Development of a Dosage form for a Photoswitchable Local Anesthetic Ethercaine
Abstract
:1. Introduction
2. Results and Discussion
2.1. Selection of Conditions for Ethercaine Solubilization
2.2. Spectrophotometric Determination of ETH·HCl Z→E Half-Transformation Time in Solutions
2.3. Comparison of the Local Anesthetic Activity of 1% Solution of ETH·HCl in 4% Solutions of Kolliphor ELP and Pluronic F127
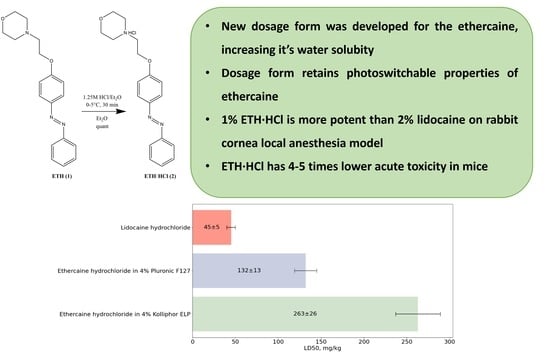
2.4. Examination of Ethercaine Acute Toxicity in 4% Solutions Kolliphor ELP and Pluronic F127
3. Materials and Methods
3.1. Materials
3.2. Chemistry
3.2.1. Synthesis of 4-(2-(N-Morpholino)-Ethoxy)-Azobenzene Hydrochloride (Ethercaine Hydrochloride, 2)
3.2.2. Preparation of a Micellar Solutions of 4-(2-(N-Morpholino)-Ethoxy)-Azobenzene Hydrochloride (2)
3.3. Investigation of Photophysical Properties
3.4. Biology
3.4.1. Preparation of Animals and Arrangement of Groups
3.4.2. Irradiation Modes
3.4.3. Determination of the Sensitivity of Rabbit Eye Cornea Using the Surface Anesthesia Method
3.4.4. Determination of the Acute Toxicity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Soyuer, F.; Varol, B.K. Quality of Life and Pain. Int. J. Fam. Community Med. 2019, 3, 110–114. [Google Scholar] [CrossRef]
- Azizabadi Farahani, M.; Assari, S. Relationship Between Pain and Quality of Life. In Handbook of Disease Burdens and Quality of Life Measures; Springer: New York, NY, USA, 2010; Volume 2010, pp. 3933–3953. [Google Scholar]
- Dysvik, E.; Lindstrøm, T.C.; Eikeland, O.-J.; Natvig, G.K. Health-Related Quality of Life and Pain Beliefs among People Suffering from Chronic Pain. Pain Manag. Nurs. 2004, 5, 66–74. [Google Scholar] [CrossRef] [PubMed]
- Rang, H.P.; Ritter, J.M.; Flower, R.J.; Henderson, G. Rang & Dale’s Pharmacology, 8th ed.; Churchill Livingstone: London, UK, 2015; Volume 13, ISBN 978-0-7020-5363-4. [Google Scholar]
- Mehra, P.; Caiazzo, A.; Maloney, P. Lidocaine Toxicity. Anesth. Prog. 1998, 45, 38–41. [Google Scholar] [CrossRef] [PubMed]
- Hasan, B.; Asif, T.; Hasan, M. Lidocaine-Induced Systemic Toxicity: A Case Report and Review of Literature. Cureus 2017, 9, e1275. [Google Scholar] [CrossRef] [PubMed]
- Macfarlane, A.J.R.; Gitman, M.; Bornstein, K.J.; El-Boghdadly, K.; Weinberg, G. Updates in Our Understanding of Local Anaesthetic Systemic Toxicity: A Narrative Review. Anaesthesia 2021, 76, 27–39. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, P.T.; Yarov-Yarovoy, V. Towards Structure-Guided Development of Pain Therapeutics Targeting Voltage-Gated Sodium Channels. Front. Pharmacol. 2022, 13, 842032. [Google Scholar] [CrossRef] [PubMed]
- Moradkhani, M.R.; Karimi, A.; Negahdari, B. Nanotechnology Application to Local Anaesthesia (LA). Artif. Cells Nanomed. Biotechnol. 2018, 46, 355–360. [Google Scholar] [CrossRef]
- Ji, M.; Liu, G.; Cui, Y.; Zhao, P. Safety and Efficacy Concerns of Modern Strategies of Local Anesthetics Delivery. 3 Biotech 2020, 10, 333. [Google Scholar] [CrossRef]
- Castro, S.R.; Ribeiro, L.N.M.; Breitkreitz, M.C.; Guilherme, V.A.; Rodrigues da Silva, G.H.; Mitsutake, H.; Alcântara, A.C.S.; Yokaichiya, F.; Franco, M.K.K.D.; Clemens, D.; et al. A Pre-Formulation Study of Tetracaine Loaded in Optimized Nanostructured Lipid Carriers. Sci. Rep. 2021, 11, 21463. [Google Scholar] [CrossRef]
- Zhang, W.; Ji, T.; Li, Y.; Zheng, Y.; Mehta, M.; Zhao, C.; Liu, A.; Kohane, D.S. Light-Triggered Release of Conventional Local Anesthetics from a Macromolecular Prodrug for on-Demand Local Anesthesia. Nat. Commun. 2020, 11, 2323. [Google Scholar] [CrossRef]
- Kobauri, P.; Dekker, F.J.; Szymanski, W.; Feringa, B.L. Rational Design in Photopharmacology with Molecular Photoswitches. Angew. Chemie Int. Ed. 2023, 62, e202300681. [Google Scholar] [CrossRef] [PubMed]
- Velema, W.A.; Szymanski, W.; Feringa, B.L. Photopharmacology: Beyond Proof of Principle. J. Am. Chem. Soc. 2014, 136, 2178–2191. [Google Scholar] [CrossRef] [PubMed]
- Gazerani, P. Shedding Light on Photo-Switchable Analgesics for Pain. Pain Manag. 2017, 7, 71–74. [Google Scholar] [CrossRef] [PubMed]
- Fuchter, M.J. On the Promise of Photopharmacology Using Photoswitches: A Medicinal Chemist’s Perspective. J. Med. Chem. 2020, 63, 11436–11447. [Google Scholar] [CrossRef] [PubMed]
- Ang, J.M.; Bin Riaz, I.; Kamal, M.U.; Paragh, G.; Zeitouni, N.C. Photodynamic Therapy and Pain: A Systematic Review. Photodiagnosis Photodyn. Ther. 2017, 19, 308–344. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.K.; Lee, K.M.; Jose, M.V.; Nakamura, M.; Ucmak, D.; Farahnik, B.; Abrouk, M.; Zhu, T.H.; Bhutani, T.; Liao, W. The Patient’s Guide to Psoriasis Treatment. Part 1: UVB Phototherapy. Dermatol. Ther. 2016, 6, 307–313. [Google Scholar] [CrossRef] [PubMed]
- Noev, A.; Kuznetsov, N.; Korenev, G.; Morozova, N.; Vasil’ev, Y.; Suvorov, N.; Diachkova, E.; Usachev, M.; Pankratov, A.; Grin, M. A Novel Photoswitchable Azobenzene-Containing Local Anesthetic Ethercaine with Light-Controlled Biological Activity In Vivo. Int. J. Mol. Sci. 2022, 23, 5352. [Google Scholar] [CrossRef] [PubMed]
- Stegemann, S.; Leveiller, F.; Franchi, D.; de Jong, H.; Lindén, H. When Poor Solubility Becomes an Issue: From Early Stage to Proof of Concept. Eur. J. Pharm. Sci. 2007, 31, 249–261. [Google Scholar] [CrossRef]
- Lipinski, C. Poor Aqueous Solubility—An Industry Wide Problem in Drug Discovery. Am. Pharm. Rev. 2002, 5, 82–85. [Google Scholar]
- Barakat, K.; Aravindhan, G. Solubility: A Speed-Breaker on the Drug Discovery Highway. MOJ Bioequivalence Bioavailab. 2017, 3, 56–58. [Google Scholar] [CrossRef]
- Williams, R.O., III; Davis, D.A., Jr.; Miller, D.A. Formulating Poorly Water Soluble Drugs, 3rd ed.; Williams, R.O., III, Davis, D.A., Miller, D.A., Eds.; AAPS Advances in the Pharmaceutical Sciences Series; Springer International Publishing: Cham, Switzerland, 2022; ISBN 978-3-030-88718-6. [Google Scholar]
- Das, B.; Baidya, A.T.K.; Mathew, A.T.; Yadav, A.K.; Kumar, R. Structural Modification Aimed for Improving Solubility of Lead Compounds in Early Phase Drug Discovery. Bioorg. Med. Chem. 2022, 56, 116614. [Google Scholar] [CrossRef] [PubMed]
- O’Driscoll, C.M.; Griffin, B.T. Biopharmaceutical Challenges Associated with Drugs with Low Aqueous Solubility—The Potential Impact of Lipid-Based Formulations. Adv. Drug Deliv. Rev. 2008, 60, 617–624. [Google Scholar] [CrossRef] [PubMed]
- Stella, V.J.; Nti-Addae, K.W. Prodrug Strategies to Overcome Poor Water Solubility. Adv. Drug Deliv. Rev. 2007, 59, 677–694. [Google Scholar] [CrossRef] [PubMed]
- Mikheev, A.A.; Shmendel, E.V.; Zhestovskaya, E.S.; Nazarov, G.V.; Maslov, M.A. Cationic Liposomes as Delivery Systems for Nucleic Acids. Fine Chem. Technol. 2020, 15, 7–27. [Google Scholar] [CrossRef]
- Vinarov, Z.; Katev, V.; Radeva, D.; Tcholakova, S.; Denkov, N.D. Micellar Solubilization of Poorly Water-Soluble Drugs: Effect of Surfactant and Solubilizate Molecular Structure. Drug Dev. Ind. Pharm. 2018, 44, 677–686. [Google Scholar] [CrossRef] [PubMed]
- Habriev, R.U. Guideline for Experimental (Preclinical) Studying of New Pharmacological Substances, 2nd ed.; JSC “Izdatelstvo Medicina”: Moscow, Russia, 2005; ISBN 5225042198. [Google Scholar]
- Plotnikova, E.A.; Stramova, V.O.; Morozova, N.B.; Plyutinskaya, A.D.; Ostroverkhov, P.V.; Grin, M.A.; Mironov, A.F.; Yakubovskaya, R.I.; Kaprin, A.D. Solubilization of Hydrophobic Bacteriochlorin-Based Photosensitizers in Micelles of Surfactants. Biomed. Photonics 2019, 8, 18–23. [Google Scholar] [CrossRef]
- Pogorilyy, V.; Plyutinskaya, A.; Suvorov, N.; Diachkova, E.; Vasil’ev, Y.; Pankratov, A.; Mironov, A.; Grin, M. The First Selenoanhydride in the Series of Chlorophyll a Derivatives, Its Stability and Photoinduced Cytotoxicity. Molecules 2021, 26, 7298. [Google Scholar] [CrossRef] [PubMed]
- Grin, M.A.; Pogorilyy, V.A.; Noev, A.N.; Tikhonov, S.I.; Majouga, A.G.; Mironov, A.F. Bacteriochlorophyll a Derivatives with Sulfur-Containing Amino Acids as Promising Photosensitizers for Cancer PDT. Macroheterocycles 2018, 11, 89–94. [Google Scholar] [CrossRef]
- Gawin-Mikołajewicz, A.; Nartowski, K.P.; Dyba, A.J.; Gołkowska, A.M.; Malec, K.; Karolewicz, B. Ophthalmic Nanoemulsions: From Composition to Technological Processes and Quality Control. Mol. Pharm. 2021, 18, 3719–3740. [Google Scholar] [CrossRef]
- Kozhushkov, S.I.; Khlebnikov, A.F.; Kostikov, R.R.; Yufit, D.S.; de Meijere, A. Scalable Synthesis of (1-Cyclopropyl)Cyclopropylamine Hydrochloride. Beilstein J. Org. Chem. 2011, 7, 1003–1006. [Google Scholar] [CrossRef]
- Vogel, H. Drug Discovery and Evaluation, 3rd ed.; Vogel, H.G., Ed.; Springer: Berlin/Heidelberg, Germany, 2008; ISBN 978-3-540-71420-0. [Google Scholar]
- De Jong, R.H.; Bonin, J.D. Deaths from Local Anesthetic-Induced Convulsions in Mice. Anesth. Analg. 1980, 59, 401–405. [Google Scholar] [CrossRef] [PubMed]
- Prozorovsky, V.B. Statistical Processing of the Results of Pharmacological Studies. Psychopharmacol. Biol. Narcol. 2007, 7, 2090–2120. (In Russian) [Google Scholar]
- Seeling, A.; Leuschner, F.; Oelschläger, H. Physikalisch-Chemische, Pharmakologische Und Toxikologische Eigenschaften von Fomocain-Metaboliten. Arzneimittelforschung 2011, 51, 7–17. [Google Scholar] [CrossRef] [PubMed]
- Felice, K.L.; Schumann, H.M. Intravenous Lipid Emulsion for Local Anesthetic Toxicity: A Review of the Literature. J. Med. Toxicol. 2008, 4, 184–191. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhang, J.; Yu, P.; Niu, J.; Yu, S. Mechanisms and Efficacy of Intravenous Lipid Emulsion Treatment for Systemic Toxicity From Local Anesthetics. Front. Med. 2021, 8. [Google Scholar] [CrossRef] [PubMed]
- Hunter, J.D. Matplotlib: A 2D Graphics Environment. Comput. Sci. Eng. 2007, 9, 90–95. [Google Scholar] [CrossRef]
- Liu, Y.; Huang, Y.; Xiao, Z.; Ren, X.; Yang, C. Guide for the Care and Use of Laboratory Animals; National Academies Press: Washington, DC, USA, 2011; ISBN 978-0-309-15400-0. [Google Scholar]
- Percie du Sert, N.; Hurst, V.; Ahluwalia, A.; Alam, S.; Avey, M.T.; Baker, M.; Browne, W.J.; Clark, A.; Cuthill, I.C.; Dirnagl, U.; et al. The ARRIVE Guidelines 2.0: Updated Guidelines for Reporting Animal Research. Exp. Physiol. 2020, 105, 1459–1466. [Google Scholar] [CrossRef]






| Chemical Form of Ethercaine | Excipients | Maximum Solubility of Ethercaine, mg/mL |
|---|---|---|
| ETH | - | <1 |
| ETH | 4% micellar solution of Kolliphor ELP | 6 |
| ETH | 10% micellar solution of Kolliphor ELP | 10 |
| ETH | 4% micellar solution of Pluronic F127 | 7 |
| ETH·HCl | - | 6 |
| ETH·HCl | 4% micellar solution of Kolliphor ELP | >20 |
| ETH·HCl | 4% micellar solution of Pluronic F127 | >20 |
| Solubilizer | Half-Transformation Time (), Min |
|---|---|
| 4% micellar solution of Kolliphor ELP | 51 |
| 4% micellar solution of Pluronic F127 | 69 |
| Compound | Regnier Index (Min—13, Max—1300) | |
|---|---|---|
| Dark | UV λ = 365 nm | |
| 4% Kolliphor® ELP | 13 (n = 4) | 13 (n = 4) |
| 4% Pluronic F127 | 13 (n = 4) | 13 (n = 4) |
| 2% lidocaine hydrochloride | 451 ± 40 (n = 8) a | 469 ± 37 (n = 8) a |
| 0.6% ETH in 4% Kolliphor ELP a | 232 ± 50 (n = 8) a | 22 ± 3 (n = 8) a |
| 1% ETH·HCl in 4% Kolliphor ELP | 644 ± 30 (n = 8) | 32 ± 16 (n = 8) |
| 1% ETH·HCl in 4% Pluronic F127 | 656 ± 18 (n = 8) | 40 ± 11 (n = 8) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Noev, A.; Morozova, N.; Suvorov, N.; Vasil’ev, Y.; Pankratov, A.; Grin, M. Development of a Dosage form for a Photoswitchable Local Anesthetic Ethercaine. Pharmaceuticals 2023, 16, 1398. https://doi.org/10.3390/ph16101398
Noev A, Morozova N, Suvorov N, Vasil’ev Y, Pankratov A, Grin M. Development of a Dosage form for a Photoswitchable Local Anesthetic Ethercaine. Pharmaceuticals. 2023; 16(10):1398. https://doi.org/10.3390/ph16101398
Chicago/Turabian StyleNoev, Alexey, Natalia Morozova, Nikita Suvorov, Yuriy Vasil’ev, Andrei Pankratov, and Mikhail Grin. 2023. "Development of a Dosage form for a Photoswitchable Local Anesthetic Ethercaine" Pharmaceuticals 16, no. 10: 1398. https://doi.org/10.3390/ph16101398
APA StyleNoev, A., Morozova, N., Suvorov, N., Vasil’ev, Y., Pankratov, A., & Grin, M. (2023). Development of a Dosage form for a Photoswitchable Local Anesthetic Ethercaine. Pharmaceuticals, 16(10), 1398. https://doi.org/10.3390/ph16101398