Modulation of Gut Microbiome Community Mitigates Multiple Sclerosis in a Mouse Model: The Promising Role of Palmaria palmata Alga as a Prebiotic
Abstract
:1. Introduction
2. Results
2.1. Weight Assessment
2.2. Palmaria p. Extract Fatty Acid and Antioxidant Assessment
2.3. Behavioral Testing Results
2.3.1. Grip Strength at W5 and W7 in Both Sexes
2.3.2. Open Field Test at W5 & W7 in Both Sexes
2.4. Gut Microbiota Analysis
2.4.1. Diversity in the Gut Microbiome Community
2.4.2. Variations in GM in Both Sexes at the End of Demyelination and Remyelination Stages
2.5. Histopathological Assessment of the Corpus Callosum in Demyelination and Remyelination Stages in Both Sexes
3. Discussion
3.1. The Neuroprotective Effect of the Palmaria p. Bioactive Constituents on the Structure and Function
3.2. Palmaria p. Affected Gut Microbiome Community Variably Regarding the Disease Stage and the Sex
3.3. The Suggested Role of Palmaria p. Active Constituents in Modulating Gut–Brain Axis
4. Methods
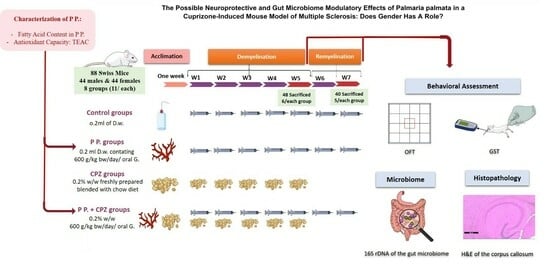
4.1. Animals
4.2. Chemicals
Cuprizone (CPZ) and Palmaria p.
4.3. Palmaria p. Extract Characterization
4.4. Experimental Design
4.5. Behavioral Testing
4.5.1. Grip Strength Test (GST)
4.5.2. Open Field Test (OFT)
4.6. Sequencing of Bacterial 16S rDNA Gene and Data Processing
4.7. Histological Assessment of the Brain Tissue
4.8. Statistical Analysis
5. Limitations and Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pereira, L.; Valado, A. The Seaweed Diet in Prevention and Treatment of the Neurodegenerative Diseases. Mar. Drugs 2021, 19, 128. [Google Scholar] [CrossRef] [PubMed]
- Luchetti, S.; van Eden, C.G.; Schuurman, K.; van Strien, M.E.; Swaab, D.F.; Huitinga, I. Gender Differences in Multiple Sclerosis: Induction of Estrogen Signaling in Male and Progesterone Signaling in Female Lesions. J. Neuropathol. Exp. Neurol. 2014, 73, 123–135. [Google Scholar] [CrossRef] [PubMed]
- Burrows, D.J.; McGown, A.; Jain, S.A.; De Felice, M.; Ramesh, T.M.; Sharrack, B.; Majid, A. Animal models of multiple sclerosis: From rodents to zebrafish. Mult. Scler. J. 2019, 25, 306–324. [Google Scholar] [CrossRef] [PubMed]
- Al-Otaibi, K.M.; Alghamdi, B.S.; Al-Ghamdi, M.A.; Mansouri, R.A.; Ashraf, G.M.; Omar, U.M. Therapeutic effect of combination vitamin D3 and siponimod on remyelination and modulate microglia activation in cuprizone mouse model of multiple sclerosis. Front. Behav. Neurosci. 2022, 16, 1068736. [Google Scholar] [CrossRef]
- Bjelobaba, I.; Begovic-Kupresanin, V.; Pekovic, S.; Lavrnja, I. Animal models of multiple sclerosis: Focus on experimental autoimmune encephalomyelitis. J. Neurosci. Res. 2018, 96, 1021–1042. [Google Scholar] [CrossRef]
- Yousof, S.M.; Awad, Y.M.; Mostafa, E.M.A.; Hosny, M.M.; Anwar, M.M.; Eldesouki, R.E.; Badawy, A.-E. The potential neuroprotective role of Amphora coffeaeformis algae against monosodium glutamate-induced neurotoxicity in adult albino rats. Food Funct. 2021, 12, 706–716. [Google Scholar] [CrossRef]
- Barbalace, M.C.; Malaguti, M.; Giusti, L.; Lucacchini, A.; Hrelia, S.; Angeloni, C. Anti-Inflammatory Activities of Marine Algae in Neurodegenerative Diseases. Int. J. Mol. Sci. 2019, 20, 3061. [Google Scholar] [CrossRef]
- Huang, C.; Zhang, Z.; Cui, W. Marine-Derived Natural Compounds for the Treatment of Parkinson’s Disease. Mar. Drugs 2019, 17, 221. [Google Scholar] [CrossRef]
- Lopes, D.; Melo, T.; Meneses, J.; Abreu, M.H.; Pereira, R.; Domingues, P.; Lillebø, A.I.; Calado, R.; Domingues, M.R. A New Look for the Red Macroalga Palmaria palmata: A Seafood with Polar Lipids Rich in EPA and with Antioxidant Properties. Mar. Drugs 2019, 17, 533. [Google Scholar] [CrossRef]
- Ismail, M.M.; Alotaibi, B.S.; El-Sheekh, M.M. Therapeutic Uses of Red Macroalgae. Molecules 2020, 25, 4411. [Google Scholar] [CrossRef]
- Vamanu, E.; Rai, S.N. The Link between Obesity, Microbiota Dysbiosis, and Neurodegenerative Pathogenesis. Diseases 2021, 9, 45. [Google Scholar] [CrossRef] [PubMed]
- Sivamaruthi, B.S.; Kesika, P. A Review on Role of Microbiome in Obesity and Antiobesity Properties of Probiotic Supplements. BioMed Res. Int. 2019, 2019, 3291367. [Google Scholar] [CrossRef] [PubMed]
- Forte, N.; Fernández-Rilo, A.C.; Palomba, L.; Di Marzo, V.; Cristino, L. Obesity Affects the Microbiota-Gut-Brain Axis and the Regulation Thereof by Endocannabinoids and Related Mediators. Int. J. Mol. Sci. 2020, 21, 1554. [Google Scholar] [CrossRef] [PubMed]
- Van Ginneken, V.J.; Helsper, J.P.; de Visser, W.; van Keulen, H.; Brandenburg, W.A. Polyunsaturated fatty acids in various macroalgal species from North Atlantic and tropical seas. Lipids Health Dis. 2011, 10, 104. [Google Scholar] [CrossRef] [PubMed]
- Andersen, A.D.; Mølbak, L.; Michaelsen, K.F.; Lauritzen, L. Molecular fingerprints of the human fecal microbiota from 9 to 18 months old and the effect of fish oil supplementation. J. Pediatr. Gastroenterol. Nutr. 2011, 53, 303–309. [Google Scholar] [CrossRef]
- Balfegó, M.; Canivell, S.; Hanzu, F.A.; Sala-Vila, A.; Martínez-Medina, M.; Murillo, S.; Mur, T.; Ruano, E.G.; Linares, F.; Porras, N.; et al. Effects of sardine-enriched diet on metabolic control, inflammation and gut microbiota in drug-naïve patients with type 2 diabetes: A pilot randomized trial. Lipids Health Dis. 2016, 15, 78. [Google Scholar] [CrossRef]
- Watson, H.; Mitra, S.; Croden, F.C.; Taylor, M. A randomised trial of the effect of omega-3 polyunsaturated fatty acid supplements on the human intestinal microbiota. Gut 2018, 67, 1974–1983. [Google Scholar] [CrossRef]
- Robertson, R.C.; Seira Oriach, C.; Murphy, K.; Moloney, G.M.; Cryan, J.F.; Dinan, T.; Ross, R.P.; Stanton, C. Omega-3 polyunsaturated fatty acids critically regulate behaviour and gut microbiota development in adolescence and adulthood. Brain Behav. Immun. 2017, 59, 21–37. [Google Scholar] [CrossRef]
- Provensi, G.; Schmidt, S.D.; Boehme, M.; Bastiaanssen, T.F.S.; Rani, B.; Costa, A.; Busca, K.; Fouhy, F.; Strain, C.; Stanton, C.; et al. Preventing adolescent stress-induced cognitive and microbiome changes by diet. Proc. Natl. Acad. Sci. USA 2019, 116, 9644–9651. [Google Scholar] [CrossRef]
- Parolini, C. Effects of Fish n-3 PUFAs on Intestinal Microbiota and Immune System. Mar. Drugs 2019, 17, 374. [Google Scholar] [CrossRef]
- Harnedy, P.A.; Soler-Vila, A.; Edwards, M.D.; FitzGerald, R.J. The effect of time and origin of harvest on the in vitro biological activity of Palmaria palmata protein hydrolysates. Food Res. Int. 2014, 62, 746–752. [Google Scholar] [CrossRef]
- Martins, B.; Vieira, M.; Delerue-Matos, C.; Grosso, C.; Soares, C. Biological Potential, Gastrointestinal Digestion, Absorption, and Bioavailability of Algae-Derived Compounds with Neuroprotective Activity: A Comprehensive Review. Mar. Drugs 2022, 20, 362. [Google Scholar] [CrossRef] [PubMed]
- Suganya, K.; Koo, B.-S. Gut–Brain Axis: Role of Gut Microbiota on Neurological Disorders and How Probiotics/Prebiotics Beneficially Modulate Microbial and Immune Pathways to Improve Brain Functions. Int. J. Mol. Sci. 2020, 21, 7551. [Google Scholar] [CrossRef] [PubMed]
- Mendez, R.L.; Miranda, C.; Armour, C.R.; Sharpton, T.J.; Stevens, J.F.; Kwon, J.Y. Supplementation with Sea Vegetables Palmaria mollis and Undaria pinnatifida Exerts Metabolic Benefits in Diet-Induced Obesity in Mice. Curr. Dev. Nutr. 2020, 4, nzaa072. [Google Scholar] [CrossRef] [PubMed]
- Davani-Davari, D.; Negahdaripour, M.; Karimzadeh, I.; Seifan, M.; Mohkam, M.; Masoumi, S.J.; Berenjian, A.; Ghasemi, Y. Prebiotics: Definition, Types, Sources, Mechanisms, and Clinical Applications. Foods 2019, 8, 92. [Google Scholar] [CrossRef]
- Allsopp, P.; Paul, C.; Strain, C.; Yadav, S.; Smyth, T.; Ross, P.; McSorley, E.; Stanton, C. An in-vitro investigation into the prebiotic potential of xylan derived from the edible red seaweed Palmaria palmata. Proc. Nutr. Soc. 2020, 79, e111. [Google Scholar] [CrossRef]
- Mayer, E.A. Gut feelings: The emerging biology of gut-brain communication. Nat. Rev. Neurosci. 2011, 12, 453–466. [Google Scholar] [CrossRef]
- Cian, R.E.; Drago, S.R.; de Medina, F.S.; Martínez-Augustin, O. Proteins and Carbohydrates from Red Seaweeds: Evidence for Beneficial Effects on Gut Function and Microbiota. Mar. Drugs 2015, 13, 5358–5383. [Google Scholar] [CrossRef]
- Olsson, T.; Barcellos, L.F.; Alfredsson, L. Interactions between genetic, lifestyle and environmental risk factors for multiple sclerosis. Nat. Rev. Neurol. 2017, 13, 25–36. [Google Scholar] [CrossRef]
- Foseid, L.; Natvik, I.; Devle, H.; Ekeberg, D. Identification of fatty acids in fractionated lipid extracts from Palmaria palmata, Alaria esculenta and Saccharina latissima by off-line SPE GC-MS. J. Appl. Phycol. 2020, 32, 4251–4262. [Google Scholar] [CrossRef]
- Giacobbe, J.; Benoiton, B.; Zunszain, P.; Pariante, C.M.; Borsini, A. The Anti-Inflammatory Role of Omega-3 Polyunsaturated Fatty Acids Metabolites in Pre-Clinical Models of Psychiatric, Neurodegenerative, and Neurological Disorders. Front. Psychiatry 2020, 11, 122. [Google Scholar] [CrossRef] [PubMed]
- Saini, R.K.; Prasad, P.; Sreedhar, R.V.; Akhilender Naidu, K.; Shang, X.; Keum, Y.-S. Omega-3 Polyunsaturated Fatty Acids (PUFAs): Emerging Plant and Microbial Sources, Oxidative Stability, Bioavailability, and Health Benefits—A Review. Antioxidants 2021, 10, 1627. [Google Scholar] [CrossRef] [PubMed]
- Ghasemi Darestani, N.; Bahrami, A. Association of Polyunsaturated Fatty Acid Intake on Inflammatory Gene Expression and Multiple Sclerosis: A Systematic Review and Meta-Analysis. Nutrients 2022, 14, 4627. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.; Shahidi, F. Methods for the assessment of antioxidant activity in foods11This chapter is reproduced to a large extent from an article in press by the authors in the Journal of Functional Foods. In Handbook of Antioxidants for Food Preservation; Woodhead Publishing: Cambridge, UK, 2015; pp. 287–333. [Google Scholar]
- Oppedisano, F.; Macrì, R.; Gliozzi, M.; Musolino, V.; Carresi, C.; Maiuolo, J.; Bosco, F.; Nucera, S.; Zito, M.C.; Guarnieri, L.; et al. The Anti-Inflammatory and Antioxidant Properties of n-3 PUFAs: Their Role in Cardiovascular Protection. Biomedicines 2020, 8, 306. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Jia, Z.; Zhu, H. Regulation of Nrf2 Signaling. React. Oxyg. Species (Apex.) 2019, 8, 312–322. [Google Scholar] [CrossRef] [PubMed]
- Albertos, I.; Martin-Diana, A.B.; Burón, M.; Rico, D. Development of functional bio-based seaweed (Himanthalia elongata and Palmaria palmata) edible films for extending the shelflife of fresh fish burgers. Food Packag. Shelf Life 2019, 22, 100382. [Google Scholar] [CrossRef]
- Yuan, Y.V.; Bone, D.E.; Carrington, M.F. Antioxidant activity of dulse (Palmaria palmata) extract evaluated in vitro. Food Chem. 2005, 91, 485–494. [Google Scholar] [CrossRef]
- Sen, M.K.; Mahns, D.A.; Coorssen, J.R.; Shortland, P.J.J.G. The roles of microglia and astrocytes in phagocytosis and myelination: Insights from the cuprizone model of multiple sclerosis. Glia 2022, 70, 1215–1250. [Google Scholar] [CrossRef]
- Mandolesi, G.; Bullitta, S.; Fresegna, D.; De Vito, F.; Rizzo, F.R.; Musella, A.; Guadalupi, L.; Vanni, V.; Bassi, M.S.; Buttari, F.; et al. Voluntary running wheel attenuates motor deterioration and brain damage in cuprizone-induced demyelination. Neurobiol. Dis. 2019, 129, 102–117. [Google Scholar] [CrossRef]
- Hahn, K.R.; Kim, W.; Jung, H.Y.; Kwon, H.J.; Kim, D.W.; Hwang, I.K.; Yoon, Y.S. Comparison of the Effects of Cuprizone on Demyelination in the Corpus Callosum and Hippocampal Progenitors in Young Adult and Aged Mice. Neurochem. Res. 2022, 47, 1073–1082. [Google Scholar] [CrossRef]
- Rahn, E.J.; Iannitti, T.; Donahue, R.R.; Taylor, B.K. Sex differences in a mouse model of multiple sclerosis: Neuropathic pain behavior in females but not males and protection from neurological deficits during proestrus. Biol. Sex. Differ. 2014, 5, 4. [Google Scholar] [CrossRef] [PubMed]
- Ysrraelit, M.C.; Correale, J. Impact of sex hormones on immune function and multiple sclerosis development. Immunology 2019, 156, 9–22. [Google Scholar] [CrossRef] [PubMed]
- Murgia, F.; Giagnoni, F.; Lorefice, L.; Caria, P.; Dettori, T.; D’alterio, M.N.; Angioni, S.; Hendren, A.J.; Caboni, P.; Pibiri, M.; et al. Sex Hormones as Key Modulators of the Immune Response in Multiple Sclerosis: A Review. Biomedicines 2022, 10, 3107. [Google Scholar] [CrossRef] [PubMed]
- Gould, T.D.; Dao, D.T.; Kovacsics, C.E. The Open Field Test. In Mood and Anxiety Related Phenotypes in Mice: Characterization Using Behavioral Tests; Gould, T.D., Ed.; Humana Press: Totowa, NJ, USA, 2009; pp. 1–20. [Google Scholar]
- Atarashi, K.; Tanoue, T.; Shima, T.; Imaoka, A.; Kuwahara, T.; Momose, Y.; Cheng, G.; Yamasaki, S.; Saito, T.; Ohba, Y.; et al. Induction of colonic regulatory T cells by indigenous Clostridium species. Science 2011, 331, 337–341. [Google Scholar] [CrossRef] [PubMed]
- Schepici, G.; Silvestro, S.; Bramanti, P.; Mazzon, E. The Gut Microbiota in Multiple Sclerosis: An Overview of Clinical Trials. Cell Transplant. 2019, 28, 1507–1527. [Google Scholar] [CrossRef] [PubMed]
- Galluzzo, P.; Capri, F.C.; Vecchioni, L.; Realmuto, S.; Scalisi, L.; Cottone, S.; Nuzzo, D.; Alduina, R. Comparison of the Intestinal Microbiome of Italian Patients with Multiple Sclerosis and Their Household Relatives. Life 2021, 11, 620. [Google Scholar] [CrossRef]
- Tang, Z.; Gao, H.; Wang, S.; Wen, S.; Qin, S. Hypolipidemic and antioxidant properties of a polysaccharide fraction from Enteromorpha prolifera. Int. J. Biol. Macromol. 2013, 58, 186–189. [Google Scholar] [CrossRef]
- Shin, N.R.; Whon, T.W.; Bae, J.W. Proteobacteria: Microbial signature of dysbiosis in gut microbiota. Trends Biotechnol. 2015, 33, 496–503. [Google Scholar] [CrossRef]
- Satokari, R. High Intake of Sugar and the Balance between Pro- and Anti-Inflammatory Gut Bacteria. Nutrients 2020, 12, 1348. [Google Scholar] [CrossRef]
- Shahi, S.K.; Freedman, S.N.; Mangalam, A.K. Gut microbiome in multiple sclerosis: The players involved and the roles they play. Gut Microbes 2017, 8, 607–615. [Google Scholar] [CrossRef]
- Chen, J.; Chia, N.; Kalari, K.R.; Yao, J.Z.; Novotna, M.; Paz Soldan, M.M.; Luckey, D.H.; Marietta, E.V.; Jeraldo, P.R.; Chen, X.; et al. Multiple sclerosis patients have a distinct gut microbiota compared to healthy controls. Sci. Rep. 2016, 6, 28484. [Google Scholar] [CrossRef] [PubMed]
- Cignarella, F.; Cantoni, C.; Ghezzi, L.; Salter, A.; Dorsett, Y.; Chen, L.; Philips, D.; Weinstock, G.M.; Fontana, L.; Cross, A.H.; et al. Intermittent Fasting Confers Protection in CNS Autoimmunity by Altering the Gut Microbiota. Cell Metab. 2018, 27, 1222–1235.e1226. [Google Scholar] [CrossRef]
- Fu, Y.; Wang, Y. Associations among Dietary Omega-3 Polyunsaturated Fatty Acids, the Gut Microbiota, and Intestinal Immunity. Mediat. Inflamm. 2021, 2021, 8879227. [Google Scholar] [CrossRef] [PubMed]
- Chappus-McCendie, H.; Chevalier, L.; Roberge, C. Plourde M Omega-3 PUFA metabolism and brain modifications during aging. Prog. Neuropsychopharmacol. Biol. Psychiatry 2019, 94, 109662. [Google Scholar] [CrossRef]
- Awoyemi, A.; Trøseid, M.; Arnesen, H.; Solheim, S.; Seljeflot, I. Effects of dietary intervention and n-3 PUFA supplementation on markers of gut-related inflammation and their association with cardiovascular events in a high-risk population. Atherosclerosis 2019, 286, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Ochoa-Repáraz, J.; Kasper, L.H. The Second Brain: Is the Gut Microbiota a Link Between Obesity and Central Nervous System Disorders? Curr. Obes. Rep. 2016, 5, 51–64. [Google Scholar] [CrossRef]
- Chen, J.; Xu, Q.; Li, Y.; Tang, Z.; Sun, W.; Zhang, X.; Sun, J.; Sun, Z. Comparative effects of dietary supplementations with sodium butyrate, medium-chain fatty acids, and n-3 polyunsaturated fatty acids in late pregnancy and lactation on the reproductive performance of sows and growth performance of suckling piglets. J. Anim. Sci. 2019, 97, 4256–4267. [Google Scholar] [CrossRef]
- Onishi, J.C.; Campbell, S.; Moreau, M.; Patel, F.; Brooks, A.I.; Zhou, Y.X.; Haggblom, M.M.; Storch, J. Bacterial communities in the small intestine respond differently to those in the caecum and colon in mice fed low- and high-fat diets. Microbiology 2017, 163, 1189–1197. [Google Scholar] [CrossRef]
- Reis, D.J.; Ilardi, S.S.; Punt, S.E.W. The anxiolytic effect of probiotics: A systematic review and meta-analysis of the clinical and preclinical literature. PLoS ONE 2018, 13, e0199041. [Google Scholar] [CrossRef]
- Bravo, J.A.; Forsythe, P.; Chew, M.V.; Escaravage, E.; Savignac, H.M.; Dinan, T.G.; Bienenstock, J.; Cryan, J.F. Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proc. Natl. Acad. Sci. USA 2011, 108, 16050–16055. [Google Scholar] [CrossRef]
- Qin, Q.; Liu, H.; Yang, Y.; Wang, Y.; Xia, C.; Tian, P.; Wei, J.; Li, S.; Chen, T. Probiotic Supplement Preparation Relieves Test Anxiety by Regulating Intestinal Microbiota in College Students. Dis. Markers 2021, 2021, 5597401. [Google Scholar] [CrossRef] [PubMed]
- Unoda, K.; Doi, Y.; Nakajima, H.; Yamane, K.; Hosokawa, T.; Ishida, S.; Kimura, F.; Hanafusa, T. Eicosapentaenoic acid (EPA) induces peroxisome proliferator-activated receptors and ameliorates experimental autoimmune encephalomyelitis. J. Neuroimmunol. 2013, 256, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Salvati, S.; Di Biase, A.; Attorri, L.; Di Benedetto, R.; Sanchez, M.; Lorenzini, L.; Alessandri, M.; Calzà, L. Ethyl-eicosapentaenoic acid ameliorates the clinical course of experimental allergic encephalomyelitis induced in dark agouti rats. J. Nutr. Biochem. 2013, 24, 1645–1654. [Google Scholar] [CrossRef] [PubMed]
- Yamate-Morgan, H.; Lauderdale, K.; Horeczko, J.; Merchant, U.; Tiwari-Woodruff, S.K. Functional Effects of Cuprizone-Induced Demyelination in the Presence of the mTOR-Inhibitor Rapamycin. Neuroscience 2019, 406, 667–683. [Google Scholar] [CrossRef]
- Alghamdi, B.S.; AboTaleb, H.A. Melatonin improves memory defects in a mouse model of multiple sclerosis by up-regulating cAMP-response element-binding protein and synapse-associated proteins in the prefrontal cortex. J. Integr. Neurosci. 2020, 19, 229–237. [Google Scholar]
- Arnao, M.B.; Cano, A.; Acosta, M. The hydrophilic and lipophilic contribution to total antioxidant activity. Food Chem. 2001, 73, 239–244. [Google Scholar] [CrossRef]
- Pimentel, F.B.; Cermeño, M.; Kleekayai, T.; Harnedy, P.A.; FitzGerald, R.J.; Alves, R.C.; Oliveira, M.B.P. Effect of in vitro simulated gastrointestinal digestion on the antioxidant activity of the red seaweed Porphyra dioica. Food Res. Int. 2020, 136, 109309. [Google Scholar] [CrossRef]
- Olsthoorn, S.E.M.; Wang, X.; Tillema, B.; Vanmierlo, T. Brown Seaweed Food Supplementation: Effects on Allergy and Inflammation and Its Consequences. Nutrients 2021, 13, 2613. [Google Scholar] [CrossRef]
- Montilla-García, Á.; Tejada, M.; Perazzoli, G.; Entrena, J.M.; Portillo-Salido, E.; Fernández-Segura, E.; Canizares, F.J.; Cobos, E.J. Grip strength in mice with joint inflammation: A rheumatology function test sensitive to pain and analgesia. Neuropharmacology 2017, 125, 231–242. [Google Scholar] [CrossRef]
- Balkaya, M.; Kröber, J.M.; Rex, A.; Endres, M. Assessing post-stroke behavior in mouse models of focal ischemia. J. Cereb. Blood Flow. Metab. 2013, 33, 330–338. [Google Scholar] [CrossRef]
- Drury, R.A.B.; Wallington, E.A. (Eds.) Carleton’s Histological Technique, 5th ed.; Oxford University Press: New York, NY, USA, 1980. [Google Scholar]
- Bancroft, J.D.; Gamble, M. Bancroft’s Theory and Practice of Histological Techniques, 7th ed.; Churchill Livingstone: London, UK; Elsevier: Amsterdam, The Nerherlands, 2013. [Google Scholar]









| Stage | Group Number | Group Name | OTUs | Chao1 | Shannon | Gini–Simpson (%) | Good’s Coverage (%) |
|---|---|---|---|---|---|---|---|
| Sacrificed at week 5 | 1. | Male-C | 238 | 250.05 | 5.23 | 93.42 | 99.89 |
| 2. | Female-C | 191 | 204.13 | 5.06 | 93.28 | 99.90 | |
| 3. | P-Male | 157 | 172.33 | 4.34 | 90.32 | 99.84 | |
| 4. | P-Female | 206 | 217.11 | 5.23 | 94.77 | 99.86 | |
| 5. | CPZ Male | 192 | 234.27 | 5.01 | 92.70 | 99.82 | |
| 6. | CPZ Female | 158 | 169.55 | 4.94 | 94.02 | 99.84 | |
| 7. | CPZ-Male-P | 187 | 238.48 | 4.84 | 93.46 | 99.68 | |
| 8. | CPZ-Female-P | 174 | 182.88 | 4.81 | 93.48 | 99.86 | |
| Sacrificed at week 7 | 9. | Male-C | 187 | 200.8 | 3.15 | 69.29 | 99.91 |
| 10. | Female-C | 215 | 233.91 | 4.42 | 88.47 | 99.87 | |
| 11. | P-Male | 238 | 281.04 | 4.93 | 93.20 | 99.79 | |
| 12. | P-Female | 196 | 222.11 | 4.76 | 91.93 | 99.83 | |
| 13. | CPZ Male | 161 | 190.18 | 4.27 | 90.65 | 99.83 | |
| 14. | CPZ Female | 146 | 190 | 4.09 | 90.34 | 99.79 | |
| 15. | CPZ-Male-P | 186 | 209.38 | 3.14 | 67.57 | 99.86 | |
| 16. | CPZ-Female-P | 207 | 227.3 | 5.07 | 94.51 | 99.82 |
| Group | Microbiome | Demyelination Stage | Remyelination Stage |
|---|---|---|---|
| Control | Bacteroidetes | 28% in ♂—40.2% in ♀ | Decreased in both sexes $ |
| Firmicutes | 38.6 in ♂—36.6% in ♀ | Increased in both sexes $ | |
| Proteobacteria | 19.2% in ♂—22.1% in ♀ | A marked decrease in ♀ $ | |
| Palmaria p. | Bacteroidetes | No marked change in both sexes * | Increased in both sexes * |
| Firmicutes | Increased in both sexes * | Decreased in ♂ * | |
| Proteobacteria | Decreased in both sexes * | Decreased in ♀ > ♂ * | |
| CPZ | Bacteroidetes | Increased in both sexes * | Increased in both sexes * |
| Firmicutes | No marked change in both sexes * | Decreased in both sexes * | |
| Proteobacteria | ++ decrease in both sexes * | Increased in both sexes * | |
| CPZ + Palmaria p. | Bacteroidetes | Decreased in ♂ ** Increased in ♀ ** | ++ Increased in ♀ ** |
| Firmicutes | Increased in ♂ ** Decreased in ♀ ** | ++ Decreased in ♀ ** | |
| Proteobacteria | Increased in ♀ > ♂ ** | ++ Increased in both sexes $ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yousof, S.M.; Alghamdi, B.S.; Alqurashi, T.; Alam, M.Z.; Tash, R.; Tanvir, I.; Kaddam, L.A. Modulation of Gut Microbiome Community Mitigates Multiple Sclerosis in a Mouse Model: The Promising Role of Palmaria palmata Alga as a Prebiotic. Pharmaceuticals 2023, 16, 1355. https://doi.org/10.3390/ph16101355
Yousof SM, Alghamdi BS, Alqurashi T, Alam MZ, Tash R, Tanvir I, Kaddam LA. Modulation of Gut Microbiome Community Mitigates Multiple Sclerosis in a Mouse Model: The Promising Role of Palmaria palmata Alga as a Prebiotic. Pharmaceuticals. 2023; 16(10):1355. https://doi.org/10.3390/ph16101355
Chicago/Turabian StyleYousof, Shimaa Mohammad, Badrah S. Alghamdi, Thamer Alqurashi, Mohammad Zubair Alam, Reham Tash, Imrana Tanvir, and Lamis AbdelGadir Kaddam. 2023. "Modulation of Gut Microbiome Community Mitigates Multiple Sclerosis in a Mouse Model: The Promising Role of Palmaria palmata Alga as a Prebiotic" Pharmaceuticals 16, no. 10: 1355. https://doi.org/10.3390/ph16101355
APA StyleYousof, S. M., Alghamdi, B. S., Alqurashi, T., Alam, M. Z., Tash, R., Tanvir, I., & Kaddam, L. A. (2023). Modulation of Gut Microbiome Community Mitigates Multiple Sclerosis in a Mouse Model: The Promising Role of Palmaria palmata Alga as a Prebiotic. Pharmaceuticals, 16(10), 1355. https://doi.org/10.3390/ph16101355