Current Advancement of Immunomodulatory Drugs as Potential Pharmacotherapies for Autoimmunity Based Neurological Diseases
Abstract
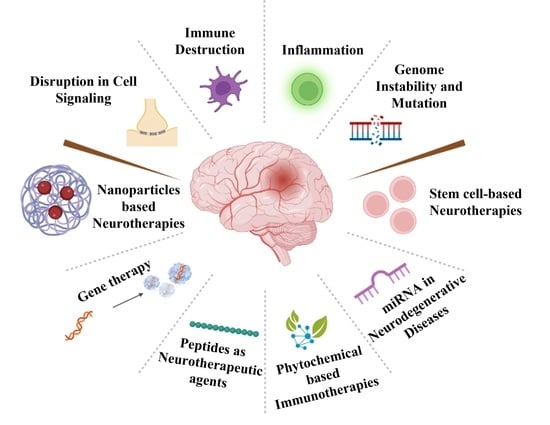
:1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Immune System Mediated Neurological Diseases
3.1.1. Multiple Sclerosis
3.1.2. NMO Spectrum Disorder (NMOSD)
3.1.3. Alzheimer’s Disease
3.1.4. Parkinson’s Disease
3.1.5. Amyotrophic Lateral Sclerosis (ALS)
4. Factors Affecting Neurodegeneration
4.1. Age
4.2. Environment
4.3. Genetic Factors
Immunomodulatory Drugs
5. Potential Pharmacotherapies for Autoimmune Neurological Diseases
5.1. miRNAs as a Potential Biomarker for Neurological Diseases
5.2. Phytochemicals for the Treatment of Autoimmune Neurological Disease
5.2.1. Flavonoids
Quercetin
Apigenin Derivatives
Diosgenin
Rosmarinic Acid
6. Gene Therapy as a Potential Treatment Strategy for Autoimmune Neurological Diseases
7. Nanotechnology for Autoimmune Neurological Diseases
7.1. Gold Nanoparticles
7.2. Magnetic Nanomaterials
7.3. Cerium Oxide Nanoparticles
7.4. Graphene Quantum Dots (GQDs)
8. Stem Cells Based Neurotherapies
8.1. Neural Stem Cells (NSCs)
8.2. Embryonic Stem Cells (ESCs)
8.3. Induced Pluripotent Stem Cells (iPSCs)
8.4. Mesenchymal Stem Cells (MSCs)
9. Peptides as Potential Neurotherapeutic Agents
9.1. Carnosine
9.2. P110
9.3. Vasoactive Intestinal Peptides
10. Etiological Concerns Related to Autoimmune Neurological Diseases (AINDs) Therapies
10.1. Novel Etiological Molecular Biomarkers in AINDs
10.2. Microbiota Dysbiosis in Autoimmune Neurological Diseases
10.2.1. The Impact of the Gut–Brain Axis, Gut Microbiota, and Probiotics in AINDs
10.2.2. Ferulic Acid (FA)
10.2.3. Manipulation of AINDs through Microbiota Produced Short-Chain Fatty Acids
10.2.4. The Impact of Gut Microbiota Producing Histamine in AINDs
11. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Walton, C.; King, R.; Rechtman, L.; Kaye, W.; Leray, E.; Marrie, R.A.; Robertson, N.; La Rocca, N.; Uitdehaag, B.; van der Mei, I. Rising prevalence of multiple sclerosis worldwide: Insights from the Atlas of MS. Mult. Scler. J. 2020, 26, 1816–1821. [Google Scholar] [CrossRef] [PubMed]
- Serra, P.; Santamaria, P. Nanoparticle-based approaches to immune tolerance for the treatment of autoimmune diseases. Eur. J. Immunol. 2018, 48, 751–756. [Google Scholar] [CrossRef] [PubMed]
- Serra, P.; Santamaria, P. Nanoparticle-based autoimmune disease therapy. Clin. Immunol. 2015, 160, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Agmon-Levin, N.; Theodor, E.; Segal, R.M.; Shoenfeld, Y. Vitamin D in systemic and organ-specific autoimmune diseases. Clin. Rev. Allergy Immunol. 2013, 45, 256–266. [Google Scholar] [CrossRef]
- Brummer, T.; Ruck, T.; Meuth, S.G.; Zipp, F.; Bittner, S. Treatment approaches to patients with multiple sclerosis and coexisting autoimmune disorders. Ther. Adv. Neurol. Disord. 2021, 14, 17562864211035542. [Google Scholar] [CrossRef]
- Eaton, W.W.; Pedersen, M.G.; Nielsen, P.R.; Mortensen, P.B. Autoimmune diseases, bipolar disorder, and non-affective psychosis. Bipolar Disord. 2010, 12, 638–646. [Google Scholar] [CrossRef]
- Saleem, K.; Azim, W. Association of vitiligo with other autoimmune disorders. Diabetes Case Rep. 2016, 1, 1–3. [Google Scholar] [CrossRef]
- Goodwin, G. Type 1 diabetes mellitus and celiac disease: Distinct autoimmune disorders that share common pathogenic mechanisms. Horm. Res. Paediatr. 2019, 92, 285–292. [Google Scholar] [CrossRef]
- Wu, J.J.; Nguyen, T.U.; Poon, K.-Y.T.; Herrinton, L.J. The association of psoriasis with autoimmune diseases. J. Am. Acad. Dermatol. 2012, 67, 924–930. [Google Scholar] [CrossRef]
- Engelhardt, B.; Vajkoczy, P.; Weller, R.O. The movers and shapers in immune privilege of the CNS. Nat. Immunol. 2017, 18, 123–131. [Google Scholar] [CrossRef]
- Sabatino, J.J.; Pröbstel, A.-K.; Zamvil, S.S. B cells in autoimmune and neurodegenerative central nervous system diseases. Nat. Rev. Neurosci. 2019, 20, 728–745. [Google Scholar] [CrossRef] [PubMed]
- Arlehamn, C.S.L.; Garretti, F.; Sulzer, D.; Sette, A. Roles for the adaptive immune system in Parkinson’s and Alzheimer’s diseases. Curr. Opin. Immunol. 2019, 59, 115–120. [Google Scholar] [CrossRef]
- Kobelt, G.; Thompson, A.; Berg, J.; Gannedahl, M.; Eriksson, J.; Group, M.S.; Platform, E.M.S. New insights into the burden and costs of multiple sclerosis in Europe. Mult. Scler. J. 2017, 23, 1123–1136. [Google Scholar] [CrossRef]
- Ascherio, A. Environmental factors in multiple sclerosis. Expert Rev. Neurother. 2013, 13, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Leray, E.; Yaouanq, J.; Le Page, E.; Coustans, M.; Laplaud, D.; Oger, J.; Edan, G. Evidence for a two-stage disability progression in multiple sclerosis. Brain 2010, 133, 1900–1913. [Google Scholar] [CrossRef] [PubMed]
- Handel, A.E.; Williamson, A.J.; Disanto, G.; Handunnetthi, L.; Giovannoni, G.; Ramagopalan, S.V. An updated meta-analysis of risk of multiple sclerosis following infectious mononucleosis. PLoS ONE 2010, 5, e12496. [Google Scholar] [CrossRef]
- Koch-Henriksen, N.; Sørensen, P.S. The changing demographic pattern of multiple sclerosis epidemiology. Lancet Neurol. 2010, 9, 520–532. [Google Scholar] [CrossRef]
- Dobson, R.; Giovannoni, G. Multiple sclerosis–a review. Eur. J. Neurol. 2019, 26, 27–40. [Google Scholar] [CrossRef]
- Wingerchuk, D.M.; Banwell, B.; Bennett, J.L.; Cabre, P.; Carroll, W.; Chitnis, T.; De Seze, J.; Fujihara, K.; Greenberg, B.; Jacob, A. International consensus diagnostic criteria for neuromyelitis optica spectrum disorders. Neurology 2015, 85, 177–189. [Google Scholar] [CrossRef]
- Wu, Y.; Zhong, L.; Geng, J. Neuromyelitis optica spectrum disorder: Pathogenesis, treatment, and experimental models. Mult. Scler. Relat. Disord. 2019, 27, 412–418. [Google Scholar] [CrossRef]
- Jarius, S.; Wildemann, B.; Paul, F. Neuromyelitis optica: Clinical features, immunopathogenesis and treatment. Clin. Exp. Immunol. 2014, 176, 149–164. [Google Scholar] [CrossRef] [PubMed]
- Verheijen, J.; Sleegers, K. Understanding Alzheimer disease at the interface between genetics and transcriptomics. Trends Genet. 2018, 34, 434–447. [Google Scholar] [CrossRef] [PubMed]
- Chu, L. Alzheimer’s disease: Early diagnosis and treatment. Hong Kong Med. J. 2012, 18, 228–237. [Google Scholar] [PubMed]
- Cedazo-Mínguez, A. Apolipoprotein E and Alzheimer’s disease: Molecular mechanisms and therapeutic opportunities. J. Cell. Mol. Med. 2007, 11, 1227–1238. [Google Scholar] [CrossRef] [PubMed]
- Lim, B.; Prassas, I.; Diamandis, E.P. Alzheimer disease pathogenesis: The role of autoimmunity. J. Appl. Lab. Med. 2021, 6, 756–764. [Google Scholar] [CrossRef]
- Ciccocioppo, F.; Lanuti, P.; Pierdomenico, L.; Simeone, P.; Bologna, G.; Ercolino, E.; Buttari, F.; Fantozzi, R.; Thomas, A.; Onofrj, M. The characterization of regulatory T-cell profiles in Alzheimer’s disease and multiple sclerosis. Sci. Rep. 2019, 9, 1–9. [Google Scholar] [CrossRef]
- Itzhaki, R. Herpes simplex virus type 1 and Alzheimer’s disease: Increasing evidence for a major role of the virus. Front. Aging Neurosci. 2014, 6, 2014. [Google Scholar] [CrossRef]
- Harrison, I.F.; Smith, A.D.; Dexter, D.T. Pathological histone acetylation in Parkinson’s disease: Neuroprotection and inhibition of microglial activation through SIRT 2 inhibition. Neurosci. Lett. 2018, 666, 48–57. [Google Scholar] [CrossRef]
- Garbes, L.; Riessland, M.; Wirth, B. Histone acetylation as a potential therapeutic target in motor neuron degenerative diseases. Curr. Pharm. Des. 2013, 19, 5093–5104. [Google Scholar] [CrossRef]
- Sherer, T.B.; Chowdhury, S.; Peabody, K.; Brooks, D.W. Overcoming obstacles in Parkinson’s disease. Mov. Disord. 2012, 27, 1606–1611. [Google Scholar] [CrossRef]
- Beitz, J.M. Parkinson’s disease: A review. Front. Biosci.-Sch. 2014, 6, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Margis, R.; Margis, R.; Rieder, C.R. Identification of blood microRNAs associated to Parkinsońs disease. J. Biotechnol. 2011, 152, 96–101. [Google Scholar] [CrossRef] [PubMed]
- Michel, P.P.; Hirsch, E.C.; Hunot, S. Understanding dopaminergic cell death pathways in Parkinson disease. Neuron 2016, 90, 675–691. [Google Scholar] [CrossRef]
- Chaudhuri, K.R.; Odin, P. The challenge of non-motor symptoms in Parkinson’s disease. Prog. Brain Res. 2010, 184, 325–341. [Google Scholar]
- Nuzziello, N.; Ciaccia, L.; Liguori, M. Precision medicine in neurodegenerative diseases: Some promising tips coming from the microRNAs’ world. Cells 2019, 9, 75. [Google Scholar] [CrossRef] [PubMed]
- Jiang, T.; Li, G.; Xu, J.; Gao, S.; Chen, X. The challenge of the pathogenesis of Parkinson’s disease: Is autoimmunity the culprit? Front. Immunol. 2018, 9, 2047. [Google Scholar] [CrossRef]
- Kiernan, M.C.; Vucic, S.; Cheah, B.C.; Turner, M.R.; Eisen, A.; Hardiman, O.; Burrell, J.R.; Zoing, M.C. Amyotrophic lateral sclerosis. Lancet 2011, 377, 942–955. [Google Scholar] [CrossRef]
- Uchino, A.; Takao, M.; Hatsuta, H.; Sumikura, H.; Nakano, Y.; Nogami, A.; Saito, Y.; Arai, T.; Nishiyama, K.; Murayama, S. Incidence and extent of TDP-43 accumulation in aging human brain. Acta Neuropathol. Commun. 2015, 3, 1–11. [Google Scholar] [CrossRef]
- Burke, S.N.; Barnes, C.A. Neural plasticity in the ageing brain. Nat. Rev. Neurosci. 2006, 7, 30–40. [Google Scholar] [CrossRef]
- Baig, M.H.; Ahmad, K.; Saeed, M.; Alharbi, A.M.; Barreto, G.E.; Ashraf, G.M.; Choi, I. Peptide based therapeutics and their use for the treatment of neurodegenerative and other diseases. Biomed. Pharmacother. 2018, 103, 574–581. [Google Scholar]
- MacArthur, J.; Bowler, E.; Cerezo, M.; Gil, L.; Hall, P.; Hastings, E.; Junkins, H.; McMahon, A.; Milano, A.; Morales, J. The new NHGRI-EBI Catalog of published genome-wide association studies (GWAS Catalog). Nucleic Acids Res. 2017, 45, D896–D901. [Google Scholar] [CrossRef] [PubMed]
- Avorn, J. Learning about the safety of drugs—A half-century of evolution. N. Engl. J. Med. 2011, 365, 2151–2153. [Google Scholar] [CrossRef] [PubMed]
- Holstein, S.A.; McCarthy, P.L. Immunomodulatory drugs in multiple myeloma: Mechanisms of action and clinical experience. Drugs 2017, 77, 505–520. [Google Scholar] [CrossRef]
- Zumla, A.; Rao, M.; Dodoo, E.; Maeurer, M. Potential of immunomodulatory agents as adjunct host-directed therapies for multidrug-resistant tuberculosis. BMC Med. 2016, 14, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Bascones-Martinez, A.; Mattila, R.; Gomez-Font, R.; Meurman, J.H. Immunomodulatory drugs: Oral and systemic adverse effects. Med. Oral Patol. Oral Y Cir. Bucal 2014, 19, e24. [Google Scholar] [CrossRef]
- Li, Z.; Liu, Y.; Fang, X.; Shu, Z. Nanomaterials enhance the immunomodulatory effect of molecular targeted therapy. Int. J. Nanomed. 2021, 16, 1631. [Google Scholar] [CrossRef]
- Wallis, R.S.; Zumla, A. Vitamin D as adjunctive host-directed therapy in tuberculosis: A systematic review. Open Forum Infect. Diseases 2016, 7, ofw151. [Google Scholar] [CrossRef]
- Silva, O.; De La Fuente-Núñez, C.; Haney, E.; Fensterseifer, I.; Ribeiro, S.; Porto, W.; Brown, P.; Faria-Junior, C.; Rezende, T.; Moreno, S. An anti-infective synthetic peptide with dual antimicrobial and immunomodulatory activities. Sci. Rep. 2016, 6, 35465. [Google Scholar] [CrossRef]
- van Dieren, J.M.; Kuipers, E.J.; Samsom, J.N.; Nieuwenhuis, E.E.; van der Woude, J.C. Revisiting the immunomodulators tacrolimus, methotrexate, and mycophenolate mofetil: Their mechanisms of action and role in the treatment of IBD. Inflamm. Bowel Dis. 2006, 12, 311–327. [Google Scholar] [CrossRef]
- Toscano, E.; Cotta, J.; Robles, M.; Lucena, M.I.; Andrade, R.J. Toxicidad hepática inducida por los nuevos fármacos inmunosupresores. Gastroenterol. Y Hepatol. 2010, 33, 54–65. [Google Scholar] [CrossRef]
- Peedicayil, J. Pharmacoepigenetics and pharmacoepigenomics: An overview. Current Drug Discov. Technol. 2019, 16, 392–399. [Google Scholar] [CrossRef] [PubMed]
- Peedicayil, J. Pharmacoepigenetics and pharmacoepigenomics. Pharmacogenomics 2008, 9, 1785–1786. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marangon, D.; Raffaele, S.; Fumagalli, M.; Lecca, D. MicroRNAs change the games in central nervous system pharmacology. Biochem. Pharmacol. 2019, 168, 162–172. [Google Scholar] [CrossRef] [PubMed]
- Nakano, M.; Nakajima, M. Current knowledge of microRNA-mediated regulation of drug metabolism in humans. Expert Opin. Drug Metab. Toxicol. 2018, 14, 493–504. [Google Scholar] [CrossRef]
- Massoud, F.; Gauthier, S. Update on the pharmacological treatment of Alzheimer’s disease. Curr. Neuropharmacol. 2010, 8, 69–80. [Google Scholar] [CrossRef]
- Li, K.X.; Picheca, L. Second-Line Therapy for Patients with Relapsing-Remitting Multiple Sclerosis: A Review of Guidelines; Canadian Agency for Drugs and Technologies in Health: Ottawa, ON, Canada, 2019. [Google Scholar]
- Montalban, X.; Gold, R.; Thompson, A.J.; Otero-Romero, S.; Amato, M.P.; Chandraratna, D.; Clanet, M.; Comi, G.; Derfuss, T.; Fazekas, F. ECTRIMS/EAN guideline on the pharmacological treatment of people with multiple sclerosis. Mult. Scler. J. 2018, 24, 96–120. [Google Scholar] [CrossRef]
- Markowitz, C.E. Interferon-beta: Mechanism of action and dosing issues. Neurology 2007, 68, S8–S11. [Google Scholar] [CrossRef]
- Hecker, M.; Thamilarasan, M.; Koczan, D.; Schröder, I.; Flechtner, K.; Freiesleben, S.; Füllen, G.; Thiesen, H.-J.; Zettl, U.K. MicroRNA expression changes during interferon-beta treatment in the peripheral blood of multiple sclerosis patients. Int. J. Mol. Sci. 2013, 14, 16087–16110. [Google Scholar] [CrossRef]
- Ehtesham, N.; Khorvash, F.; Kheirollahi, M. miR-145 and miR20a-5p potentially mediate pleiotropic effects of interferon-beta through mitogen-activated protein kinase signaling pathway in multiple sclerosis patients. J. Mol. Neurosci. 2017, 61, 16–24. [Google Scholar] [CrossRef]
- Waschbisch, A.; Atiya, M.; Linker, R.A.; Potapov, S.; Schwab, S.; Derfuss, T. Glatiramer acetate treatment normalizes deregulated microRNA expression in relapsing remitting multiple sclerosis. PLoS ONE 2011, 6, e24604. [Google Scholar] [CrossRef]
- Singh, J.; Deshpande, M.; Suhail, H.; Rattan, R.; Giri, S. Targeted stage-specific inflammatory microRNA profiling in urine during disease progression in experimental autoimmune encephalomyelitis: Markers of disease progression and drug response. J. Neuroimmune Pharmacol. 2016, 11, 84–97. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Culla, M.; Irizar, H.; Castillo-Triviño, T.; Sáenz-Cuesta, M.; Sepúlveda, L.; Lopetegi, I.; de Munain, A.L.; Olascoaga, J.; Baranzini, S.; Otaegui, D. Blood miRNA expression pattern is a possible risk marker for natalizumab-associated progressive multifocal leukoencephalopathy in multiple sclerosis patients. Mult. Scler. J. 2014, 20, 1851–1859. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Chen, M.; Simpson, S.; Lucas, R.M.; Charlesworth, J.C.; Blackburn, N.; Van Der Mei, I.; Ponsonby, A.-L.; Taylor, B.V. Common genetic variation within miR-146a predicts disease onset and relapse in multiple sclerosis. Neurol. Sci. 2018, 39, 297–304. [Google Scholar] [CrossRef] [PubMed]
- Kalinderi, K.; Fidani, L.; Katsarou, Z.; Bostantjopoulou, S. Pharmacological treatment and the prospect of pharmacogenetics in Parkinson’s disease. Int. J. Clin. Pract. 2011, 65, 1289–1294. [Google Scholar] [CrossRef] [PubMed]
- Weller, J.; Budson, A. Current understanding of Alzheimer’s disease diagnosis and treatment. F1000Research 2018, 7, F1000. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.-N.; Wang, Y.-J.; Wang, H.; Song, L.; Chen, Y.; Wang, J.-L.; Ye, Y.; Jiang, B. The anti-dementia effects of donepezil involve miR-206-3p in the hippocampus and cortex. Biol. Pharm. Bull. 2017, 40, 465–472. [Google Scholar] [CrossRef]
- Jiao, Y.; Kong, L.; Yao, Y.; Li, S.; Tao, Z.; Yan, Y.; Yang, J. Osthole decreases beta amyloid levels through up-regulation of miR-107 in Alzheimer’s disease. Neuropharmacology 2016, 108, 332–344. [Google Scholar] [CrossRef]
- Zhang, Z.-R.; Leung, W.N.; Cheung, H.Y.; Chan, C.W. Osthole: A review on its bioactivities, pharmacological properties, and potential as alternative medicine. Evid.-Based Complementary Altern. Med. 2015, 2015, 919616. [Google Scholar] [CrossRef]
- Li, S.; Yan, Y.; Jiao, Y.; Gao, Z.; Xia, Y.; Kong, L.; Yao, Y.; Tao, Z.; Song, J.; Yan, Y. Neuroprotective effect of osthole on neuron synapses in an Alzheimer’s disease cell model via upregulation of microRNA-9. J. Mol. Neurosci. 2016, 60, 71–81. [Google Scholar] [CrossRef]
- Shioya, M.; Obayashi, S.; Tabunoki, H.; Arima, K.; Saito, Y.; Ishida, T.; Satoh, J.-i. Aberrant microRNA expression in the brains of neurodegenerative diseases: miR-29a decreased in Alzheimer disease brains targets neurone navigator 3. Neuropathol. Appl. Neurobiol. 2010, 36, 320–330. [Google Scholar] [CrossRef]
- Butovsky, O.; Siddiqui, S.; Gabriely, G.; Lanser, A.; Dake, B.; Gopal, M.; Gali, R.; Iyer, L.; Lawson, R.; Berry, J. Modulation of inflammatory monocytes with a unique microRNA-gene signature ameliorates ALS mice. J. Neuroimmunol. 2012, 122, 63. [Google Scholar]
- Maciotta, S.; Meregalli, M.; Torrente, Y. The involvement of microRNAs in neurodegenerative diseases. Front. Cell. Neurosci. 2013, 7, 265. [Google Scholar] [CrossRef] [PubMed]
- Patel, D.K. Medicinal importance, pharmacological activities and analytical aspects of a flavonoid glycoside’nicotiflorin’in the medicine. Drug Metab. Lett. 2022, 15, 2–11. [Google Scholar] [CrossRef]
- Shen, N.; Wang, T.; Gan, Q.; Liu, S.; Wang, L.; Jin, B. Plant flavonoids: Classification, distribution, biosynthesis, and antioxidant activity. Food Chem. 2022, 383, 132531. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Zhong, T.; Zhang, Y.; Meng, C.; Gao, J.; Han, T.; Chen, M.; Liu, J.; Fan, Y.; Xu, Y. Flavonoid-rich extract of Toxicodendron vernicifluum served as a natural neuroprotective agent. Ind. Crops Prod. 2022, 186, 115137. [Google Scholar] [CrossRef]
- Kim, S.Y.; Gao, J.J.; Lee, W.-C.; Ryu, K.S.; Lee, K.R.; Kim, Y.C. Antioxidative flavonoids from the leaves ofMorus alba. Arch. Pharmacal Res. 1999, 22, 81–85. [Google Scholar] [CrossRef]
- Elumalai, P.; Lakshmi, S. Role of quercetin benefits in neurodegeneration. In The Benefits of Natural Products for Neurodegenerative Diseases; Springer: Berlin/Heidelberg, Germany, 2016; pp. 229–245. [Google Scholar]
- Xu, F.; Wang, C.; Yang, L.; Luo, H.; Fan, W.; Zi, C.; Dong, F.; Hu, J.; Zhou, J. C-dideoxyhexosyl flavones from the stems and leaves of Passiflora edulis Sims. Food Chem. 2013, 136, 94–99. [Google Scholar] [CrossRef]
- Yang, Y.; Bai, L.; Li, X.; Xiong, J.; Xu, P.; Guo, C.; Xue, M. Transport of active flavonoids, based on cytotoxicity and lipophilicity: An evaluation using the blood–brain barrier cell and Caco-2 cell models. Toxicol. Vitr. 2014, 28, 388–396. [Google Scholar] [CrossRef]
- Woo, K.W.; Kwon, O.W.; Kim, S.Y.; Choi, S.Z.; Son, M.W.; Kim, K.H.; Lee, K.R. Phenolic derivatives from the rhizomes of Dioscorea nipponica and their anti-neuroinflammatory and neuroprotective activities. J. Ethnopharmacol. 2014, 155, 1164–1170. [Google Scholar] [CrossRef]
- Kang, T.H.; Moon, E.; Hong, B.N.; Choi, S.Z.; Son, M.; Park, J.-H.; Kim, S.Y. Diosgenin from Dioscorea nipponica ameliorates diabetic neuropathy by inducing nerve growth factor. Biol. Pharm. Bull. 2011, 34, 1493–1498. [Google Scholar] [CrossRef]
- Bayat, M.; Azami Tameh, A.; Hossein Ghahremani, M.; Akbari, M.; Mehr, S.E.; Khanavi, M.; Hassanzadeh, G. Neuroprotective properties of Melissa officinalis after hypoxic-ischemic injury both in vitro and in vivo. DARU J. Pharm. Sci. 2012, 20, 42. [Google Scholar] [CrossRef] [PubMed]
- Sayed, N.; Allawadhi, P.; Khurana, A.; Singh, V.; Navik, U.; Pasumarthi, S.K.; Khurana, I.; Banothu, A.K.; Weiskirchen, R.; Bharani, K.K. Gene therapy: Comprehensive overview and therapeutic applications. Life Sci. 2022, 294, 120375. [Google Scholar] [CrossRef] [PubMed]
- Foldvari, M.; Chen, D.W.; Nafissi, N.; Calderon, D.; Narsineni, L.; Rafiee, A. Non-viral gene therapy: Gains and challenges of non-invasive administration methods. J. Control. Release 2016, 240, 165–190. [Google Scholar] [CrossRef]
- Shu, S.-A.; Wang, J.; Tao, M.-H.; Leung, P.S. Gene therapy for autoimmune disease. Clin. Rev. Allergy Immunol. 2015, 49, 163–176. [Google Scholar] [CrossRef] [PubMed]
- Sloane, E.; Ledeboer, A.; Seibert, W.; Coats, B.; Van Strien, M.; Maier, S.; Johnson, K.; Chavez, R.; Watkins, L.; Leinwand, L. Anti-inflammatory cytokine gene therapy decreases sensory and motor dysfunction in experimental Multiple Sclerosis: MOG-EAE behavioral and anatomical symptom treatment with cytokine gene therapy. Brain Behav. Immun. 2009, 23, 92–100. [Google Scholar] [CrossRef] [PubMed]
- Keeler, G.D.; Kumar, S.; Palaschak, B.; Silverberg, E.L.; Markusic, D.M.; Jones, N.T.; Hoffman, B.E. Gene therapy-induced antigen-specific Tregs inhibit neuro-inflammation and reverse disease in a mouse model of multiple sclerosis. Mol. Ther. 2018, 26, 173–183. [Google Scholar] [CrossRef] [PubMed]
- Skarica, M.; Wang, T.; McCadden, E.; Kardian, D.; Calabresi, P.A.; Small, D.; Whartenby, K.A. Signal transduction inhibition of APCs diminishes th17 and Th1 responses in experimental autoimmune encephalomyelitis. J. Immunol. 2009, 182, 4192–4199. [Google Scholar] [CrossRef]
- Leung, P.S.; Dhirapong, A.; Wu, P.-Y.; Tao, M.-H. Gene therapy in autoimmune diseases: Challenges and opportunities. Autoimmun. Rev. 2010, 9, 170–174. [Google Scholar] [CrossRef]
- Lobell, A.; Weissert, R.; Storch, M.K.; Svanholm, C.; de Graaf, K.L.; Lassmann, H.; Andersson, R.; Olsson, T.; Wigzell, H. Vaccination with DNA encoding an immunodominant myelin basic protein peptide targeted to Fc of immunoglobulin G suppresses experimental autoimmune encephalomyelitis. J. Exp. Med. 1998, 187, 1543–1548. [Google Scholar] [CrossRef]
- Yoo, T.J. Anti-Inflammatory Gene Therapy Improves Spatial Memory Performance in a Mouse Model of Alzheimer’s Disease. J. Alzheimer’s Dis. 2022, 85, 1001–1008. [Google Scholar]
- Ashraf, H.; Meer, B.; Iqbal, J.; Ali, J.S.; Andleeb, A.; Butt, H.; Zia, M.; Mehmood, A.; Nadeem, M.; Drouet, S. Comparative evaluation of chemically and green synthesized zinc oxide nanoparticles: Their in vitro antioxidant, antimicrobial, cytotoxic and anticancer potential towards HepG2 cell line. J. Nanostructure Chem. 2022, 12, 1–19. [Google Scholar] [CrossRef]
- Wu, C.-C.; Chen, D.-H. Facile green synthesis of gold nanoparticles with gum arabic as a stabilizing agent and reducing agent. Gold Bull. 2010, 43, 234–240. [Google Scholar] [CrossRef]
- Rudramurthy, G.R.; Swamy, M.K. Potential applications of engineered nanoparticles in medicine and biology: An update. JBIC J. Biol. Inorg. Chem. 2018, 23, 1185–1204. [Google Scholar] [CrossRef]
- Nagaich, U. Theranostic nanomedicine: Potential therapeutic epitome. J. Adv. Pharm. Technol. Res. 2015, 6, 1. [Google Scholar] [CrossRef] [PubMed]
- Tosi, G.; Vandelli, M.A.; Forni, F.; Ruozi, B. Nanomedicine and Neurodegenerative Disorders: So Close Yet So Far; Taylor & Francis: Abingdon, UK, 2015; Volume 12, pp. 1041–1044. [Google Scholar]
- Azevedo, R.S.; de Sousa, J.R.; Araujo, M.T.; Martins Filho, A.J.; de Alcantara, B.N.; Araujo, F.; Queiroz, M.G.; Cruz, A.C.; Vasconcelos, B.H.B.; Chiang, J.O. In situ immune response and mechanisms of cell damage in central nervous system of fatal cases microcephaly by Zika virus. Sci. Rep. 2018, 8, 1. [Google Scholar] [CrossRef]
- Asil, S.M.; Ahlawat, J.; Barroso, G.G.; Narayan, M. Nanomaterial based drug delivery systems for the treatment of neurodegenerative diseases. Biomater. Sci. 2020, 8, 4109–4128. [Google Scholar] [CrossRef]
- Ajnai, G.; Chiu, A.; Kan, T.; Cheng, C.-C.; Tsai, T.-H.; Chang, J. Trends of gold nanoparticle-based drug delivery system in cancer therapy. J. Exp. Clin. Med. 2014, 6, 172–178. [Google Scholar] [CrossRef]
- Sanati, M.; Khodagholi, F.; Aminyavari, S.; Ghasemi, F.; Gholami, M.; Kebriaeezadeh, A.; Sabzevari, O.; Hajipour, M.J.; Imani, M.; Mahmoudi, M. Impact of gold nanoparticles on amyloid β-induced Alzheimer’s disease in a rat animal model: Involvement of STIM proteins. ACS Chem. Neurosci. 2019, 10, 2299–2309. [Google Scholar] [CrossRef]
- Gao, G.; Zhang, M.; Gong, D.; Chen, R.; Hu, X.; Sun, T. The size-effect of gold nanoparticles and nanoclusters in the inhibition of amyloid-β fibrillation. Nanoscale 2017, 9, 4107–4113. [Google Scholar] [CrossRef]
- Li, H.; Yang, S.; Hui, D.; Hong, R. Progress in magnetic Fe3O4 nanomaterials in magnetic resonance imaging. Nanotechnol. Rev. 2020, 9, 1265–1283. [Google Scholar] [CrossRef]
- Ryabchikova, E. Advances in nanomaterials in biomedicine. Nanomaterials 2021, 11, 118. [Google Scholar] [CrossRef] [PubMed]
- Basak, G.; Hazra, C.; Sen, R. Biofunctionalized nanomaterials for in situ clean-up of hydrocarbon contamination: A quantum jump in global bioremediation research. J. Environ. Manag. 2020, 256, 109913. [Google Scholar] [CrossRef] [PubMed]
- Mazur, A.; Pleshakov, I.; Khudyakov, A.; Bibik, E.; Kuzmin, Y.I. NMR investigation of iron-containing magnetically ordered nanomaterial used for preparing of magnetic fluid. J. Phys. Conf. Ser. 2019, 1326, 012009. [Google Scholar] [CrossRef]
- Gu, M.; Zhang, Q.; Lamon, S. Nanomaterials for optical data storage. Nat. Rev. Mater. 2016, 1, 16070. [Google Scholar] [CrossRef]
- Neuberger, T.; Schöpf, B.; Hofmann, H.; Hofmann, M.; Von Rechenberg, B. Superparamagnetic nanoparticles for biomedical applications: Possibilities and limitations of a new drug delivery system. J. Magn. Magn. Mater. 2005, 293, 483–496. [Google Scholar] [CrossRef]
- Assa, F.; Jafarizadeh-Malmiri, H.; Ajamein, H.; Vaghari, H.; Anarjan, N.; Ahmadi, O.; Berenjian, A. Chitosan magnetic nanoparticles for drug delivery systems. Crit. Rev. Biotechnol. 2017, 37, 492–509. [Google Scholar] [CrossRef]
- Nelson, B.C.; Johnson, M.E.; Walker, M.L.; Riley, K.R.; Sims, C.M. Antioxidant cerium oxide nanoparticles in biology and medicine. Antioxidants 2016, 5, 15. [Google Scholar] [CrossRef]
- Dowding, J.; Song, W.; Bossy, K.; Karakoti, A.; Kumar, A.; Kim, A.; Bossy, B.; Seal, S.; Ellisman, M.; Perkins, G. Cerium oxide nanoparticles protect against Aβ-induced mitochondrial fragmentation and neuronal cell death. Cell Death Differ. 2014, 21, 1622–1632. [Google Scholar] [CrossRef]
- Ruotolo, R.; De Giorgio, G.; Minato, I.; Bianchi, M.G.; Bussolati, O.; Marmiroli, N. Cerium oxide nanoparticles rescue α-synuclein-induced toxicity in a yeast model of Parkinson’s disease. Nanomaterials 2020, 10, 235. [Google Scholar] [CrossRef]
- Tian, P.; Tang, L.; Teng, K.; Lau, S. Graphene quantum dots from chemistry to applications. Mater. Today Chem. 2018, 10, 221–258. [Google Scholar] [CrossRef]
- Kim, D.; Yoo, J.M.; Hwang, H.; Lee, J.; Lee, S.H.; Yun, S.P.; Park, M.J.; Lee, M.; Choi, S.; Kwon, S.H. Graphene quantum dots prevent α-synucleinopathy in Parkinson’s disease. Nat. Nanotechnol. 2018, 13, 812–818. [Google Scholar] [CrossRef] [PubMed]
- Pichla, M.; Bartosz, G.; Sadowska-Bartosz, I. The antiaggregative and antiamyloidogenic properties of nanoparticles: A promising tool for the treatment and diagnostics of neurodegenerative diseases. Oxidative Med. Cell. Longev. 2020, 2020, 3534570. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Xu, L.-P.; Wang, Q.; Yang, B.; Zhang, X. Synergistic inhibitory effect of GQDs–tramiprosate covalent binding on amyloid aggregation. ACS Chem. Neurosci. 2017, 9, 817–823. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, R.J.; Barker, R.A. Neurodegeneration: A failure of neuroregeneration? Lancet 2001, 358, 1174–1176. [Google Scholar] [CrossRef]
- Ager, R.R.; Davis, J.L.; Agazaryan, A.; Benavente, F.; Poon, W.W.; LaFerla, F.M.; Blurton-Jones, M. Human neural stem cells improve cognition and promote synaptic growth in two complementary transgenic models of Alzheimer’s disease and neuronal loss. Hippocampus 2015, 25, 813–826. [Google Scholar] [CrossRef] [PubMed]
- Vissers, C.; Ming, G.-l.; Song, H. Nanoparticle technology and stem cell therapy team up against neurodegenerative disorders. Adv. Drug Deliv. Rev. 2019, 148, 239–251. [Google Scholar] [CrossRef]
- Carradori, D.; Eyer, J.; Saulnier, P.; Préat, V.; Des Rieux, A. The therapeutic contribution of nanomedicine to treat neurodegenerative diseases via neural stem cell differentiation. Biomaterials 2017, 123, 77–91. [Google Scholar] [CrossRef]
- Mazzini, L.; Gelati, M.; Profico, D.C.; Sgaravizzi, G.; Projetti Pensi, M.; Muzi, G.; Ricciolini, C.; Rota Nodari, L.; Carletti, S.; Giorgi, C. Human neural stem cell transplantation in ALS: Initial results from a phase I trial. J. Transl. Med. 2015, 13, 17. [Google Scholar] [CrossRef]
- McIntyre, L.L.; Greilach, S.A.; Othy, S.; Sears-Kraxberger, I.; Wi, B.; Ayala-Angulo, J.; Vu, E.; Pham, Q.; Silva, J.; Dang, K. Regulatory T cells promote remyelination in the murine experimental autoimmune encephalomyelitis model of multiple sclerosis following human neural stem cell transplant. Neurobiol. Dis. 2020, 140, 104868. [Google Scholar] [CrossRef]
- Wang, Y.; Ji, X.; Leak, R.K.; Chen, F.; Cao, G. Stem cell therapies in age-related neurodegenerative diseases and stroke. Ageing Res. Rev. 2017, 34, 39–50. [Google Scholar] [CrossRef]
- Carson, C.T.; Aigner, S.; Gage, F.H. Stem cells: The good, bad and barely in control. Nat. Med. 2006, 12, 1237–1238. [Google Scholar] [CrossRef] [PubMed]
- Kazmerova, Z.; Zilka, N.; Cente, M.; Neradil, P.; Zilkova, M.; Novak, M. Can we teach old dogs new tricks? neuroprotective cell therapy in Alzheimer’s and Parkinson’s disease. J. Alzheimer’s Dis. 2013, 37, 251–272. [Google Scholar] [CrossRef] [PubMed]
- Lindvall, O.; Kokaia, Z.; Martinez-Serrano, A. Stem cell therapy for human neurodegenerative disorders–how to make it work. Nat. Med. 2004, 10, S42–S50. [Google Scholar] [CrossRef] [PubMed]
- Kerr, D.A.; Lladó, J.; Shamblott, M.J.; Maragakis, N.J.; Irani, D.N.; Crawford, T.O.; Krishnan, C.; Dike, S.; Gearhart, J.D.; Rothstein, J.D. Human embryonic germ cell derivatives facilitate motor recovery of rats with diffuse motor neuron injury. J. Neurosci. 2003, 23, 5131–5140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takahashi, K.; Yamanaka, S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 2006, 126, 663–676. [Google Scholar] [CrossRef] [PubMed]
- Sonntag, K.-C.; Song, B.; Lee, N.; Jung, J.H.; Cha, Y.; Leblanc, P.; Neff, C.; Kong, S.W.; Carter, B.S.; Schweitzer, J. Pluripotent stem cell-based therapy for Parkinson’s disease: Current status and future prospects. Prog. Neurobiol. 2018, 168, 1–20. [Google Scholar] [CrossRef]
- Feng, B.; Ng, J.-H.; Heng, J.-C.D.; Ng, H.-H. Molecules that promote or enhance reprogramming of somatic cells to induced pluripotent stem cells. Cell stem Cell 2009, 4, 301–312. [Google Scholar] [CrossRef]
- Selim, A.O.; Selim, S.A.; Shalaby, S.M.; Mosaad, H.; Saber, T. Neuroprotective effects of placenta-derived mesenchymal stromal cells in a rat model of experimental autoimmune encephalomyelitis. Cytotherapy 2016, 18, 1100–1113. [Google Scholar] [CrossRef]
- Mandal, S.M.; Roy, A.; Ghosh, A.K.; Hazra, T.K.; Basak, A.; Franco, O.L. Challenges and future prospects of antibiotic therapy: From peptides to phages utilization. Front. Pharmacol. 2014, 5, 105. [Google Scholar] [CrossRef]
- Vlieghe, P.; Lisowski, V.; Martinez, J.; Khrestchatisky, M. Synthetic therapeutic peptides: Science and market. Drug Discov. Today 2010, 15, 40–56. [Google Scholar] [CrossRef]
- Cararo, J.H.; Streck, E.L.; Schuck, P.F.; da C Ferreira, G. Carnosine and related peptides: Therapeutic potential in age-related disorders. Aging Dis. 2015, 6, 369. [Google Scholar]
- Prokopieva, V.; Yarygina, E.; Bokhan, N.; Ivanova, S. Use of carnosine for oxidative stress reduction in different pathologies. Oxidative Med. Cell. Longev. 2016, 2016, 2939087. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Shi, L.; Zhang, L.R. Neuroprotective effect of carnosine against salsolinol-induced Parkinson’s disease. Exp. Ther. Med. 2017, 14, 664–670. [Google Scholar] [CrossRef] [PubMed]
- Spaas, J.; Van Noten, P.; Keytsman, C.; Nieste, I.; Blancquaert, L.; Derave, W.; Eijnde, B.O. Carnosine and skeletal muscle dysfunction in a rodent multiple sclerosis model. Amino Acids 2021, 53, 1749–1761. [Google Scholar] [CrossRef] [PubMed]
- Hegazy, M.A.; Abdelmonsif, D.A.; Zeitoun, T.M.; El-Sayed, N.S.; Samy, D.M. Swimming exercise versus L-carnosine supplementation for Alzheimer’s dementia in rats: Implication of circulating and hippocampal FNDC5/irisin. J. Physiol. Biochem. 2022, 78, 109–124. [Google Scholar] [CrossRef] [PubMed]
- Hobart, L.J.; Seibel, I.; Yeargans, G.S.; Seidler, N.W. Anti-crosslinking properties of carnosine: Significance of histidine. Life Sci. 2004, 75, 1379–1389. [Google Scholar] [CrossRef]
- Filichia, E.; Hoffer, B.; Qi, X.; Luo, Y. Inhibition of Drp1 mitochondrial translocation provides neural protection in dopaminergic system in a Parkinson’s disease model induced by MPTP. Sci. Rep. 2016, 6, 32656. [Google Scholar] [CrossRef]
- White, C.M.; Ji, S.; Cai, H.; Maudsley, S.; Martin, B. Therapeutic potential of vasoactive intestinal peptide and its receptors in neurological disorders. CNS Neurol. Disord.-Drug Targets (Former. Curr. Drug Targets-CNS Neurol. Disord.) 2010, 9, 661–666. [Google Scholar] [CrossRef]
- Delgado, M.; Abad, C.; Martinez, C.; Juarranz, M.; Arranz, A.; Gomariz, R.; Leceta, J. Vasoactive intestinal peptide in the immune system: Potential therapeutic role in inflammatory and autoimmune diseases. J. Mol. Med. 2002, 80, 16–24. [Google Scholar] [CrossRef]
- Korkmaz, O.T.; Ay, H.; Aytan, N.; Carreras, I.; Kowall, N.W.; Dedeoglu, A.; Tuncel, N. Vasoactive intestinal peptide decreases β-amyloid accumulation and prevents brain atrophy in the 5xFAD mouse model of Alzheimer’s disease. J. Mol. Neurosci. 2019, 68, 389–396. [Google Scholar] [CrossRef]
- Korkmaz, O.T.; Tunçel, N. Advantages of vasoactive intestinal peptide for the future treatment of Parkinson’s disease. Curr. Pharm. Des. 2018, 24, 4693–4701. [Google Scholar] [CrossRef] [PubMed]
- Kurlan, R.; Kaplan, E.L. The pediatric autoimmune neuropsychiatric disorders associated with streptococcal infection (PANDAS) etiology for tics and obsessive-compulsive symptoms: Hypothesis or entity? Practical considerations for the clinician. Pediatrics 2004, 113, 883–886. [Google Scholar] [CrossRef] [PubMed]
- Olek, M. Multiple Sclerosis: Etiology, Diagnosis, and New Treatment Strategies; Springer: Berlin/Heidelberg, Germany, 2007. [Google Scholar]
- Hornig, M. The role of microbes and autoimmunity in the pathogenesis of neuropsychiatric illness. Curr. Opin. Rheumatol. 2013, 25, 488–795. [Google Scholar] [CrossRef] [PubMed]
- Ercolini, A.; Miller, S. The role of infections in autoimmune disease. Clin. Exp. Immunol. 2009, 155, 1–15. [Google Scholar] [CrossRef]
- Hughes, J.F.; Coffin, J.M. A novel endogenous retrovirus-related element in the human genome resembles a DNA transposon: Evidence for an evolutionary link? Genomics 2002, 80, 453–455. [Google Scholar] [CrossRef]
- Simula, E.R.; Arru, G.; Zarbo, I.R.; Solla, P.; Sechi, L.A. TDP-43 and HERV-K Envelope-Specific Immunogenic Epitopes Are Recognized in ALS Patients. Viruses 2021, 13, 2301. [Google Scholar] [CrossRef]
- Arru, G.; Mameli, G.; Deiana, G.; Rassu, A.; Piredda, R.; Sechi, E.; Caggiu, E.; Bo, M.; Nako, E.; Urso, D. Humoral immunity response to human endogenous retroviruses K/W differentiates between amyotrophic lateral sclerosis and other neurological diseases. Eur. J. Neurol. 2018, 25, 1076-e84. [Google Scholar] [CrossRef]
- Arru, G.; Galleri, G.; Deiana, G.A.; Zarbo, I.R.; Sechi, E.; Bo, M.; Cadoni, M.P.L.; Corda, D.G.; Frau, C.; Simula, E.R. HERV-K modulates the immune response in ALS patients. Microorganisms 2021, 9, 1784. [Google Scholar] [CrossRef]
- Douville, R.; Liu, J.; Rothstein, J.; Nath, A. Identification of active loci of a human endogenous retrovirus in neurons of patients with amyotrophic lateral sclerosis. Ann. Neurol. 2011, 69, 141–151. [Google Scholar] [CrossRef]
- Li, W.; Lee, M.-H.; Henderson, L.; Tyagi, R.; Bachani, M.; Steiner, J.; Campanac, E.; Hoffman, D.A.; Von Geldern, G.; Johnson, K. Human endogenous retrovirus-K contributes to motor neuron disease. Sci. Transl. Med. 2015, 7, 307ra153. [Google Scholar] [CrossRef]
- Manghera, M.; Ferguson-Parry, J.; Lin, R.; Douville, R.N. NF-κB and IRF1 induce endogenous retrovirus K expression via interferon-stimulated response elements in its 5′ long terminal repeat. J. Virol. 2016, 90, 9338–9349. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Montojo, M.; Fathi, S.; Norato, G.; Smith, B.; Rowe, D.; Kiernan, M.; Vucic, S.; Mathers, S.; van Eijk, R.; Santamaria, U. Inhibition of HERV-K (HML-2) in amyotrophic lateral sclerosis patients on antiretroviral therapy. J. Neurol. Sci. 2021, 423, 117358. [Google Scholar] [CrossRef] [PubMed]
- Mameli, G.; Cocco, E.; Frau, J.; Marrosu, M.G.; Sechi, L.A. Epstein Barr Virus and Mycobacterium avium subsp. paratuberculosis peptides are recognized in sera and cerebrospinal fluid of MS patients. Sci. Rep. 2016, 6, 22401. [Google Scholar]
- Bjornevik, K.; Cortese, M.; Healy, B.C.; Kuhle, J.; Mina, M.J.; Leng, Y.; Elledge, S.J.; Niebuhr, D.W.; Scher, A.I.; Munger, K.L. Longitudinal analysis reveals high prevalence of Epstein-Barr virus associated with multiple sclerosis. Science 2022, 375, 296–301. [Google Scholar] [CrossRef] [PubMed]
- Robinson, W.H.; Steinman, L. Epstein-Barr virus and multiple sclerosis. Science 2022, 375, 264–265. [Google Scholar] [CrossRef]
- Keightley, P.C.; Koloski, N.A.; Talley, N.J. Pathways in gut-brain communication: Evidence for distinct gut-to-brain and brain-to-gut syndromes. Aust. N. Z. J. Psychiatry 2015, 49, 207–214. [Google Scholar] [CrossRef]
- Patterson, E.; Cryan, J.F.; Fitzgerald, G.F.; Ross, R.P.; Dinan, T.G.; Stanton, C. Gut microbiota, the pharmabiotics they produce and host health. Proc. Nutr. Soc. 2014, 73, 477–489. [Google Scholar] [CrossRef]
- Fang, X. Potential role of gut microbiota and tissue barriers in Parkinson’s disease and amyotrophic lateral sclerosis. Int. J. Neurosci. 2016, 126, 771–776. [Google Scholar] [CrossRef]
- Hill, J.M.; Bhattacharjee, S.; Pogue, A.I.; Lukiw, W.J. The gastrointestinal tract microbiome and potential link to Alzheimer’s disease. Front. Neurol. 2014, 5, 43. [Google Scholar] [CrossRef]
- Li, D.; Wang, P.; Wang, P.; Hu, X.; Chen, F. The gut microbiota: A treasure for human health. Biotechnol. Adv. 2016, 34, 1210–1224. [Google Scholar] [CrossRef]
- Dinan, T.G.; Cryan, J.F. Gut instincts: Microbiota as a key regulator of brain development, ageing and neurodegeneration. J. Physiol. 2017, 595, 489–503. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Miller, R.G.; Gascon, R.; Champion, S.; Katz, J.; Lancero, M.; Narvaez, A.; Honrada, R.; Ruvalcaba, D.; McGrath, M.S. Circulating endotoxin and systemic immune activation in sporadic amyotrophic lateral sclerosis (sALS). J. Neuroimmunol. 2009, 206, 121–124. [Google Scholar] [CrossRef] [PubMed]
- Bataveljic, D.; Milosevic, M.; Radenovic, L.; Andjus, P. Novel molecular biomarkers at the blood-brain barrier in ALS. BioMed Res. Int. 2014, 2014, 907545. [Google Scholar] [CrossRef] [PubMed]
- Garbuzova-Davis, S.; Hernandez-Ontiveros, D.G.; Rodrigues, M.C.; Haller, E.; Frisina-Deyo, A.; Mirtyl, S.; Sallot, S.; Saporta, S.; Borlongan, C.V.; Sanberg, P.R. Impaired blood–brain/spinal cord barrier in ALS patients. Brain Res. 2012, 1469, 114–128. [Google Scholar] [CrossRef] [PubMed]
- Graves, M.; Fiala, M.; Dinglasan, L.A.; Liu, N.; Sayre, J.; Chiappelli, F.; van Kooten, C.; Vinters, H. Inflammation in amyotrophic lateral sclerosis spinal cord and brain is mediated by activated macrophages, mast cells and T cells. Amyotroph. Lateral Scler. Other Mot. Neuron Disord. 2004, 5, 213–219. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Yi, J.; Zhang, Y.g.; Zhou, J.; Sun, J. Leaky intestine and impaired microbiome in an amyotrophic lateral sclerosis mouse model. Physiol. Rep. 2015, 3, e12356. [Google Scholar] [CrossRef] [Green Version]
- Szablewski, L. Human gut microbiota in health and Alzheimer’s disease. J. Alzheimer’s Dis. 2018, 62, 549–560. [Google Scholar] [CrossRef]
- Zlokovic, B.V. Neurovascular mechanisms of Alzheimer’s neurodegeneration. Trends Neurosci. 2005, 28, 202–208. [Google Scholar]
- Spielman, L.J.; Gibson, D.L.; Klegeris, A. Unhealthy gut, unhealthy brain: The role of the intestinal microbiota in neurodegenerative diseases. Neurochem. Int. 2018, 120, 149–163. [Google Scholar] [CrossRef]
- Smith, P.; Smythies, L.; Shen, R.; Greenwell-Wild, T.; Gliozzi, M.; Wahl, S. Intestinal macrophages and response to microbial encroachment. Mucosal Immunol. 2011, 4, 31–42. [Google Scholar] [CrossRef]
- Faden, A.I.; Loane, D.J. Chronic neurodegeneration after traumatic brain injury: Alzheimer disease, chronic traumatic encephalopathy, or persistent neuroinflammation? Neurotherapeutics 2015, 12, 143–150. [Google Scholar] [CrossRef] [PubMed]
- Vivekanantham, S.; Shah, S.; Dewji, R.; Dewji, A.; Khatri, C.; Ologunde, R. Neuroinflammation in Parkinson’s disease: Role in neurodegeneration and tissue repair. Int. J. Neurosci. 2015, 125, 717–725. [Google Scholar] [CrossRef] [PubMed]
- Clarke, G.; Stilling, R.M.; Kennedy, P.J.; Stanton, C.; Cryan, J.F.; Dinan, T.G. Minireview: Gut microbiota: The neglected endocrine organ. Mol. Endocrinol. 2014, 28, 1221–1238. [Google Scholar] [CrossRef] [PubMed]
- Mancuso, C.; Santangelo, R. Ferulic acid: Pharmacological and toxicological aspects. Food Chem. Toxicol. 2014, 65, 185–195. [Google Scholar] [CrossRef] [PubMed]
- Trombino, S.; Cassano, R.; Ferrarelli, T.; Barone, E.; Picci, N.; Mancuso, C. Trans-ferulic acid-based solid lipid nanoparticles and their antioxidant effect in rat brain microsomes. Colloids Surf. B Biointerfaces 2013, 109, 273–279. [Google Scholar] [CrossRef] [PubMed]
- Szwajgier, D.; Dmowska, A. Novel ferulic acid esterases from Bifidobacterium sp. produced on selected synthetic and natural carbon sources. Acta Sci. Pol. Technol. Aliment. 2010, 9, 305–318. [Google Scholar]
- Tomaro-Duchesneau, C.; Saha, S.; Malhotra, M.; Coussa-Charley, M.; Kahouli, I.; Jones, M.L.; Labbé, A.; Prakash, S. Probiotic ferulic acid esterase active Lactobacillus fermentum NCIMB 5221 APA microcapsules for oral delivery: Preparation and in vitro characterization. Pharmaceuticals 2012, 5, 236–248. [Google Scholar] [CrossRef]
- Mori, T.; Koyama, N.; Guillot-Sestier, M.-V.; Tan, J.; Town, T. Ferulic acid is a nutraceutical β-secretase modulator that improves behavioral impairment and alzheimer-like pathology in transgenic mice. PLoS ONE 2013, 8, e55774. [Google Scholar] [CrossRef] [Green Version]
- Yan, J.-J.; Jung, J.-S.; Kim, T.-K.; Hasan, M.A.; Hong, C.-W.; Nam, J.-S.; Song, D.-K. Protective effects of ferulic acid in amyloid precursor protein plus presenilin-1 transgenic mouse model of Alzheimer disease. Biol. Pharm. Bull. 2013, 36, 140–143. [Google Scholar] [CrossRef]
- Mori, T.; Koyama, N.; Tan, J.; Segawa, T.; Maeda, M.; Town, T. Combination therapy with octyl gallate and ferulic acid improves cognition and neurodegeneration in a transgenic mouse model of Alzheimer’s disease. J. Biol. Chem. 2017, 292, 11310–11325. [Google Scholar] [CrossRef]
- Verbeke, K.A.; Boobis, A.R.; Chiodini, A.; Edwards, C.A.; Franck, A.; Kleerebezem, M.; Nauta, A.; Raes, J.; Van Tol, E.A.; Tuohy, K.M. Towards microbial fermentation metabolites as markers for health benefits of prebiotics. Nutr. Res. Rev. 2015, 28, 42–66. [Google Scholar] [CrossRef] [PubMed]
- Tagliabue, A.; Elli, M. The role of gut microbiota in human obesity: Recent findings and future perspectives. Nutr. Metab. Cardiovasc. Dis. 2013, 23, 160–168. [Google Scholar] [CrossRef] [PubMed]
- DeCastro, M.; Nankova, B.B.; Shah, P.; Patel, P.; Mally, P.V.; Mishra, R.; La Gamma, E.F. Short chain fatty acids regulate tyrosine hydroxylase gene expression through a cAMP-dependent signaling pathway. Mol. Brain Res. 2005, 142, 28–38. [Google Scholar] [CrossRef] [PubMed]
- Govindarajan, N.; Agis-Balboa, R.C.; Walter, J.; Sananbenesi, F.; Fischer, A. Sodium butyrate improves memory function in an Alzheimer’s disease mouse model when administered at an advanced stage of disease progression. J. Alzheimer’s Dis. 2011, 26, 187–197. [Google Scholar] [CrossRef]
- Westfall, S.; Lomis, N.; Kahouli, I.; Dia, S.Y.; Singh, S.P.; Prakash, S. Microbiome, probiotics and neurodegenerative diseases: Deciphering the gut brain axis. Cell. Mol. Life Sci. 2017, 74, 3769–3787. [Google Scholar] [CrossRef]
- Del Rio, R.; Noubade, R.; Saligrama, N.; Wall, E.H.; Krementsov, D.N.; Poynter, M.E.; Zachary, J.F.; Thurmond, R.L.; Teuscher, C. Histamine H4 receptor optimizes T regulatory cell frequency and facilitates anti-inflammatory responses within the central nervous system. J. Immunol. 2012, 188, 541–547. [Google Scholar] [CrossRef]
- Naddafi, F.; Mirshafiey, A. The neglected role of histamine in Alzheimer’s disease. Am. J. Alzheimer’s Dis. Other Dement. 2013, 28, 327–336. [Google Scholar] [CrossRef]
- Dong, H.; Zhang, W.; Zeng, X.; Hu, G.; Zhang, H.; He, S.; Zhang, S. Histamine induces upregulated expression of histamine receptors and increases release of inflammatory mediators from microglia. Mol. Neurobiol. 2014, 49, 1487–1500. [Google Scholar] [CrossRef]
- Landete, J.M.; De Las Rivas, B.; Marcobal, A.; Munoz, R. Updated molecular knowledge about histamine biosynthesis by bacteria. Crit. Rev. Food Sci. Nutr. 2008, 48, 697–714. [Google Scholar] [CrossRef]
- Jadidi-Niaragh, F.; Mirshafiey, A. Histamine and histamine receptors in pathogenesis and treatment of multiple sclerosis. Neuropharmacology 2010, 59, 180–189. [Google Scholar] [CrossRef]




| Advantages and Limitations of Immunomodulatory Drugs | |
|---|---|
| Advantages | Limitations |
| A combination of drugs within nutraceuticals has clinical advantages for the treatment of infections [44]. | Immunomodulator drugs increase the risk of infection as they cause both mild and adverse effects on human health [45]. |
| Nanomaterials in combination with molecular targeted therapy enhanced the immunomodulatory effect [46]. | The major limitations related to immunomodulatory drugs are in vivo toxicity, routes of administration, and suitable formulations. |
| The combinations of vitamin D3 and phenylbutyrate activate innate immunity [47] and it produces antimicrobial peptides which can be used for the treatment of tuberculosis [48] as they produce both immunomodulatory and antibacterial responses. | Medullar suppression is caused by using immunomodulators such as azathioprine and 6-mercaptopurine. It is also recommended to take gastric protectors to avoid possible gastric irritation. |
| The major advantage of using immunomodulators is their well-known mechanism of action and long-term side effects [49]. | Side effects of using immunomodulators are pancreatitis, dizziness, hepatitis, and myalgia [50]. |
| A combination of drugs within nutraceuticals has clinical advantages for the treatment of infections [44]. | Immunomodulator drugs increase the risk of infection as they cause both mild and adverse effects on human health [45]. |
| The major limitations related to immunomodulatory drugs are in vivo toxicity, routes of administration, and suitable formulations. | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ashraf, H.; Solla, P.; Sechi, L.A. Current Advancement of Immunomodulatory Drugs as Potential Pharmacotherapies for Autoimmunity Based Neurological Diseases. Pharmaceuticals 2022, 15, 1077. https://doi.org/10.3390/ph15091077
Ashraf H, Solla P, Sechi LA. Current Advancement of Immunomodulatory Drugs as Potential Pharmacotherapies for Autoimmunity Based Neurological Diseases. Pharmaceuticals. 2022; 15(9):1077. https://doi.org/10.3390/ph15091077
Chicago/Turabian StyleAshraf, Hajra, Paolo Solla, and Leonardo Atonio Sechi. 2022. "Current Advancement of Immunomodulatory Drugs as Potential Pharmacotherapies for Autoimmunity Based Neurological Diseases" Pharmaceuticals 15, no. 9: 1077. https://doi.org/10.3390/ph15091077
APA StyleAshraf, H., Solla, P., & Sechi, L. A. (2022). Current Advancement of Immunomodulatory Drugs as Potential Pharmacotherapies for Autoimmunity Based Neurological Diseases. Pharmaceuticals, 15(9), 1077. https://doi.org/10.3390/ph15091077