Sustainable Extraction, Chemical Profile, Cytotoxic and Antileishmanial Activities In-Vitro of Some Citrus Species Leaves Essential Oils
Abstract
:1. Introduction
2. Results
2.1. Extraction Yield
2.2. Quantitative and Qualitative Analysis
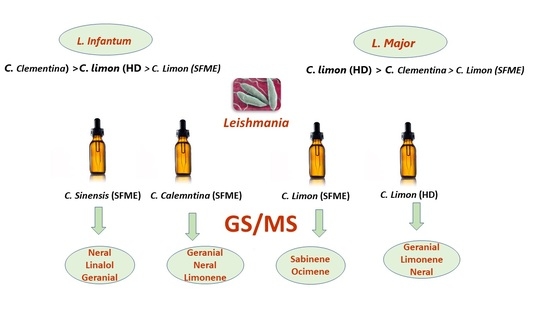
2.3. Anti-Leishmanial Activity
2.4. Cytotoxicity
3. Discussion
3.1. Essential Oil Analysis
3.2. Anti-Leishmanial Activity
4. Materials and Methods
4.1. Reagents
4.2. Plant Material
- -
- The “Eureka” lemon cultivar is an ever-bearing and basically seedless variety. Throughout the year, the tree produces fruits of a medium size with a yellow and smooth peel. The first “Eureka” selection originated from seed in the 19th century in the USA [39].
- -
- The “Thomson Washington Navel” orange is a “Washington Navel” cultivar improved in the USA from bud selections, in the 19th century. Fruits are generally seedless and less colored; the fruit apex is usually protruded, with the famous large open navel. They mature approximately two weeks earlier [40].
- -
- The “Cassar” Clementina cultivar is a local clone discovered in the region of La Sokra [38], Tunisia, in the 20th century. The tree canopy port is basically spherical, with an average vigor. Fruit shape is generally flattened at the apex and rounded on the peduncle side, with a medium caliber, thin skin, and easy coat. Fruits are generally seedless but contain seeds when the orchard has other genotypes.
4.3. Essential Oil Extraction by Hydro-Distillation
4.4. Solvent-Free Microwave Extraction (SFME)
4.5. GC-MS Analysis
4.6. Identification of EO Components
4.7. Leishmania Parasite Culture
4.8. Anti-Leishmanial Activity
4.9. Cell Viability Assay
4.10. Cell and Leishmania Parasite Treatment
4.11. Cytotoxicity Assay and Selectivity Index
4.12. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- WHO (World Health Organization). Leishmaniasis: Geneva: Special Programme for Research and Training in Tropical Diseases; WHO: Geneva, Switzerland, 2012. [Google Scholar]
- Weninger, B.; Robledo, S.; Arango, G.J.; Deharo, E.; Arango, R.; Munoz, V.; Callapa, J.; Lobstein, A.; Anton, R. Antiprotozoal activities of Colombianplants. J. Ethnopharmacol. 2001, 78, 193–200. [Google Scholar] [CrossRef]
- Berman, J.D. Current treatment approaches to leishmaniasis. Curr. Opin. Infect. Dis. 2003, 5, 397–401. [Google Scholar] [CrossRef] [PubMed]
- Anthony, J.; Fyfe, L.; Smith, H. Plant active components—A resource for anti-parasitic agents. Trends Parasitol. 2005, 21, 462–468. [Google Scholar] [CrossRef] [PubMed]
- Monzote, L.; Piñón, A.; Setzer, W.N. Antileishmanial potential of tropical Rain Forest plant extracts. Medicines 2014, 1, 32–55. [Google Scholar] [CrossRef] [PubMed]
- Youssefi, M.R.; Moghaddas, E.; Tabari, M.A.; Moghadamnia, A.A.; Hosseini, S.M.; Farash, B.R.H.; Petrelli, R. In vitro and in vivo effectiveness of carvacrol, thymol and linalool against Leishmania infantum. Molecules 2019, 24, 2072. [Google Scholar] [CrossRef]
- Arias, B.A.; Ramon-Laca, L. Pharmacological properties of Citrus and their ancient and medieval uses in the Mediterranean region. J. Ethnopharmacol. 2005, 97, 89–95. [Google Scholar] [CrossRef]
- Fisher, T.K.; Phillipsm, C.A. The effect of lemon, orange and bergamot essential oils and their components on the survival of Campylobacter jejuni, Escherichia coli O157, Listeria monocytogenes, Bacillus cereus and Staphylococcus aureus in vitro and in food systems. J. Appl. Microbiol. 2006, 101, 1232. [Google Scholar] [CrossRef]
- Eldahshan, O.A.; Halim, A.F. Comparison of the Composition and Antimicrobial Activities of the Essential Oils of Green Branches and Leaves of Egyptian Navel Orange (Citrus sinensis (L.) Osbeck var. malesy). Chem. Biodivers. 2016, 13, 681–685. [Google Scholar] [CrossRef]
- Gavahian, M.; Farahnaky, A.; Farhoosh, R.; Javidnia, K.; Shahidi, F. Extraction of essential oils from Mentha piperita using advanced techniques: Microwave versus ohmic assisted hydrodistillation. Food Bioprod. Process. 2015, 94, 50–58. [Google Scholar] [CrossRef]
- Faucon, M. Traité D’aromathérapie Scientifique et Médicale: Fondements & Aide à la Prescription; Sang de la Terre: Paris, France, 2015; pp. 39–455. [Google Scholar]
- Festy, D. Ma Bible des Huiles Essentielles; Leduc: Paris, France, 2017; p. 122. [Google Scholar]
- Oliveira, A.C.S.D.; Fernandes, C.C.; Santos, L.S.; Candido, A.C.B.B.; Magalhães, L.G.; Miranda, M.L.D. Chemical composition, in vitro larvicidal and antileishmanial activities of the essential oil from Citrus reticulata Blanco fruit peel. Braz. J. Biol. 2023, 83, e247539. [Google Scholar] [CrossRef]
- Zhao, X.; Zhang, X.; Fu, L.; Zhu, H.; Zhang, B. Effect of extraction and drying methods on antioxidant activity of astaxanthin from Haematococcus pluvialis. Food Bioprod. Process. 2016, 99, 197–203. [Google Scholar] [CrossRef]
- Rombaut, N.; Tixier, A.S.; Bily, A.B.; Chemat, F. Green extraction processes of natural products as tools for biorefinery. Biofuels Bioprod. Bioref. 2014, 8, 530–544. [Google Scholar] [CrossRef]
- Chemat, F.; Abert Vian, M.; Ravi, H.K.; Khadhraoui, B.; Hilali, S.; Perino, S.; Tixier, A.F. Review of Alternative Solvents for Green Extraction of Food and Natural Products: Panorama, Principles, Applications and Prospects. Molecules 2019, 24, 3007. [Google Scholar] [CrossRef]
- Chemat, F.; Vian, M.A.; Fabiano-Tixier, A.-S.; Nutrizio, M.; Jambrak, A.R.; Munekata, P.E.S.; Lorenzo, J.M.; Barba, F.J.; Binello, A.; Cravotto, G. A review of sustainable and intensified techniques for extraction of food and natural products. Green Chem. 2020, 22, 2325–2353. [Google Scholar] [CrossRef]
- Tigrine-Kordjani, N.; Meklati, B.Y.; Chemat, F. Contribution of microwave accelerated distillation in the extraction of the essential oil of Zygophyllum album L. Phytochem. Anal. 2011, 22, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Cardoso-Ugarte, G.A.; Juárez-Becerra, G.P.; Sosa-Morales, M.E.; López-Malo, A. Microwave-assisted extraction of essential oils from herbs. J. Microw. Power Electromagn. Energy 2013, 47, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Mossman, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Favela-Hernández, J.M.; González-Santiago, O.; Ramírez-Cabrera, M.A.; Esquivel-Ferriño, P.C.; Camacho-Corona, R.M.d. Chemistry and Pharmacology of Citrus sinensis. Molecules 2016, 21, 247. [Google Scholar] [CrossRef]
- Klimek-Szczykutowicz, M.; Szopa, A.; Ekiert, H. Citrus limon (Lemon) Phenomenon—A Review of the Chemistry, Pharmacological Properties, Applications in the Modern Pharmaceutical, Food, and Cosmetics Industries, and Biotechnological Studies. Plants 2020, 9, 119. [Google Scholar] [CrossRef]
- Available online: https://www.healthbenefitstimes.com/clementine (accessed on 31 July 2021).
- Millet, F. Huiles essentielles et essence de citronnier (Citrus limon (L.) Burm. f.). Phytothérapie 2014, 12, 89–97. [Google Scholar] [CrossRef]
- Janoti, D.S.; Rana, M.; Rawat, A.K.S. Comparative antioxidant activity of essential oil of leaves of Citrus limettioides and Citrus pseudolimon of Nainital District. J. Pharmacogn. Phytochem. 2014, 2, 24–26. [Google Scholar]
- Vekiari, S.A.; Protopapadakis, E.E.; Parthena, P.; Dimitrios, P.; Panou, C.; Vamvakias, M. Composition and seasonal variation of the essential oil from leaves and peel of a Cretan lemon variety. J. Agric. Food Chem. 2002, 50, 147–153. [Google Scholar] [CrossRef] [PubMed]
- Ayedoun, A.M.; Sossou, P.V.; Mardarowicz, M.; Leclercq, P.A. Volatile constituents of the peel and leaf oils of Citrus limon L. Burm. f. from Benin. J. Essent. Oil Res. 1996, 8, 441–444. [Google Scholar] [CrossRef]
- Owolabi, M.S.; Avoseh, O.N.; Ogunwande, I.A.; Setzer, W.N.; Ogungbo, R.; Ogundajo, A.L.; Lawal, O.A.; Flamini, G. Chemical composition of Citrus limon (L.) Osbeck growing in southwestern Nigeria: Essential oil chemotypes of both peel and leaf of lemon. Am. J. Essent. Oils Nat. Prod. 2018, 6, 36–40. [Google Scholar]
- Hamdani, S. Chemical Study and Antioxidant Activity of Essentials Oils of Citrus Fruits Grown in the Region of Tlemcen. Ph.D. Thesis, University Abou-Bekr Belkaid-Tlemcen, Chetouane, Algeria, 2018. [Google Scholar]
- Nayak, B.; Dahmoune, F.; Moussi, K.; Remini, H.; Dairi, S.; Aoun, O.; Khodir, M. Comparison of microwave, ultrasound and accelerated-assisted solvent extraction for recovery of polyphenols from Citrus sinensis peels. Food Chem. 2015, 187, 507–513. [Google Scholar] [CrossRef] [PubMed]
- Li, C.Y. Comparison between Solvent-Free Microwave Extraction and Hydro-Distillation Extraction in the Compositions and Biological Activities of Essential Oils from Guava (Psidium guajava L.) Leaves. Ph.D. Thesis, Institute of Cosmetic Science, Chia-Nan University of Pharmacy and Science, Tainan City, Taiwan, 2013. [Google Scholar]
- Filly, A.; Fabiano-Tixier, A.S.; Louis, C.; Fernandez, X.; Chemat, F. Water as a green solvent combined with different techniques for extraction of essential oil from lavender flowers. Comptes Rendus Chim. 2016, 19, 707–717. [Google Scholar] [CrossRef]
- Maaroufi, Z.; Cojean, S.; Loiseau, P.M.; Yahyaoui, M.; Agnely, F.; Abderraba, M.; Mekhloufi, G. In vitro antileishmanial potentialities of essential oils from Citrus limon and Pistacia lentiscus harvested in Tunisia. Parasitol. Res. 2021, 120, 1455–1469. [Google Scholar] [CrossRef]
- Arruda, D.C.; Miguel, D.C.; Yokoyama-Yasunaka, J.K.; Katzin, A.M.; Uliana, S.R. Inhibitory activity of limonene against Leishmania parasites in vitro and in vivo. Biomed. Pharmacother. 2009, 63, 643–649. [Google Scholar] [CrossRef]
- Rosa, M.D.S.S.; Mendonça-Filho, R.R.; Bizzo, H.R.; Rodrigues, I.D.A.; Soares, R.M.A.; Souto-Padrόn, T.; Alviano, C.S.; Lopes, A.H.C.S. Antileishmanial activity of a linalool-rich essential oil from Croton cajucara. Antimicrob. Agents Chemother. 2003, 47, 1895–1901. [Google Scholar] [CrossRef]
- Gomes, G.A.; Martins-Cardoso, K.; dos Santos, F.R.; Florencio, M.; Rosa, D.; Zuma, A.A.; Santiago, G.M.P.; Motta, M.C.M.; de Carvalho, M.G.; Fampa, P. Antileishmanial activity of the essential oils of Myrcia ovata Cambess. and Eremanthus erythropappus (DC) McLeisch leads to parasite mitochondrial damage. Nat. Prod. Res. 2021, 35, 6117–6121. [Google Scholar] [CrossRef]
- Essid, R.; Rahali, F.Z.; Msaada, K.; Sghair, I.; Hammami, M.; Bouratbine, A.; Aoun, K.; Limam, F. Antileishmanial and cytotoxic potential of essential oils from medicinal plants in Northern Tunisia. Ind. Crops Prod. 2015, 77, 795–802. [Google Scholar] [CrossRef]
- Aleza, P.; Juárez, J.; Hernández, M.; Pina, J.A.; Ollitrault, P.; Navarro, L. Recovery and characterization of a Citrus clementina Hort. ex Tan. “Clemenules” haploid plant selected to establish the reference whole Citrus genome sequence. BMC Plant Biol. 2009, 9, 110–129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Petit, M.; Montaigne, E.; El Hadad-Gauthier, F.; García Álvarez-Coque, J.M.; Mattas, K.; Mili, S. Sustainable Agricultural Development: Challenges and Approaches in Southern and Eastern Mediterranean Countries; Springer: Cham, Switzerland, 2015; 334p. [Google Scholar]
- Lovel, K.; Paull, R.E. Fruits, Nuts, and Beverage Crops; F_N-25; College of Tropical Agriculture and Human Resources, University of Hawai’I at Manoa: Honolulu, HI, USA, 2013; pp. 1–6. [Google Scholar]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry, 4th ed.; Allured Publishing Corp.: Carol Stream, IL, USA, 2007. [Google Scholar]
- Todirascu-Ciornea, E.; El-Nashar, H.A.S.; Mostafa, N.M.; Eldahshan, O.A.; Boiangiu, R.S.; Dumitru, G.; Hritcu, L.; Singab, A.N.B. Schinus terebinthifolius Essential Oil Attenuates Scopolamine-Induced Memory Deficits via Cholinergic Modulation and Antioxidant Properties in a Zebrafish Model. Evid.-Based Complement. Alternat. Med. 2019, 2019, 5256781. [Google Scholar] [CrossRef] [PubMed]
- Singab, A.N.; Mostafa, N.M.; Eldahshan, O.A.; Ashour, M.L.; Wink, M. Profile of volatile components of hydrodistilled and extracted leaves of Jacaranda acutifolia and their antimicrobial activity against foodborne pathogens. Nat. Prod. Commun. 2014, 9, 1007–1010. [Google Scholar] [CrossRef] [PubMed]
- Shahat, E.A.; Bakr, R.O.; Eldahshan, O.A.; Ayoub, N.A. Chemical Composition and Biological Activities of the Essential Oil from Leaves and Flowers of Pulicaria incisa sub. candolleana (Family Asteraceae). Chem. Biodivers. 2017, 14, e1600156. [Google Scholar] [CrossRef]
- Bouabdallah, S.; Sgheier, R.M.; Selmi, S.; Khalifi, D.; Laouni, D.; Ben-Attia, M. Current Approaches and Challenges for Chemical Characterization of inhibitory effect against cancer Cell line isolated from Gokshur Extract. J. Chrom. B 2015, 1026, 279–285. [Google Scholar] [CrossRef]
- Bouabdallah, S.; Laouini, D.; Bouzouita, N.; El Bok, S.; Sghaier, R.M.; Selmi, S.; Ben-Attia, M. Separation and evaluation of natural antileishmanial potential against Leishmania major and infantum isolated from the Tunisia strains. Bangladesh J. Pharmacol. 2018, 13, 74–81. [Google Scholar] [CrossRef] [Green Version]





| N | Compounds | RI a Cal | RI a Rep | Class b | % C. clementina SFME c | % C. sinensis SFME c | % C. limon SFME c | % C. limon HD d |
|---|---|---|---|---|---|---|---|---|
| 1 | α-Pinene | 939 | 932 | MH | 0.19 ± 0.01 | 1.15 ± 0.01 | 0.33 ± 0.01 | 0.27 ± 0.00 |
| 2 | Sabinene | 975 | 969 | MH | 0.55 ± 0.01 | 1.59 ± 0.01 | 14.22 ± 0.03 | 0.53 ± 0.01 |
| 3 | β-Pinene | 979 | 974 | MH | 1.81 ± 0.02 | - | 0.45 ± 0.04 | 2.77 ± 0.05 |
| 4 | Myrcene | 990 | 988 | MH | 0.32 ± 0.04 | - | 1.29 ± 0.11 | 0.46 ± 0.01 |
| 5 | δ-3-Carene | 1011 | 1008 | MH | 0.52 ± 0.02 | 1.1 ± 0.02 | 4.65 ± 0.01 | 0.97 ± 0.01 |
| 6 | p-Cymene | 1024 | 1020 | MH | 0.03 ± 0.14 | - | 0.32 ± 0.01 | 0.19 ± 0.01 |
| 7 | Limonene | 1029 | 1024 | MH | 14.48 ± 0.01 | 0.53 ± 0.01 | 4.34 ± 0.09 | 27.09 ± 1.89 |
| 8 | (E)-β-Ocimene | 1050 | 1044 | MH | 0.83 ± 0.02 | - | 6.09 ± 0.01 | 1.14 ± 0.03 |
| 9 | γ-Terpinene | 1059 | 1054 | MH | 0.03 ± 0.01 | - | 0.48 ± 0.04 | - |
| 10 | Terpinolene | 1088 | 1086 | MH | 0.12 ± 0.16 | - | 1.95 ± 0.04 | 0.15 ± 0.00 |
| 11 | Linalool | 1096 | 1095 | MO | 1.68 ± 0.16 | 25.83 ± 0.32 | 44.21 ± 0.52 | 1.59 ± 0.07 |
| 12 | cis-Limonene oxide | 1132 | 1132 | MO | 0.06 ± 0.01 | - | - | 0.24 ± 0.02 |
| 13 | Citronellal | 1153 | 1148 | MO | 0.47 ± 0.01 | 2.26 ± 0.05 | 1.22 ± 0.03 | 0.48 ± 0.03 |
| 14 | Terpinen-4-ol | 1177 | 1174 | MO | 0.1 ± 0.16 | - | 1.84 ± 0.01 | 0.23 ± 0.01 |
| 15 | α-Terpineol | 1188 | 1186 | MO | 0.83 ± 0.03 | 3.47 ± 0.03 | 4.71 ± 0.03 | 0.47 ± 0.02 |
| 16 | Neral | 1238 | 1235 | MO | 26.79 ± 0.01 | 27.52 ± 0.32 | 2.74 ± 0.31 | 22.87 ± 0.55 |
| 17 | Geraniol | 1249 | 1249 | MO | - | - | 0.77 ± 0.15 | - |
| 18 | Geranial | 1264 | 1264 | MO | 42.40 ± 0.24 | 23.44 ± 0.19 | 2.85 ± 0.15 | 30.08 ± 0.75 |
| 19 | Neryl acetate | 1361 | 1359 | MO | 0.89 ± 0.06 | 0.6 ± 0.02 | - | 2.14 ± 0.06 |
| 20 | Geranyl acetate | 1381 | 1379 | MO | 2.27 ± 0.4 | - | 1.65 ± 0.07 | 4.02 ± 0.1 |
| 21 | (Z)-Caryophyllene | 1408 | 1408 | SH | 0.24 ± 0.03 | 0.85 ± 0.03 | 0.38 ± 0.07 | 0.65 ± 0.02 |
| 22 | α-trans-Bergamotene | 1434 | 1432 | SH | 0.05 ± 0.01 | - | - | - |
| 23 | Germacrene D | 1485 | 1484 | SH | - | 0.04 | - | |
| 24 | (E, E)-α-Farnensene | 1505 | 1505 | SH | 0.05 ± 0.01 | - | 0.12 ± 0.01 | - |
| 25 | δ-Cadinene | 1523 | 1522 | SH | 0.25 ± 0.01 | - | - | - |
| 26 | (E)-γ-Bisabolene | 1531 | 1529 | SH | 0.15 ± 0.01 | - | - | 0.14 ± 0.01 |
| 27 | Caryophyllene oxide | 1583 | 1583 | SO | 0.14 ± 0.01 | - | - | - |
| 28 | (2E,6Z)-Farnesol | 1715 | 1714 | SO | 0.08 ± 0.01 | - | - | - |
| 29 | α-Sinensal | 1756 | 1755 | SO | - | 1.48 ± 0.05 | 0.6 ± 0.06 | 0.13 ± 0.01 |
| Total identified (%) | 68.54 | 89.82 | 93.96 | 96.67 | ||||
| EOs | IC50 ± SD (µg/mL) | IC80 ± SD (µg/mL) | SI | ||
|---|---|---|---|---|---|
| GLC94 | Lv50 | RAW 264.7 | GLC94 | Lv50 | |
| E1 | 1.13 ± 0.3 | 0.57 ± 0.09 | 0.30± 0.39 | 0.26 | 0.78 |
| E2 | 1.03 ± 0.27 | 0.32 ± 0.18 | 0.18 ± 0.11 | 0.17 | 0.56 |
| E3 | 5.25 ± 0.56 | 9.48 ± 0.25 | 0.16 ± 0.09 | 0.03 | 0.016 |
| E4 | 0.9 ± 0.29 | 0.52 ± 0.15 | 3.32 ± 0.24 | 3.68 | 6.38 |
| AB | 0.80 ± 0.18 | 0.23 ± 0.13 | 9.23 ± 0.13 | 41.95 | 11.53 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bouabdallah, S.; Cianfaglione, K.; Azzouz, M.; Batiha, G.E.-S.; Alkhuriji, A.F.; Al-Megrin, W.A.I.; Ben-Attia, M.; Eldahshan, O.A. Sustainable Extraction, Chemical Profile, Cytotoxic and Antileishmanial Activities In-Vitro of Some Citrus Species Leaves Essential Oils. Pharmaceuticals 2022, 15, 1163. https://doi.org/10.3390/ph15091163
Bouabdallah S, Cianfaglione K, Azzouz M, Batiha GE-S, Alkhuriji AF, Al-Megrin WAI, Ben-Attia M, Eldahshan OA. Sustainable Extraction, Chemical Profile, Cytotoxic and Antileishmanial Activities In-Vitro of Some Citrus Species Leaves Essential Oils. Pharmaceuticals. 2022; 15(9):1163. https://doi.org/10.3390/ph15091163
Chicago/Turabian StyleBouabdallah, Salwa, Kevin Cianfaglione, Myriam Azzouz, Gaber El-Saber Batiha, Afrah Fahad Alkhuriji, Wafa Abdullah I. Al-Megrin, Mossadok Ben-Attia, and Omayma A. Eldahshan. 2022. "Sustainable Extraction, Chemical Profile, Cytotoxic and Antileishmanial Activities In-Vitro of Some Citrus Species Leaves Essential Oils" Pharmaceuticals 15, no. 9: 1163. https://doi.org/10.3390/ph15091163
APA StyleBouabdallah, S., Cianfaglione, K., Azzouz, M., Batiha, G. E. -S., Alkhuriji, A. F., Al-Megrin, W. A. I., Ben-Attia, M., & Eldahshan, O. A. (2022). Sustainable Extraction, Chemical Profile, Cytotoxic and Antileishmanial Activities In-Vitro of Some Citrus Species Leaves Essential Oils. Pharmaceuticals, 15(9), 1163. https://doi.org/10.3390/ph15091163