRef] [PubMed]
- Umbricht, C.A.; Benešová, M.; Hasler, R.; Schibli, R.; Van Der Meulen, N.P.; Müller, C. Design and Preclinical Evaluation of an Albumin-Binding PSMA Ligand for 64Cu-Based PET Imaging. Mol. Pharm. 2018, 15, 5556–5564. [Google Scholar] [CrossRef] [PubMed]
- Cantiello, F.; Gangemi, V.; Cascini, G.L.; Calabria, F.; Moschini, M.; Ferro, M.; Musi, G.; Butticè, S.; Salonia, A.; Briganti, A.; et al. Diagnostic Accuracy of 64copper Prostate-Specific Membrane Antigen Positron Emission Tomography/Computed Tomography for Primary Lymph Node Staging of Intermediate- to High-Risk Prostate Cancer: Our Preliminary Experience. Urology 2017, 106, 139–145. [Google Scholar] [CrossRef]
- Cantiello, F.; Crocerossa, F.; Russo, G.I.; Gangemi, V.; Ferro, M.; Vartolomei, M.D.; Lucarelli, G.; Mirabelli, M.; Scafuro, C.; Ucciero, G.; et al. Comparison Between 64Cu-PSMA-617 PET/CT and 18F-Choline PET/CT Imaging in Early Diagnosis of Prostate Cancer Biochemical Recurrence. Clin. Genitourin. Cancer 2018, 16, 385–391. [Google Scholar] [CrossRef]
- Grubmüller, B.; Baum, R.P.; Capasso, E.; Singh, A.; Ahmadi, Y.; Knoll, P.; Floth, A.; Righi, S.; Zandieh, S.; Meleddu, C.; et al. 64Cu-PSMA-617 PET/CT Imaging of Prostate Adenocarcinoma: First in-Human Studies. Cancer Biother. Radiopharm. 2016, 31, 277–286. [Google Scholar] [CrossRef]
- Ait-Mohand, S.; Denis, C.; Tremblay, G.; Paquette, M.; Guérin, B. Development of Bifunctional Chelates Bearing Hydroxamate Arms for Highly Efficient 64Cu Radiolabeling. Org. Lett. 2014, 16, 4512–4515. [Google Scholar] [CrossRef]
- Mansour, N.; Paquette, M.; Ait-Mohand, S.; Dumulon-Perreault, V.; Guérin, B. Evaluation of a Novel GRPR Antagonist for Prostate Cancer PET Imaging: [64Cu]-DOTHA2-PEG-RM26. Nucl. Med. Biol. 2018, 56, 31–38. [Google Scholar] [CrossRef]
- Hirner, S.; Kirchner, D.K.; Somfai, P. Synthesis of α-Amino Acids by Umpolung of Weinreb Amide Enolates. European J. Org. Chem. 2008, 2008, 5583–5589. [Google Scholar] [CrossRef]
- Shayegan, B.; Zukotynski, K.; Bénard, F.; Ménard, C.; Kuk, J.; Sistani, G.; Bauman, G.; Veit-Haibach, P.; Metser, U. Canadian Urological Association Best Practice Report: Prostate Specific Membrane Antigen Positron Emission Tomography/ Computed Tomography (PSMA PET/CT) and PET/Magnetic Resonance (MR) in Prostate Cancer. Can. Urol. Assoc. J. 2021, 15, 162–173. [Google Scholar] [CrossRef] [PubMed]
- Sharifi, M.; Yousefnia, H.; Bahrami-Samani, A.; Jalilian, A.R.; Zolghadri, S.; Alirezapour, B.; Geramifar, P.; Maus, S.; Beiki, D. Optimized Production, Quality Control, Biological Evaluation and PET/CT Imaging of 68Ga-PSMA-617 in Breast Adenocarcinoma Model. Radiochim. Acta 2017, 105, 399–407. [Google Scholar] [CrossRef]
- Baker, M.; Parton, T. Kinetic Determinants of Hepatic Clearance: Plasma Protein Binding and Hepatic Uptake. Xenobiotica 2008, 37, 1110–1134. [Google Scholar] [CrossRef] [PubMed]
- Kuo, H.T.; Merkens, H.; Zhang, Z.; Uribe, C.F.; Lau, J.; Zhang, C.; Colpo, N.; Lin, K.S.; Bénard, F. Enhancing Treatment Efficacy of 177Lu-PSMA-617 with the Conjugation of an Albumin-Binding Motif: Preclinical Dosimetry and Endoradiotherapy Studies. Mol. Pharm. 2018, 15, 5183–5191. [Google Scholar] [CrossRef] [PubMed]
- Dash, R.P.; Tichý, T.; Veeravalli, V.; Lam, J.; Alt, J.; Wu, Y.; Tenora, L.; Majer, P.; Slusher, B.S.; Rais, R. Enhanced Oral Bioavailability of 2-(Phosphonomethyl)-Pentanedioic Acid (2-PMPA) from Its (5-Methyl-2-Oxo-1,3-Dioxol-4-Yl)Methyl (ODOL)-Based Prodrugs. Mol. Pharm. 2019, 16, 4292–4301. [Google Scholar] [CrossRef]
- Rais, R.; Rojas, C.; Wozniak, K.; Wu, Y.; Zhao, M.; Tsukamoto, T.; Rudek, M.A.; Slusher, B.S. Bioanalytical Method for Evaluating the Pharmacokinetics of the GCP-II Inhibitor 2-Phosphonomethyl Pentanedioic Acid (2-PMPA). J. Pharm. Biomed. Anal. 2014, 88, 162–169. [Google Scholar] [CrossRef] [PubMed]
- Bouchelouche, K.; Capala, J. Prostate Specific Membrane Antigen-A Target for Imaging and Therapy with Radionuclides. Discov. Med. 2010, 9, 55–61. [Google Scholar] [PubMed]
- Palleria, C.; Di Paolo, A.; Giofrè, C.; Caglioti, C.; Leuzzi, G.; Siniscalchi, A.; De Sarro, G.; Gallelli, L. Pharmacokinetic Drug-Drug Interaction and Their Implication in Clinical Management. J. Res. Med. Sci. 2013, 18, 601. [Google Scholar] [PubMed]
- Zeisler, S.K.; Pavan, R.A.; Orzechowski, J.; Langlois, R.; Rodrigue, S.; Van Lier, J.E. Production of 64Cu on the Sherbrooke TR-PET Cyclotron. J. Radioanal. Nucl. Chem. 2003, 257, 175–177. [Google Scholar] [CrossRef]
- McCarthy, D.; Shefer, R.; Klinkowstein, R.; Bass, L.; Margeneau, W.; Cutler, C.; Anderson, C.; Welch, M. Efficient Production of High Specific Activity 64Cu Using a Biomedical Cyclotron. Nucl. Med. Biol. 1997, 24, 35–43. [Google Scholar] [CrossRef]
- Benešová, M.; Bauder-Wüst, U.; Schäfer, M.; Klika, K.; Mier, W.; Haberkorn, U.; Kopka, K.; Eder, M. Linker Modification Strategies to Control the Prostate-Specific Membrane Antigen (PSMA)-Targeting and Pharmacokinetic Properties of DOTA-Conjugated PSMA Inhibitors. J. Med. Chem. 2016, 59, 1761–1775. [Google Scholar] [CrossRef]
- Maykel, J.; Jian, H.; Liu, H.; Li, H.; Shultz, L.; Greiner, D.L.; Houghton, J. NOD-ScidIl2rg Tm1Wjl and NOD-Rag1 Null Il2rg Tm1Wjl: A Model for Stromal Cell-Tumor Cell Interaction for Human Colon Cancer. Dig. Dis. Sci. 2014, 59, 1169–1179. [Google Scholar] [CrossRef]
- Pearson, T.; Shultz, L.D.; Miller, D.; King, M.; Laning, J.; Fodor, W.; Cuthbert, A.; Burzenski, L.; Gott, B.; Lyons, B.; et al. Non-Obese Diabetic-Recombination Activating Gene-1 (NOD-Rag1 Null ) Interleukin (IL)-2 Receptor Common Gamma Chain (IL2rg Null ) Null Mice: A Radioresistant Model for Human Lymphohaematopoietic Engraftment. Clin. Exp. Immunol. 2008, 154, 270–284. [Google Scholar] [CrossRef]
- Kahn, J.; Tofilon, P.J.; Camphausen, K. Preclinical Models in Radiation Oncology. Radiat. Oncol. 2012, 7, 223. [Google Scholar] [CrossRef]





| Time (h) | 64Cu in the Supernatant (%) | 64Cu and 64Cu-DOTHA2-PSMA Bound to Plasma Proteins (%) | 64Cu-DOTHA2-PSMA Released from Proteins d (%) | 64Cu released from Proteins d (%) |
|---|---|---|---|---|
| 1 a | 0 | 24 | 15 | 0.79 |
| 4 a | 1.98 | 34 | 21 | 0.64 |
| 24 a | 1.28 | 36 | 21 | 0.65 |
| 1 b | 0 | 24 | 0.82 | |
| 4 b | 3.35 | 33 | 0.84 | |
| 24 b | 5.60 | 30 | 2.14 | |
| 1 c | 2.60 | 48 | 31 | 1.98 |
| Entry | PSMA Ligand | IC50 on LNCaP (nM) |
|---|---|---|
| 1 | natCu-DOTHA2-PSMA a | 11.3 ± 14.3 |
| 2 | PMPA-2 a | 81.3 ± 59.5 |
| 3 | PMPA-2 b | 43.5 ± 16.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Milot, M.-C.; Benesty, O.B.; Dumulon-Perreault, V.; Ait-Mohand, S.; Richard, P.O.; Rousseau, É.; Guérin, B. 64Cu-DOTHA2-PSMA, a Novel PSMA PET Radiotracer for Prostate Cancer with a Long Imaging Time Window. Pharmaceuticals 2022, 15, 996. https://doi.org/10.3390/ph15080996
Milot M-C, Benesty OB, Dumulon-Perreault V, Ait-Mohand S, Richard PO, Rousseau É, Guérin B. 64Cu-DOTHA2-PSMA, a Novel PSMA PET Radiotracer for Prostate Cancer with a Long Imaging Time Window. Pharmaceuticals. 2022; 15(8):996. https://doi.org/10.3390/ph15080996
Chicago/Turabian StyleMilot, Marie-Christine, Ophélie Bélissant Benesty, Véronique Dumulon-Perreault, Samia Ait-Mohand, Patrick O. Richard, Étienne Rousseau, and Brigitte Guérin. 2022. "64Cu-DOTHA2-PSMA, a Novel PSMA PET Radiotracer for Prostate Cancer with a Long Imaging Time Window" Pharmaceuticals 15, no. 8: 996. https://doi.org/10.3390/ph15080996
APA StyleMilot, M. -C., Benesty, O. B., Dumulon-Perreault, V., Ait-Mohand, S., Richard, P. O., Rousseau, É., & Guérin, B. (2022). 64Cu-DOTHA2-PSMA, a Novel PSMA PET Radiotracer for Prostate Cancer with a Long Imaging Time Window. Pharmaceuticals, 15(8), 996. https://doi.org/10.3390/ph15080996