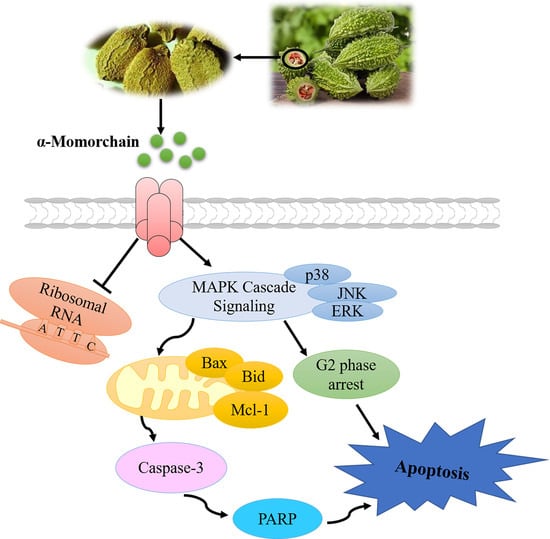
MAPK Cascade Signaling Is Involved in α-MMC Induced Growth Inhibition of Multiple Myeloma MM.1S Cells via G2 Arrest and Mitochondrial-Pathway-Dependent Apoptosis In Vitro
Abstract
:1. Introduction
2. Results
2.1. α-MMC Inhibited the Proliferation of MM.1S Cells
2.2. α-MMC Induced Morphological Changes in MM.1S Cells
2.3. α-MMC Induced MM.1S Cell Cycle Arrest at the G2 Phase
2.4. α-MMC Induced MM.1S Cell Apoptosis via the Mitochondrial Pathway
2.5. MAPK Cascade Signaling Was Involved in the Apoptosis-Inducing Effect of α-MMC on MM.1S Cells
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Cell Culture
4.3. Separation of PBMCs
4.4. Cell Proliferation and Cytotoxicity Assay
4.5. Cell Cycle Assay
4.6. Cell Apoptosis Assay
4.7. Mitochondrial Membrane Potential Assay
4.8. Western Blot Analysis
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rajkumar, S.V. Multiple myeloma: 2020 update on diagnosis, risk-stratification and management. Am. J. Hematol. 2020, 95, 548–567. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gandolfi, S.; Laubach, J.P.; Hideshima, T.; Chauhan, D.; Anderson, K.C.; Richardson, P.G. The proteasome and proteasome inhibitors in multiple myeloma. Cancer Metastasis Rev. 2017, 36, 561–584. [Google Scholar] [CrossRef] [PubMed]
- Holstein, S.A.; Mccarthy, P.L. Immunomodulatory Drugs in Multiple Myeloma: Mechanisms of Action and Clinical Experience. Drugs 2017, 77, 505–520. [Google Scholar] [CrossRef]
- Varga, C.; Maglio, M.; Ghobrial, I.M.; Richardson, P.G. Current use of monoclonal antibodies in the treatment of multiple myeloma. Br. J. Haematol. 2018, 181, 447–459. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chim, C.S.; Kumar, S.K.; Orlowski, R.Z.; Cook, G.; Richardson, P.G.; Gertz, M.A.; Giralt, S.; Mateos, M.V.; Leleu, X.; Anderson, K.C. Management of relapsed and refractory multiple myeloma: Novel agents, antibodies, immunotherapies and beyond. Leukemia 2018, 32, 252–262. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gandhi, U.H.; Cornell, R.F.; Lakshman, A.; Gahvari, Z.J.; McGehee, E.; Jagosky, M.H.; Gupta, R.; Varnado, W.; Fiala, M.A.; Chhabra, S.; et al. Outcomes of patients with multiple myeloma refractory to CD38-targeted monoclonal antibody therapy. Leukemia 2019, 33, 2266–2275. [Google Scholar] [CrossRef]
- Surh, Y.J. Cancer chemoprevention with dietary phytochemicals. Nat. Rev. Cancer 2003, 3, 768–780. [Google Scholar] [CrossRef] [PubMed]
- Bolognesi, A.; Bortolotti, M.; Maiello, S.; Battelli, M.G.; Polito, L. Ribosome-Inactivating Proteins from Plants: A Historical Overview. Molecules 2016, 21, 1627. [Google Scholar] [CrossRef] [Green Version]
- Shi, W.W.; Mak, A.N.; Wong, K.B.; Shaw, P.-C. Structures and Ribosomal Interaction of Ribosome-Inactivating Proteins. Molecules 2016, 21, 1588. [Google Scholar] [CrossRef] [Green Version]
- Setayesh-Mehr, Z.; Poorsargol, M. Toxic proteins application in cancer therapy. Mol. Biol. Rep. 2021, 48, 3827–3840. [Google Scholar] [CrossRef]
- Asrorov, A.M.; Gu, Z.; Min, K.A.; Shin, M.C.; Huang, Y. Advances on Tumor-Targeting Delivery of Cytotoxic Proteins. ACS Pharmacol. Transl. Sci. 2020, 3, 107–118. [Google Scholar] [CrossRef] [PubMed]
- Dandawate, P.R.; Subramaniam, D.; Padhye, S.B.; Anant, S. Bitter melon: A panacea for inflammation and cancer. Chin. J. Nat. Med. 2016, 14, 81–100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, S.; Zheng, Y.; Yan, J.; Zhu, Z.; Wu, Z.; Ding, Y. Alpha-momorcharin: A ribosome-inactivating protein from Momordica charantia, possessing DNA cleavage properties. Protein Pept. Lett. 2013, 20, 1257–1263. [Google Scholar] [CrossRef] [PubMed]
- Manoharan, G.; Jaiswal, S.R.; Singh, J. Effect of alpha, beta momorcharin on viability, caspase activity, cytochrome c release and on cytosolic calcium levels in different cancer cell lines. Mol. Cell Biochem. 2014, 388, 233–240. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; He, L.; Meng, Y.; Li, G.; Li, L.; Meng, Y. Alpha-MMC and MAP30, two ribosome-inactivating proteins extracted from Momordica charantia, induce cell cycle arrest and apoptosis in A549 human lung carcinoma cells. Mol. Med. Rep. 2015, 11, 3553–3558. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cao, D.; Sun, Y.; Wang, L.; He, Q.; Zheng, J.; Deng, F.; Deng, S.; Chang, S.; Yu, X.; Li, M.; et al. Alpha-momorcharin (alpha-MMC) exerts effective anti-human breast tumor activities but has a narrow therapeutic window in vivo. Fitoterapia 2015, 100, 139–149. [Google Scholar] [CrossRef] [PubMed]
- Deng, N.H.; Wang, L.; He, Q.C.; Zheng, J.-C.; Meng, Y.; Meng, Y.-F.; Zhang, C.-J.; Shen, F.-B. PEGylation alleviates the non-specific toxicities of Alpha-Momorcharin and preserves its antitumor efficacy in vivo. Drug Deliv. 2016, 23, 95–100. [Google Scholar] [CrossRef] [Green Version]
- Deng, N.; Li, M.; Shen, D.; He, Q.; Sun, W.; Liu, M.; Liu, Y.; Zhou, Y.; Zheng, J.; Shen, F. LRP1 receptor-mediated immunosuppression of alpha-MMC on monocytes. Int. Immunopharmacol. 2019, 70, 80–87. [Google Scholar] [CrossRef]
- Peng, K.; Deng, N.; Meng, Y.; He, Q.; Meng, H.; Luo, T.; Wei, Y.; Kang, Y.; Zhou, X.; Shen, F. Alpha-Momorcharin Inhibits Proinflammatory Cytokine Expression by M1 Macrophages but Not Anti-Inflammatory Cytokine Expression by M2 Macrophages. J. Inflamm. Res. 2022, 15, 4853–4872. [Google Scholar] [CrossRef]
- Shapira, A.; Benhar, I. Toxin-based therapeutic approaches. Toxins 2010, 2, 2519–2583. [Google Scholar] [CrossRef]
- Pizzo, E.; Di Maro, A. A new age for biomedical applications of Ribosome Inactivating Proteins (RIPs): From bioconjugate to nanoconstructs. J. Biomed. Sci. 2016, 23, 54. [Google Scholar] [CrossRef] [Green Version]
- Liu, B.; Zhang, Z.; Lu, S.; He, Q.; Deng, N.; Meng, H.; Pan, C.; Li, H.; Liu, M.; Huang, A.; et al. In-silico analysis of ligand-receptor binding patterns of α-MMC, TCS and MAP30 protein to LRP1 receptor. J. Mol. Graph. Model 2020, 98, 107619. [Google Scholar] [CrossRef] [PubMed]
- Xing, P.; Liao, Z.; Ren, Z.; Zhao, J.; Song, F.; Wang, G.; Chen, K.; Yang, J. Roles of low-density lipoprotein receptor-related protein 1 in tumors. Chin. J. Cancer 2016, 35, 6. [Google Scholar] [CrossRef] [Green Version]
- Obeng, E. Apoptosis (programmed cell death) and its signals—A review. Braz. J. Biol. 2021, 81, 1133–1143. [Google Scholar] [CrossRef] [PubMed]
- Pistritto, G.; Trisciuoglio, D.; Ceci, C.; Garufi, A.; D’Orazi, G. Apoptosis as anticancer mechanism: Function and dysfunction of its modulators and targeted therapeutic strategies. Aging 2016, 8, 603–619. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thornberry, N.A.; Lazebnik, Y. Caspases: Enemies within. Science 1998, 281, 1312–1316. [Google Scholar] [CrossRef] [PubMed]
- Slade, D. PARP and PARG inhibitors in cancer treatment. Genes Dev. 2020, 34, 360–394. [Google Scholar] [CrossRef] [Green Version]
- Birkinshaw, R.W.; Czabotar, P.E. The BCL-2 family of proteins and mitochondrial outer membrane permeabilisation. Semin. Cell Dev. Biol. 2017, 72, 152–162. [Google Scholar] [CrossRef]
- Adams, J.M.; Cory, S. The Bcl-2 apoptotic switch in cancer development and therapy. Oncogene 2007, 26, 1324–1337. [Google Scholar] [CrossRef] [Green Version]
- Cosentino, K.; Garcia-Saez, A.J. Bax and Bak Pores: Are We Closing the Circle? Trends Cell Biol. 2017, 27, 266–275. [Google Scholar] [CrossRef]
- Kale, J.; Osterlund, E.J.; Andrews, D.W. BCL-2 family proteins: Changing partners in the dance towards death. Cell Death Differ. 2018, 25, 65–80. [Google Scholar] [CrossRef] [Green Version]
- Warren, C.F.A.; Wong-Brown, M.W.; Bowden, N.A. BCL-2 family isoforms in apoptosis and cancer. Cell Death Dis. 2019, 10, 177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rasche, L.; Chavan, S.S.; Stephens, O.W.; Patel, P.H.; Tytarenko, R.; Ashby, C.; Bauer, M.; Stein, C.; Deshpande, S.; Wardell, C.; et al. Spatial genomic heterogeneity in multiple myeloma revealed by multi-region sequencing. Nat. Commun. 2017, 8, 268. [Google Scholar] [CrossRef] [Green Version]
- Walker, B.A.; Mavrommatis, K.; Wardell, C.P.; Ashby, T.C.; Bauer, M.; Davies, F.E.; Rosenthal, A.; Wang, H.; Qu, P.; Hoering, A.; et al. Identification of novel mutational drivers reveals oncogene dependencies in multiple myeloma. Blood 2018, 132, 587–597. [Google Scholar] [CrossRef] [PubMed]
- Ullah, R.; Yin, Q.; Snell, A.H.; Wan, L. RAF-MEK-ERK pathway in cancer evolution and treatment. Semin. Cancer Biol. 2021, 85, 123–154. [Google Scholar] [CrossRef] [PubMed]
- Sui, X.; Kong, N.; Ye, L.; Han, W.; Zhou, J.; Zhang, Q.; He, C.; Pan, H. p38 and JNK MAPK pathways control the balance of apoptosis and autophagy in response to chemotherapeutic agents. Cancer Lett. 2014, 344, 174–179. [Google Scholar] [CrossRef]
- Wu, X.; Liu, Y.; Zhang, E.; Chen, J.; Huang, X.; Yan, H.; Cao, W.; Qu, J.; Gu, H.; Xu, R.; et al. Dihydroartemisinin Modulates Apoptosis and Autophagy in Multiple Myeloma through the P38/MAPK and Wnt/β-Catenin Signaling Pathways. Oxid. Med. Cell Longev. 2020, 2020, 6096391. [Google Scholar] [CrossRef]
- Salimi, A.; Schroeder, K.M.; Schemionek-Reinders, M.; Vieri, M.; Maletzke, S.; Gezer, D.; Masouleh, B.K.; Appelmann, I. Targeting autophagy increases the efficacy of proteasome inhibitor treatment in multiple myeloma by induction of apoptosis and activation of JNK. BMC Cancer 2022, 22, 735. [Google Scholar] [CrossRef]
- Gentile, M.; Martino, M.; Recchia, A.G.; Vigna, E.; Morabito, L.; Morabito, F. Sorafenib for the treatment of multiple myeloma. Expert. Opin. Investig. Drugs 2016, 25, 743–749. [Google Scholar] [CrossRef]
- Fong, W.P.; Poon, Y.T.; Wong, T.M.; Mock, J.W.Y.; Ng, T.B.; Wong, R.N.S.; Yao, Q.Z.; Yeung, H.W. A highly efficient procedure for purifying the ribosome-inactivating proteins alpha- and beta-momorcharins from Momordica charantia seeds, N-terminal sequence comparison and establishment of their N-glycosidase activity. Life Sci. 1996, 59, 901–909. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cai, Z.-W.; Ye, T.; Jiang, P.-W.; Liao, Y.-J.; Wang, L.; Zhang, Q.-L.; Du, W.-Q.; Huang, M.; Yang, P.; Li, M.-H. MAPK Cascade Signaling Is Involved in α-MMC Induced Growth Inhibition of Multiple Myeloma MM.1S Cells via G2 Arrest and Mitochondrial-Pathway-Dependent Apoptosis In Vitro. Pharmaceuticals 2023, 16, 124. https://doi.org/10.3390/ph16010124
Cai Z-W, Ye T, Jiang P-W, Liao Y-J, Wang L, Zhang Q-L, Du W-Q, Huang M, Yang P, Li M-H. MAPK Cascade Signaling Is Involved in α-MMC Induced Growth Inhibition of Multiple Myeloma MM.1S Cells via G2 Arrest and Mitochondrial-Pathway-Dependent Apoptosis In Vitro. Pharmaceuticals. 2023; 16(1):124. https://doi.org/10.3390/ph16010124
Chicago/Turabian StyleCai, Zi-Wei, Ting Ye, Pei-Wen Jiang, Yu-Jiao Liao, Lin Wang, Qing-Liang Zhang, Wen-Qian Du, Min Huang, Ping Yang, and Min-Hui Li. 2023. "MAPK Cascade Signaling Is Involved in α-MMC Induced Growth Inhibition of Multiple Myeloma MM.1S Cells via G2 Arrest and Mitochondrial-Pathway-Dependent Apoptosis In Vitro" Pharmaceuticals 16, no. 1: 124. https://doi.org/10.3390/ph16010124
APA StyleCai, Z. -W., Ye, T., Jiang, P. -W., Liao, Y. -J., Wang, L., Zhang, Q. -L., Du, W. -Q., Huang, M., Yang, P., & Li, M. -H. (2023). MAPK Cascade Signaling Is Involved in α-MMC Induced Growth Inhibition of Multiple Myeloma MM.1S Cells via G2 Arrest and Mitochondrial-Pathway-Dependent Apoptosis In Vitro. Pharmaceuticals, 16(1), 124. https://doi.org/10.3390/ph16010124