Potential of Lactoferrin in the Treatment of Lung Diseases
Abstract
:1. Introduction
2. Lactoferrin Characteristics and Its Properties
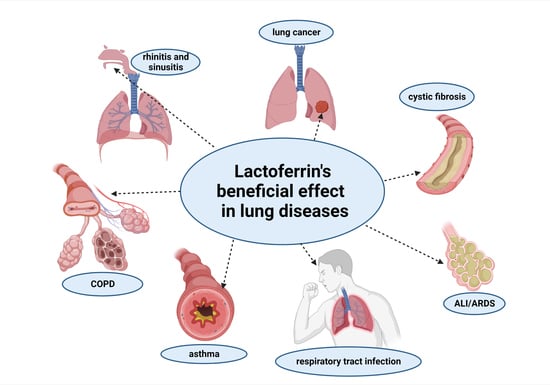
3. The Role of Lactoferrin in Lung Diseases
3.1. Rhinitis and Sinusitis
3.1.1. Allergic Rhinitis
3.1.2. Rhinosinusitis—Viral Infection
3.1.3. Bacterial Rhinosinusitis
3.2. Infectious Respiratory Diseases
3.2.1. Influenza
3.2.2. COVID-19
3.3. Asthma
Breastfeeding and/or Lactoferrin-Enriched Formulae and Risk Factors for Developing Asthma in the Childhood
3.4. Chronic Obstructive Pulmonary Disease
3.5. Cystic Fibrosis
3.6. Acute Respiratory Distress Syndrome
3.7. Lung Cancer
4. LF Forms and Application
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Forum of International Respiratory Societies. The Global Impact of Respiratory Disease, 3rd ed.; European Respiratory Society: Lausanne, Switzerland, 2021; Available online: Firsnet.Org/Images/Publications/FIRS_Master_09202021.Pdf (accessed on 22 September 2021).
- Soriano, J.B.; Kendrick, P.J.; Paulson, K.R.; Gupta, V.; Abrams, E.M.; Adedoyin, R.A.; Adhikari, T.B.; Advani, S.M.; Agrawal, A.; Ahmadian, E.; et al. Prevalence and Attributable Health Burden of Chronic Respiratory Diseases, 1990–2017: A Systematic Analysis for the Global Burden of Disease Study 2017. Lancet Respir. Med. 2020, 8, 585–596. [Google Scholar] [CrossRef] [PubMed]
- Levine, S.M.; Marciniuk, D.D. Global Impact of Respiratory Disease. Chest 2022, 161, 1153–1154. [Google Scholar] [CrossRef] [PubMed]
- Annesi-Maesano, I.; Forastiere, F.; Balmes, J.; Garcia, E.; Harkema, J.; Holgate, S.; Kelly, F.; Khreis, H.; Hoffmann, B.; Maesano, C.N.; et al. The Clear and Persistent Impact of Air Pollution on Chronic Respiratory Diseases: A Call for Interventions. Eur. Respir. J. 2021, 57, 2002981. [Google Scholar] [CrossRef] [PubMed]
- Kaczyńska, K.; Zając, D.; Wojciechowski, P.; Jampolska, M. Regulatory Peptides in Asthma. Int. J. Mol. Sci. 2021, 22, 13656. [Google Scholar] [CrossRef]
- Travis, S.M.; Conway, B.-A.D.; Zabner, J.; Smith, J.J.; Anderson, N.N.; Singh, P.K.; Peter Greenberg, E.; Welsh, M.J. Activity of Abundant Antimicrobials of the Human Airway. Am. J. Respir. Cell Mol. Biol. 1999, 20, 872–879. [Google Scholar] [CrossRef]
- Valenti, P.; Antonini, G. Lactoferrin: Lactoferrin: An Important Host Defence against Microbial and Viral Attack. Cell. Mol. Life Sci. 2005, 62, 2576–2587. [Google Scholar] [CrossRef]
- Bukowska-Ośko, I.; Sulejczak, D.; Kaczyńska, K.; Kleczkowska, P.; Kramkowski, K.; Popiel, M.; Wietrak, E.; Kowalczyk, P. Lactoferrin as a Human Genome “Guardian”—An Overall Point of View. Int. J. Mol. Sci. 2022, 23, 5248. [Google Scholar] [CrossRef]
- Kowalczyk, P.; Kaczyńska, K.; Kleczkowska, P.; Bukowska-Ośko, I.; Kramkowski, K.; Sulejczak, D. The Lactoferrin Phenomenon—A Miracle Molecule. Molecules 2022, 27, 2941. [Google Scholar] [CrossRef]
- Kruzel, M.L.; Zimecki, M.; Actor, J.K. Lactoferrin in a Context of Inflammation-Induced Pathology. Front. Immunol. 2017, 8, 1438. [Google Scholar] [CrossRef]
- Cutone, A.; Rosa, L.; Ianiro, G.; Lepanto, M.S.; Bonaccorsi di Patti, M.C.; Valenti, P.; Musci, G. Lactoferrin’s Anti-Cancer Properties: Safety, Selectivity, and Wide Range of Action. Biomolecules 2020, 10, 456. [Google Scholar] [CrossRef]
- Li, Y.-Q.; Guo, C. A Review on Lactoferrin and Central Nervous System Diseases. Cells 2021, 10, 1810. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.S.; Hasan, S.S.; Kow, C.S.; Merchant, H.A. Lactoferrin Reduces the Risk of Respiratory Tract Infections: A Meta-Analysis of Randomized Controlled Trials. Clin. Nutr. ESPEN 2021, 45, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Berthon, B.S.; Williams, L.M.; Williams, E.J.; Wood, L.G. Effect of Lactoferrin Supplementation on Inflammation, Immune Function, and Prevention of Respiratory Tract Infections in Humans: A Systematic Review and Meta-Analysis. Adv. Nutr. 2022, 13, 1799–1819. [Google Scholar] [CrossRef]
- Eschenbacher, W.; Straesser, M.; Knoeddler, A.; Li, R.-C.; Borish, L. Biologics for the Treatment of Allergic Rhinitis, Chronic Rhinosinusitis, and Nasal Polyposis. Immunol. Allergy Clin. North Am. 2020, 40, 539–547. [Google Scholar] [CrossRef] [PubMed]
- Bernstein, D.I.; Schwartz, G.; Bernstein, J.A. Allergic Rhinitis: Mechanisms and Treatment. Immunol. Allergy Clin. North Am. 2016, 36, 261–278. [Google Scholar] [CrossRef] [PubMed]
- Anon, J.B.; Jacobs, M.R.; Poole, M.D.; Ambrose, P.G.; Benninger, M.S.; Hadley, J.A.; Craig, W.A. Sinus And Allergy Health Partnership Antimicrobial Treatment Guidelines for Acute Bacterial Rhinosinusitis. Otolaryngol. –Head Neck Surg. 2004, 130, 1–45. [Google Scholar] [CrossRef]
- Heath, J.; Hartzell, L.; Putt, C.; Kennedy, J.L. Chronic Rhinosinusitis in Children: Pathophysiology, Evaluation, and Medical Management. Curr. Allergy Asthma Rep. 2018, 18, 37. [Google Scholar] [CrossRef]
- Uyeki, T.M.; Bernstein, H.H.; Bradley, J.S.; Englund, J.A.; File, T.M.; Fry, A.M.; Gravenstein, S.; Hayden, F.G.; Harper, S.A.; Hirshon, J.M.; et al. Clinical Practice Guidelines by the Infectious Diseases Society of America: 2018 Update on Diagnosis, Treatment, Chemoprophylaxis, and Institutional Outbreak Management of Seasonal Influenzaa. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2019, 68, 895–902. [Google Scholar] [CrossRef]
- CDC What You Need to Know about Influenza (Flu) from CDC. Available online: https://www.cdc.gov/flu/index.htm (accessed on 17 January 2023).
- Chalmers, J.D.; Crichton, M.L.; Goeminne, P.C.; Cao, B.; Humbert, M.; Shteinberg, M.; Antoniou, K.M.; Ulrik, C.S.; Parks, H.; Wang, C.; et al. Management of Hospitalised Adults with Coronavirus Disease 2019 (COVID-19): A European Respiratory Society Living Guideline. Eur. Respir. J. 2021, 57, 2100048. [Google Scholar] [CrossRef]
- Global Initiative for Asthma. Global Strategy for Asthma Management and Prevention. 2022. Available online: Www.Ginasthma.Org (accessed on 10 January 2023).
- Holguin, F.; Cardet, J.C.; Chung, K.F.; Diver, S.; Ferreira, D.S.; Fitzpatrick, A.; Gaga, M.; Kellermeyer, L.; Khurana, S.; Knight, S.; et al. Management of Severe Asthma: A European Respiratory Society/American Thoracic Society Guideline. Eur. Respir. J. 2020, 55, 1900588. [Google Scholar] [CrossRef]
- Wedzicha, J.A.; Calverley, P.M.A.; Albert, R.K.; Anzueto, A.; Criner, G.J.; Hurst, J.R.; Miravitlles, M.; Papi, A.; Rabe, K.F.; Rigau, D.; et al. Prevention of COPD Exacerbations: A European Respiratory Society/American Thoracic Society Guideline. Eur. Respir. J. 2017, 50, 1602265. [Google Scholar] [CrossRef] [Green Version]
- Ergan, B.; Oczkowski, S.; Rochwerg, B.; Carlucci, A.; Chatwin, M.; Clini, E.; Elliott, M.; Gonzalez-Bermejo, J.; Hart, N.; Lujan, M.; et al. European Respiratory Society Guidelines on Long-Term Home Non-Invasive Ventilation for Management of COPD. Eur. Respir. J. 2019, 54, 1901003. [Google Scholar] [CrossRef] [Green Version]
- Wedzicha, J.A.; Miravitlles, M.; Hurst, J.R.; Calverley, P.M.A.; Albert, R.K.; Anzueto, A.; Criner, G.J.; Papi, A.; Rabe, K.F.; Rigau, D.; et al. Management of COPD Exacerbations: A European Respiratory Society/American Thoracic Society Guideline. Eur. Respir. J. 2017, 49, 1600791. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chalmers, J.D.; Laska, I.F.; Franssen, F.M.E.; Janssens, W.; Pavord, I.; Rigau, D.; McDonnell, M.J.; Roche, N.; Sin, D.D.; Stolz, D.; et al. Withdrawal of Inhaled Corticosteroids in COPD: A European Respiratory Society Guideline. Eur. Respir. J. 2020, 55, 2000351. [Google Scholar] [CrossRef] [PubMed]
- Brown, S.D.; White, R.; Tobin, P. Keep Them Breathing: Cystic Fibrosis Pathophysiology, Diagnosis, and Treatment. JAAPA Off. J. Am. Acad. Physician Assist. 2017, 30, 23–27. [Google Scholar] [CrossRef] [PubMed]
- Rafeeq, M.M.; Murad, H.A.S. Cystic Fibrosis: Current Therapeutic Targets and Future Approaches. J. Transl. Med. 2017, 15, 84. [Google Scholar] [CrossRef] [Green Version]
- Oczkowski, S.; Ergan, B.; Bos, L.; Chatwin, M.; Ferrer, M.; Gregoretti, C.; Heunks, L.; Frat, J.-P.; Longhini, F.; Nava, S.; et al. ERS Clinical Practice Guidelines: High-Flow Nasal Cannula in Acute Respiratory Failure. Eur. Respir. J. 2022, 59, 2101574. [Google Scholar] [CrossRef]
- Torres, A.; Niederman, M.S.; Chastre, J.; Ewig, S.; Fernandez-Vandellos, P.; Hanberger, H.; Kollef, M.; Li Bassi, G.; Luna, C.M.; Martin-Loeches, I.; et al. International ERS/ESICM/ESCMID/ALAT Guidelines for the Management of Hospital-Acquired Pneumonia and Ventilator-Associated Pneumonia: Guidelines for the Management of Hospital-Acquired Pneumonia (HAP)/Ventilator-Associated Pneumonia (VAP) of the European Respiratory Society (ERS), European Society of Intensive Care Medicine (ESICM), European Society of Clinical Microbiology and Infectious Diseases (ESCMID) and Asociación Latinoamericana Del Tórax (ALAT). Eur. Respir. J. 2017, 50, 1700582. [Google Scholar] [CrossRef] [Green Version]
- Vinod, S.K.; Hau, E. Radiotherapy Treatment for Lung Cancer: Current Status and Future Directions. Respirol. Carlton Vic. 2020, 25 (Suppl. 2), 61–71. [Google Scholar] [CrossRef]
- Scherpereel, A.; Opitz, I.; Berghmans, T.; Psallidas, I.; Glatzer, M.; Rigau, D.; Astoul, P.; Bölükbas, S.; Boyd, J.; Coolen, J.; et al. ERS/ESTS/EACTS/ESTRO Guidelines for the Management of Malignant Pleural Mesothelioma. Eur. Respir. J. 2020, 55, 1900953. [Google Scholar] [CrossRef]
- Gesthalter, Y.; Smyth, R.; Sullivan, D. Treatment of Early-Stage Non-Small Cell Lung Cancer. Am. J. Respir. Crit. Care Med. 2022, 205, P7–P8. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Cordero, R.; Devine, W.P. Targeted Therapy and Checkpoint Immunotherapy in Lung Cancer. Surg. Pathol. Clin. 2020, 13, 17–33. [Google Scholar] [CrossRef] [PubMed]
- Baker, E.N.; Baker, H.M. Lactoferrin: Molecular Structure, Binding Properties and Dynamics of Lactoferrin. Cell. Mol. Life Sci. 2005, 62, 2531–2539. [Google Scholar] [CrossRef] [PubMed]
- Karav, S.; German, J.B.; Rouquié, C.; Le Parc, A.; Barile, D. Studying Lactoferrin N-Glycosylation. Int. J. Mol. Sci. 2017, 18, 870. [Google Scholar] [CrossRef] [Green Version]
- Shoji, H.; Oguchi, S.; Shinohara, K.; Shimizu, T.; Yamashiro, Y. Effects of Iron-Unsaturated Human Lactoferrin on Hydrogen Peroxide-Induced Oxidative Damage in Intestinal Epithelial Cells. Pediatr. Res. 2007, 61, 89–92. [Google Scholar] [CrossRef] [Green Version]
- Arnold, R.R.; Brewer, M.; Gauthier, J.J. Bactericidal Activity of Human Lactoferrin: Sensitivity of a Variety of Microorganisms. Infect. Immun. 1980, 28, 893–898. [Google Scholar] [CrossRef]
- Mayadas, T.N.; Cullere, X.; Lowell, C.A. The Multifaceted Functions of Neutrophils. Annu. Rev. Pathol. Mech. Dis. 2014, 9, 181–218. [Google Scholar] [CrossRef] [Green Version]
- Cacho, N.T.; Lawrence, R.M. Innate Immunity and Breast Milk. Front. Immunol. 2017, 8, 584. [Google Scholar] [CrossRef] [Green Version]
- Legrand, D.; Elass, E.; Carpentier, M.; Mazurier, J. Lactoferrin: Lactoferrin: A Modulator of Immune and Inflammatory Responses. Cell. Mol. Life Sci. 2005, 62, 2549. [Google Scholar] [CrossRef]
- Rosa, L.; Cutone, A.; Lepanto, M.; Paesano, R.; Valenti, P. Lactoferrin: A Natural Glycoprotein Involved in Iron and Inflammatory Homeostasis. Int. J. Mol. Sci. 2017, 18, 1985. [Google Scholar] [CrossRef]
- Siqueiros-Cendón, T.; Arévalo-Gallegos, S.; Iglesias-Figueroa, B.F.; García-Montoya, I.A.; Salazar-Martínez, J.; Rascón-Cruz, Q. Immunomodulatory Effects of Lactoferrin. Acta Pharmacol. Sin. 2014, 35, 557–566. [Google Scholar] [CrossRef] [PubMed]
- Zimecki, M.; Spiegel, K.; Właszczyk, A.; Kübler, A.; Kruzel, M.L. Lactoferrin Increases the Output of Neutrophil Precursors and Attenuates the Spontaneous Production of TNF-Alpha and IL-6 by Peripheral Blood Cells. Arch. Immunol. Ther. Exp. (Warsz.) 1999, 47, 113–118. [Google Scholar]
- Ward, P.P.; Paz, E.; Conneely, O.M. Lactoferrin: Multifunctional Roles of Lactoferrin: A Critical Overview. Cell. Mol. Life Sci. 2005, 62, 2540–2548. [Google Scholar] [CrossRef] [PubMed]
- Gruden, Š.; Poklar Ulrih, N. Diverse Mechanisms of Antimicrobial Activities of Lactoferrins, Lactoferricins, and Other Lactoferrin-Derived Peptides. Int. J. Mol. Sci. 2021, 22, 11264. [Google Scholar] [CrossRef] [PubMed]
- Berlutti, F.; Pantanella, F.; Natalizi, T.; Frioni, A.; Paesano, R.; Polimeni, A.; Valenti, P. Antiviral Properties of Lactoferrin—A Natural Immunity Molecule. Molecules 2011, 16, 6992–7018. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Lima, C.F.; Rodrigues, L.R. Anticancer Effects of Lactoferrin: Underlying Mechanisms and Future Trends in Cancer Therapy. Nutr. Rev. 2014, 72, 763–773. [Google Scholar] [CrossRef] [Green Version]
- Chea, C.; Haing, S.; Miyauchi, M.; Shrestha, M.; Imanaka, H.; Takata, T. Molecular Mechanisms Underlying the Inhibitory Effects of Bovine Lactoferrin on Osteosarcoma. Biochem. Biophys. Res. Commun. 2019, 508, 946–952. [Google Scholar] [CrossRef]
- Kosim, M.Y.; Fukazawa, T.; Miyauchi, M.; Hirohashi, N.; Tanimoto, K. P53 Status Modifies Cytotoxic Activity of Lactoferrin under Hypoxic Conditions. Front. Pharmacol. 2022, 13, 988335. [Google Scholar] [CrossRef]
- Oh, S.-M.; Pyo, C.-W.; Kim, Y.; Choi, S.-Y. Neutrophil Lactoferrin Upregulates the Human P53 Gene through Induction of NF-ΚB Activation Cascade. Oncogene 2004, 23, 8282–8291. [Google Scholar] [CrossRef] [Green Version]
- Cho, D.-Y.; Hunter, R.C.; Ramakrishnan, V.R. The Microbiome and Chronic Rhinosinusitis. Immunol. Allergy Clin. North Am. 2020, 40, 251–263. [Google Scholar] [CrossRef]
- Ramakrishnan, V.R.; Feazel, L.M.; Gitomer, S.A.; Ir, D.; Robertson, C.E.; Frank, D.N. The Microbiome of the Middle Meatus in Healthy Adults. PLoS ONE 2013, 8, e85507. [Google Scholar] [CrossRef] [PubMed]
- Shusterman, D. The Effects of Air Pollutants and Irritants on the Upper Airway. Proc. Am. Thorac. Soc. 2011, 8, 101–105. [Google Scholar] [CrossRef] [PubMed]
- Loss, G.; Depner, M.; Ulfman, L.H.; van Neerven, R.J.J.; Hose, A.J.; Genuneit, J.; Karvonen, A.M.; Hyvärinen, A.; Kaulek, V.; Roduit, C.; et al. Consumption of Unprocessed Cow’s Milk Protects Infants from Common Respiratory Infections. J. Allergy Clin. Immunol. 2015, 135, 56–62.e2. [Google Scholar] [CrossRef] [PubMed]
- van Neerven, R.J.J.; Knol, E.F.; Heck, J.M.L.; Savelkoul, H.F.J. Which Factors in Raw Cow’s Milk Contribute to Protection against Allergies? J. Allergy Clin. Immunol. 2012, 130, 853–858. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Chai, L.; Li, H.; Zhang, Y.; Xie, H.-M.; Shang, J.; Tian, W.; Yang, P.; Jiang, A.C. Effect of Bovine Lactoferrin from Iron-Fortified Formulas on Diarrhea and Respiratory Tract Infections of Weaned Infants in a Randomized Controlled Trial. Nutrition 2016, 32, 222–227. [Google Scholar] [CrossRef] [PubMed]
- King, J.C.; Cummings, G.E.; Guo, N.; Trivedi, L.; Readmond, B.X.; Keane, V.; Feigelman, S.; Waard, R. de A Double-Blind, Placebo-Controlled, Pilot Study of Bovine Lactoferrin Supplementation in Bottle-Fed Infants. J. Pediatr. Gastroenterol. Nutr. 2007, 44, 245–251. [Google Scholar] [CrossRef] [Green Version]
- Perdijk, O.; van Splunter, M.; Savelkoul, H.F.J.; Brugman, S.; van Neerven, R.J.J. Cow’s Milk and Immune Function in the Respiratory Tract: Potential Mechanisms. Front. Immunol. 2018, 9, 143. [Google Scholar] [CrossRef] [Green Version]
- Nadolska, B.; Frączek, M.; Kręcicki, T.; Kocięba, M.; Zimecki, M. Lactoferrin Inhibits the Growth of Nasal Polyp Fibroblasts. Pharmacol. Rep. 2010, 62, 1139–1147. [Google Scholar] [CrossRef]
- Raphael, G.D.; Jeney, E.V.; Baraniuk, J.N.; Kim, I.; Meredith, S.D.; Kaliner, M.A. Pathophysiology of Rhinitis. Lactoferrin and Lysozyme in Nasal Secretions. J. Clin. Invest. 1989, 84, 1528–1535. [Google Scholar] [CrossRef] [Green Version]
- Raphael, G.D.; Igarashi, Y.; White, M.V.; Kaliner, M.A. The Pathophysiology of Rhinitis. J. Allergy Clin. Immunol. 1991, 88, 33–42. [Google Scholar] [CrossRef]
- Tomazic, P.V.; Darnhofer, B.; Birner-Gruenberger, R. Nasal Mucus Proteome and Its Involvement in Allergic Rhinitis. Expert Rev. Proteom. 2020, 17, 191–199. [Google Scholar] [CrossRef] [PubMed]
- Choi, G.-S.; Shin, S.-Y.; Kim, J.-H.; Lee, H.-Y.; Palikhe, N.S.; Ye, Y.-M.; Kim, S.-H.; Park, H.-S. Serum Lactoferrin Level as a Serologic Biomarker for Allergic Rhinitis. Clin. Exp. Allergy 2010, 40, 403–410. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.B.; Deng, Y.Q.; Ren, J.; Xiao, B.K.; Chen, Z.; Tao, Z.Z. Lactoferrin Administration into the Nostril Alleviates Murine Allergic Rhinitis and Its Mechanisms. Scand. J. Immunol. 2013, 78, 507–515. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wakabayashi, H.; Oda, H.; Yamauchi, K.; Abe, F. Lactoferrin for Prevention of Common Viral Infections. J. Infect. Chemother. 2014, 20, 666–671. [Google Scholar] [CrossRef] [Green Version]
- Denani, C.B.; Real-Hohn, A.; de Carvalho, C.A.M.; Gomes, A.M.d.O.; Gonçalves, R.B. Lactoferrin Affects Rhinovirus B-14 Entry into H1-HeLa Cells. Arch. Virol. 2021, 166, 1203–1211. [Google Scholar] [CrossRef]
- Grover, M.; Giouzeppos, O.; May, J. Effect of Human Milk Prostaglandins and Lactoferrin on Respiratory Syncytial Virus and Rotavirus. Acta Paediatr. 1997, 86, 315–316. [Google Scholar] [CrossRef]
- Sano, H.; Nagai, K.; Tsutsumi, H.; Kuroki, Y. Lactoferrin and Surfactant Protein A Exhibit Distinct Binding Specificity to F Protein and Differently Modulate Respiratory Syncytial Virus Infection. Eur. J. Immunol. 2003, 33, 2894–2902. [Google Scholar] [CrossRef]
- Gualdi, L.; Mertz, S.; Gomez, A.M.; Ramilo, O.; Wittke, A.; Mejias, A. Lack of Effect of Bovine Lactoferrin in Respiratory Syncytial Virus Replication and Clinical Disease Severity in the Mouse Model. Antiviral Res. 2013, 99, 188–195. [Google Scholar] [CrossRef]
- Weng, T.; Chen, L.; Shyu, H.; Chen, S.; Wang, J.; Yu, C.; Lei, H.; Yeh, T. Lactoferrin Inhibits Enterovirus 71 Infection by Binding to VP1 Protein and Host Cells. Antiviral Res. 2005, 67, 31–37. [Google Scholar] [CrossRef]
- Di Biase, A.M.; Pietrantoni, A.; Tinari, A.; Siciliano, R.; Valenti, P.; Antonini, G.; Seganti, L.; Superti, F. Heparin-Interacting Sites of Bovine Lactoferrin Are Involved in Anti-Adenovirus Activity. J. Med. Virol. 2003, 69, 495–502. [Google Scholar] [CrossRef]
- Brook, I. Microbiology of Chronic Rhinosinusitis. Eur. J. Clin. Microbiol. Infect. Dis. 2016, 35, 1059–1068. [Google Scholar] [CrossRef] [PubMed]
- André, G.O.; Politano, W.R.; Mirza, S.; Converso, T.R.; Ferraz, L.F.C.; Leite, L.C.C.; Darrieux, M. Combined Effects of Lactoferrin and Lysozyme on Streptococcus Pneumoniae Killing. Microb. Pathog. 2015, 89, 7–17. [Google Scholar] [CrossRef] [PubMed]
- Qiu, J.; Hendrixson, D.R.; Baker, E.N.; Murphy, T.F.; Geme, J.W.S.; Plaut, A.G. Human Milk Lactoferrin Inactivates Two Putative Colonization Factors Expressed by Haemophilus Influenzae. Proc. Natl. Acad. Sci. 1998, 95, 12641–12646. [Google Scholar] [CrossRef] [Green Version]
- Aguila, A.; Herrera, A.G.; Morrison, D.; Cosgrove, B.; Perojo, A.; Montesinos, I.; Pérez, J.; Sierra, G.; Gemmell, C.G.; Brock, J.H. Bacteriostatic Activity of Human Lactoferrin against Staphylococcus Aureus Is a Function of Its Iron-Binding Properties and Is Not Influenced by Antibiotic Resistance. FEMS Immunol. Med. Microbiol. 2001, 31, 145–152. [Google Scholar] [CrossRef]
- Kaiser, L.; Fritz, R.S.; Straus, S.E.; Gubareva, L.; Hayden, F.G. Symptom Pathogenesis during Acute Influenza: Interleukin-6 and Other Cytokine Responses. J. Med. Virol. 2001, 64, 262–268. [Google Scholar] [CrossRef]
- Mogensen, T.H.; Paludan, S.R. Molecular Pathways in Virus-Induced Cytokine Production. Microbiol. Mol. Biol. Rev. MMBR 2001, 65, 131–150. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Yue, L.; Dang, L.; Yang, J.; Chen, Z.; Wang, X.; Shu, J.; Li, Z. Role of Sialylated Glycans on Bovine Lactoferrin against Influenza Virus. Glycoconj. J. 2021, 38, 689–696. [Google Scholar] [CrossRef] [PubMed]
- Marchetti, M.; Trybala, E.; Superti, F.; Johansson, M.; Bergström, T. Inhibition of Herpes Simplex Virus Infection by Lactoferrin Is Dependent on Interference with the Virus Binding to Glycosaminoglycans. Virology 2004, 318, 405–413. [Google Scholar] [CrossRef] [Green Version]
- Ammendolia, M.G.; Agamennone, M.; Pietrantoni, A.; Lannutti, F.; Siciliano, R.A.; De Giulio, B.; Amici, C.; Superti, F. Bovine Lactoferrin-Derived Peptides as Novel Broad-Spectrum Inhibitors of Influenza Virus. Pathog. Glob. Health 2012, 106, 12–19. [Google Scholar] [CrossRef] [Green Version]
- Scala, M.C.; Agamennone, M.; Pietrantoni, A.; Di Sarno, V.; Bertamino, A.; Superti, F.; Campiglia, P.; Sala, M. Discovery of a Novel Tetrapeptide against Influenza A Virus: Rational Design, Synthesis, Bioactivity Evaluation and Computational Studies. Pharmaceuticals 2021, 14, 959. [Google Scholar] [CrossRef]
- Sayers, E.J.; Palmer, I.; Hope, L.; Hope, P.; Watson, P.; Jones, A.T. Fluid-Phase Endocytosis and Lysosomal Degradation of Bovine Lactoferrin in Lung Cells. Pharmaceutics 2022, 14, 855. [Google Scholar] [CrossRef] [PubMed]
- van der Strate, B.W.; Beljaars, L.; Molema, G.; Harmsen, M.C.; Meijer, D.K. Antiviral Activities of Lactoferrin. Antiviral Res. 2001, 52, 225–239. [Google Scholar] [CrossRef] [PubMed]
- Pietrantoni, A.; Dofrelli, E.; Tinari, A.; Ammendolia, M.; Puzelli, S.; Fabiani, C.; Donatelli, I.; Superti, F. Bovine Lactoferrin Inhibits Influenza A Virus Induced Programmed Cell Death in Vitro. Biometals Int. J. Role Met. Ions Biol. Biochem. Med. 2010, 23, 465–475. [Google Scholar] [CrossRef]
- Shin, K.; Wakabayashi, H.; Yamauchi, K.; Teraguchi, S.; Tamura, Y.; Kurokawa, M.; Shiraki, K. Effects of Orally Administered Bovine Lactoferrin and Lactoperoxidase on Influenza Virus Infection in Mice. J. Med. Microbiol. 2005, 54, 717–723. [Google Scholar] [CrossRef] [Green Version]
- Yamauchi, K.; Wakabayashi, H.; Shin, K.; Takase, M. Bovine Lactoferrin: Benefits and Mechanism of Action against InfectionsThis Paper Is One of a Selection of Papers Published in This Special Issue, Entitled 7th International Conference on Lactoferrin: Structure, Functions, and Applications, and Has Undergone the Journal’s Usual Peer Review Process. Biochem. Cell Biol. 2006, 84, 291–296. [Google Scholar] [CrossRef] [PubMed]
- Sherman, M.P.; Pritzl, C.J.; Xia, C.; Miller, M.M.; Zaghouani, H.; Hahm, B. Lactoferrin Acts as an Adjuvant during Influenza Vaccination of Neonatal Mice. Biochem. Biophys. Res. Commun. 2015, 467, 766–770. [Google Scholar] [CrossRef] [Green Version]
- Pregliasco, F.; Anselmi, G.; Fonte, L.; Giussani, F.; Schieppati, S.; Soletti, L. A New Chance of Preventing Winter Diseases by the Administration of Synbiotic Formulations. J. Clin. Gastroenterol. 2008, 42, S224–S233. [Google Scholar] [CrossRef] [PubMed]
- Hartenian, E.; Nandakumar, D.; Lari, A.; Ly, M.; Tucker, J.M.; Glaunsinger, B.A. The Molecular Virology of Coronaviruses. J. Biol. Chem. 2020, 295, 12910–12934. [Google Scholar] [CrossRef]
- Rosa, L.; Cutone, A.; Conte, M.P.; Campione, E.; Bianchi, L.; Valenti, P. An Overview on in Vitro and in Vivo Antiviral Activity of Lactoferrin: Its Efficacy against SARS-CoV-2 Infection. Biometals Int. J. Role Met. Ions Biol. Biochem. Med. 2022, 1–20. [Google Scholar] [CrossRef]
- Shang, W.; Yang, Y.; Rao, Y.; Rao, X. The Outbreak of SARS-CoV-2 Pneumonia Calls for Viral Vaccines. Npj Vaccines 2020, 5, 18. [Google Scholar] [CrossRef] [Green Version]
- Hu, Y.; Meng, X.; Zhang, F.; Xiang, Y.; Wang, J. The in Vitro Antiviral Activity of Lactoferrin against Common Human Coronaviruses and SARS-CoV-2 Is Mediated by Targeting the Heparan Sulfate Co-Receptor. Emerg. Microbes Infect. 2021, 10, 317–330. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, Y.; Wu, L.; Niu, S.; Song, C.; Zhang, Z.; Lu, G.; Qiao, C.; Hu, Y.; Yuen, K.-Y.; et al. Structural and Functional Basis of SARS-CoV-2 Entry by Using Human ACE2. Cell 2020, 181, 894–904.e9. [Google Scholar] [CrossRef]
- Cascella, M.; Rajnik, M.; Aleem, A.; Dulebohn, S.C.; Di Napoli, R. Features, Evaluation, and Treatment of Coronavirus (COVID-19). In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Ou, X.; Liu, Y.; Lei, X.; Li, P.; Mi, D.; Ren, L.; Guo, L.; Guo, R.; Chen, T.; Hu, J.; et al. Characterization of Spike Glycoprotein of SARS-CoV-2 on Virus Entry and Its Immune Cross-Reactivity with SARS-CoV. Nat. Commun. 2020, 11, 1620. [Google Scholar] [CrossRef] [Green Version]
- Fan, H.; Hong, B.; Luo, Y.; Peng, Q.; Wang, L.; Jin, X.; Chen, Y.; Hu, Y.; Shi, Y.; Li, T.; et al. The Effect of Whey Protein on Viral Infection and Replication of SARS-CoV-2 and Pangolin Coronavirus in Vitro. Signal Transduct. Target. Ther. 2020, 5, 275. [Google Scholar] [CrossRef]
- He, S.-T.; Qin, H.; Guan, L.; Liu, K.; Hong, B.; Zhang, X.; Lou, F.; Li, M.; Lin, W.; Chen, Y.; et al. Bovine Lactoferrin Inhibits SARS-CoV-2 and SARS-CoV-1 by Targeting the RdRp Complex and Alleviates Viral Infection in the Hamster Model. J. Med. Virol. 2023, 95, e28281. [Google Scholar] [CrossRef]
- Pang, Z.; Hu, R.; Tian, L.; Lou, F.; Chen, Y.; Wang, S.; He, S.; Zhu, S.; An, X.; Song, L.; et al. Overview of Breastfeeding Under COVID-19 Pandemic. Front. Immunol. 2022, 13, 896068. [Google Scholar] [CrossRef]
- Salaris, C.; Scarpa, M.; Elli, M.; Bertolini, A.; Guglielmetti, S.; Pregliasco, F.; Blandizzi, C.; Brun, P.; Castagliuolo, I. Protective Effects of Lactoferrin against SARS-CoV-2 Infection In Vitro. Nutrients 2021, 13, 328. [Google Scholar] [CrossRef]
- Shafqat, F.; Rehman, S.U.; Niaz, K. Lactoferrin Can Attenuate SARS-CoV-2: An Analysis of Evidential Relations. Biomed. Res. Ther. 2022, 9, 4901–4919. [Google Scholar] [CrossRef]
- Campione, E.; Lanna, C.; Cosio, T.; Rosa, L.; Conte, M.P.; Iacovelli, F.; Romeo, A.; Falconi, M.; Del Vecchio, C.; Franchin, E.; et al. Lactoferrin Against SARS-CoV-2: In Vitro and In Silico Evidences. Front. Pharmacol. 2021, 12, 666600. [Google Scholar] [CrossRef]
- Campione, E.; Cosio, T.; Rosa, L.; Lanna, C.; Di Girolamo, S.; Gaziano, R.; Valenti, P.; Bianchi, L. Lactoferrin as Protective Natural Barrier of Respiratory and Intestinal Mucosa against Coronavirus Infection and Inflammation. Int. J. Mol. Sci. 2020, 21, 4903. [Google Scholar] [CrossRef]
- Mancinelli, R.; Rosa, L.; Cutone, A.; Lepanto, M.S.; Franchitto, A.; Onori, P.; Gaudio, E.; Valenti, P. Viral Hepatitis and Iron Dysregulation: Molecular Pathways and the Role of Lactoferrin. Molecules 2020, 25, 1997. [Google Scholar] [CrossRef] [PubMed]
- Campione, E.; Lanna, C.; Cosio, T.; Rosa, L.; Conte, M.P.; Iacovelli, F.; Romeo, A.; Falconi, M.; Del Vecchio, C.; Franchin, E.; et al. Lactoferrin as Antiviral Treatment in COVID-19 Management: Preliminary Evidence. Int. J. Environ. Res. Public. Health 2021, 18, 10985. [Google Scholar] [CrossRef] [PubMed]
- Rosa, L.; Tripepi, G.; Naldi, E.; Aimati, M.; Santangeli, S.; Venditto, F.; Caldarelli, M.; Valenti, P. Ambulatory COVID-19 Patients Treated with Lactoferrin as a Supplementary Antiviral Agent: A Preliminary Study. J. Clin. Med. 2021, 10, 4276. [Google Scholar] [CrossRef]
- Serrano, G.; Kochergina, I.; Albors, A.; Diaz, E.; Oroval, M.; Hueso, G.; Serrano, J.M. Liposomal Lactoferrin as Potential Preventative and Cure for COVID-19. Int. J. Res. Health Sci. 2020, 8, 08–15. [Google Scholar] [CrossRef]
- Gans, M.D.; Gavrilova, T. Understanding the Immunology of Asthma: Pathophysiology, Biomarkers, and Treatments for Asthma Endotypes. Paediatr. Respir. Rev. 2020, 36, 118–127. [Google Scholar] [CrossRef]
- Papi, A.; Brightling, C.; Pedersen, S.E.; Reddel, H.K. Asthma. Lancet 2018, 391, 783–800. [Google Scholar] [CrossRef]
- Barcik, W.; Boutin, R.C.T.; Sokolowska, M.; Finlay, B.B. The Role of Lung and Gut Microbiota in the Pathology of Asthma. Immunity 2020, 52, 241–255. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Actor, J.; Hwang, S.-A.; Kruzel, M. Lactoferrin as a Natural Immune Modulator. Curr. Pharm. Des. 2009, 15, 1956–1973. [Google Scholar] [CrossRef] [Green Version]
- van de Graaf, E.A.; Out, T.A.; Kobesen, A.; Jansen, H.M. Lactoferrin and Secretory IgA in the Bronchoalveolar Lavage Fluid from Patients with a Stable Asthma. Lung 1991, 169, 275–283. [Google Scholar] [CrossRef]
- Tsokos, M.; Paulsen, F. Expression of Pulmonary Lactoferrin in Sudden-Onset and Slow-Onset Asthma with Fatal Outcome. Virchows Arch. 2002, 441, 494–499. [Google Scholar] [CrossRef]
- Fernández-Delgado, L.; Vega-Rioja, A.; Ventura, I.; Chamorro, C.; Aroca, R.; Prados, M.; Bobadilla, P.; Rodríguez, D.; Palacios, R.; Monteseirín, J. Allergens Induce the Release of Lactoferrin by Neutrophils from Asthmatic Patients. PLOS ONE 2015, 10, e0141278. [Google Scholar] [CrossRef] [PubMed]
- Ghio, A.J.; Carter, J.D.; Samet, J.M.; Reed, W.; Quay, J.; Dailey, L.A.; Richards, J.H.; Devlin, R.B. Metal-Dependent Expression of Ferritin and Lactoferrin by Respiratory Epithelial Cells. Am. J. Physiol.-Lung Cell. Mol. Physiol. 1998, 274, L728–L736. [Google Scholar] [CrossRef] [PubMed]
- Kowalski, M.; Dietrich-miłobędzki, A.; Majkowska-wojciechowska, B.; Jarzębska, M. Nasal Reactivity to Capsaicin in Patients with Seasonal Allergic Rhinitis during and after the Pollen Season. Allergy 1999, 54, 804–810. [Google Scholar] [CrossRef] [PubMed]
- Kruzel, M.L.; Zimecki, M. Lactoferrin and Immunologic Dissonance: Clinical Implications. Arch. Immunol. Ther. Exp. (Warsz.) 2002, 50, 399–410. [Google Scholar]
- Chodaczek, G.; Saavedra-Molina, A.; Bacsi, A.; Kruzel, M.L.; Sur, S.; Boldogh, I. Iron-Mediated Dismutation of Superoxide Anion Augments Antigen-Induced Allergic Inflammation: Effect of Lactoferrin. Postep. Hig. Med. Dosw. Online 2007, 61, 268–276. [Google Scholar]
- Kruzel, M.L.; Bacsi, A.; Choudhury, B.; Sur, S.; Boldogh, I. Lactoferrin Decreases Pollen Antigen-Induced Allergic Airway Inflammation in a Murine Model of Asthma. Immunology 2006, 119, 159–166. [Google Scholar] [CrossRef]
- Lin, C.-C.; Chuang, K.-C.; Chen, S.-W.; Chao, Y.-H.; Yen, C.-C.; Yang, S.-H.; Chen, W.; Chang, K.-H.; Chang, Y.-K.; Chen, C.-M. Lactoferrin Ameliorates Ovalbumin-Induced Asthma in Mice through Reducing Dendritic-Cell-Derived Th2 Cell Responses. Int. J. Mol. Sci. 2022, 23, 14185. [Google Scholar] [CrossRef]
- van Scott, M.; Glynn, P.; Varadhachary, A. Oral Recombinant Human Lactoferrin (RhLF) in a Non-Human Primate Model of Asthma. J. Allergy Clin. Immunol. 2004, 113, S222–S223. [Google Scholar] [CrossRef]
- Nagaoka, K.; Ito, T.; Ogino, K.; Eguchi, E.; Fujikura, Y. Human Lactoferrin Induces Asthmatic Symptoms in NC/Nga Mice. Physiol. Rep. 2017, 5, e13365. [Google Scholar] [CrossRef]
- Shinagawa, K.; Oshikata, C.; Kaneko, T.; Tsurikisawa, N. A Case of Lactoferrin-Induced Occupational Asthma. J. Allergy Clin. Immunol. Pract. 2020, 8, 3600–3602. [Google Scholar] [CrossRef]
- Kruzel, M.L.; Actor, J.K.; Boldogh, I.; Zimecki, M. Lactoferrin in Health and Disease. Postep. Hig. Med. Dosw. Online 2007, 61, 261–267. [Google Scholar]
- Bournazou, I.; Mackenzie, K.J.; Duffin, R.; Rossi, A.G.; Gregory, C.D. Inhibition of Eosinophil Migration by Lactoferrin. Immunol. Cell Biol. 2010, 88, 220–223. [Google Scholar] [CrossRef]
- Bournazou, I.; Pound, J.D.; Duffin, R.; Bournazos, S.; Melville, L.A.; Brown, S.B.; Rossi, A.G.; Gregory, C.D. Apoptotic Human Cells Inhibit Migration of Granulocytes via Release of Lactoferrin. J. Clin. Invest. 2009, 119, 20–32. [Google Scholar] [CrossRef] [Green Version]
- Fischer, R.; Debbabi, H.; Dubarry, M.; Boyaka, P.; Tomé, D. Regulation of Physiological and Pathological Th1 and Th2 Responses by Lactoferrin. Biochem. Cell Biol. Biochim. Biol. Cell. 2006, 84, 303–311. [Google Scholar] [CrossRef]
- Abbring, S.; Verheijden, K.A.T.; Diks, M.A.P.; Leusink-Muis, A.; Hols, G.; Baars, T.; Garssen, J.; van Esch, B.C.A.M. Raw Cow’s Milk Prevents the Development of Airway Inflammation in a Murine House Dust Mite-Induced Asthma Model. Front. Immunol. 2017, 8, 1045. [Google Scholar] [CrossRef] [Green Version]
- Elrod, K.C.; Moore, W.R.; Abraham, W.M.; Tanaka, R.D. Lactoferrin, a Potent Tryptase Inhibitor, Abolishes Late-Phase Airway Responses in Allergic Sheep. Am. J. Respir. Crit. Care Med. 1997, 156, 375–381. [Google Scholar] [CrossRef]
- He, S.; McEuen, A.R.; Blewett, S.A.; Li, P.; Buckley, M.G.; Leufkens, P.; Walls, A.F. The Inhibition of Mast Cell Activation by Neutrophil Lactoferrin: Uptake by Mast Cells and Interaction with Tryptase, Chymase and Cathepsin G. Biochem. Pharmacol. 2003, 65, 1007–1015. [Google Scholar] [CrossRef]
- Glynn, P.; Varadhachary, A. Oral Lactoferrin in the Treatment of Respiratory Disorders. U.S. Patent No 7,238,661, 3 July 2007. [Google Scholar]
- Varadhachary, A. Phase II Clinical Trial of Lactoferrin in Asthma. Available online: https://Grantome.Com/Grant/NIH/R44-AI058553-02 (accessed on 19 December 2022).
- Dogaru, C.M.; Nyffenegger, D.; Pescatore, A.M.; Spycher, B.D.; Kuehni, C.E. Breastfeeding and Childhood Asthma: Systematic Review and Meta-Analysis. Am. J. Epidemiol. 2014, 179, 1153–1167. [Google Scholar] [CrossRef] [Green Version]
- Duijts, L.; Reiss, I.K.; Brusselle, G.; de Jongste, J.C. Early Origins of Chronic Obstructive Lung Diseases across the Life Course. Eur. J. Epidemiol. 2014, 29, 871–885. [Google Scholar] [CrossRef]
- Elliott, L.; Henderson, J.; Northstone, K.; Chiu, G.Y.; Dunson, D.; London, S.J. Prospective Study of Breast-Feeding in Relation to Wheeze, Atopy, and Bronchial Hyperresponsiveness in the Avon Longitudinal Study of Parents and Children (ALSPAC). J. Allergy Clin. Immunol. 2008, 122, 49–54. [Google Scholar] [CrossRef] [Green Version]
- Kramer, M.S.; Matush, L.; Vanilovich, I.; Platt, R.; Bogdanovich, N.; Sevkovskaya, Z.; Dzikovich, I.; Shishko, G.; Mazer, B. Promotion of Breastfeeding Intervention Trial (PROBIT) Study Group Effect of Prolonged and Exclusive Breast Feeding on Risk of Allergy and Asthma: Cluster Randomised Trial. BMJ 2007, 335, 815. [Google Scholar] [CrossRef] [Green Version]
- Sonnenschein-van der Voort, A.M.M.; Jaddoe, V.W.V.; van der Valk, R.J.P.; Willemsen, S.P.; Hofman, A.; Moll, H.A.; de Jongste, J.C.; Duijts, L. Duration and Exclusiveness of Breastfeeding and Childhood Asthma-Related Symptoms. Eur. Respir. J. 2012, 39, 81–89. [Google Scholar] [CrossRef] [Green Version]
- Greer, F.R.; Sicherer, S.H.; Burks, A.W.; Committee on Nutrition; Section on Allergy and Immunology; Abrams, S.A.; Fuchs, G.J.; Kim, J.H.; Lindsey, C.W.; Magge, S.N.; et al. The Effects of Early Nutritional Interventions on the Development of Atopic Disease in Infants and Children: The Role of Maternal Dietary Restriction, Breastfeeding, Hydrolyzed Formulas, and Timing of Introduction of Allergenic Complementary Foods. Pediatrics 2019, 143, e20190281. [Google Scholar] [CrossRef] [Green Version]
- Ip, S.; Chung, M.; Raman, G.; Chew, P.; Magula, N.; DeVine, D.; Trikalinos, T.; Lau, J. Breastfeeding and Maternal and Infant Health Outcomes in Developed Countries. Evid. Rep. Assess. 2007, 153, 1–186. [Google Scholar]
- Lodge, C.; Tan, D.; Lau, M.; Dai, X.; Tham, R.; Lowe, A.; Bowatte, G.; Allen, K.; Dharmage, S. Breastfeeding and Asthma and Allergies: A Systematic Review and Meta-Analysis. Acta Paediatr. 2015, 104, 38–53. [Google Scholar] [CrossRef]
- Miliku, K.; Azad, M. Breastfeeding and the Developmental Origins of Asthma: Current Evidence, Possible Mechanisms, and Future Research Priorities. Nutrients 2018, 10, 995. [Google Scholar] [CrossRef] [Green Version]
- Victora, C.G.; Bahl, R.; Barros, A.J.D.; França, G.V.A.; Horton, S.; Krasevec, J.; Murch, S.; Sankar, M.J.; Walker, N.; Rollins, N.C. Breastfeeding in the 21st Century: Epidemiology, Mechanisms, and Lifelong Effect. Lancet 2016, 387, 475–490. [Google Scholar] [CrossRef] [Green Version]
- Sozańska, B.; Pearce, N.; Dudek, K.; Cullinan, P. Consumption of Unpasteurized Milk and Its Effects on Atopy and Asthma in Children and Adult Inhabitants in Rural Poland. Allergy 2013, 68, 644–650. [Google Scholar] [CrossRef]
- Friedman, N.J.; Zeiger, R.S. The Role of Breast-Feeding in the Development of Allergies and Asthma. J. Allergy Clin. Immunol. 2005, 115, 1238–1248. [Google Scholar] [CrossRef]
- Oddy, W.H. Breastfeeding, Childhood Asthma, and Allergic Disease. Ann. Nutr. Metab. 2017, 70, 26–36. [Google Scholar] [CrossRef]
- Cloonan, S.M.; Mumby, S.; Adcock, I.M.; Choi, A.M.K.; Chung, K.F.; Quinlan, G.J. The “Iron”-y of Iron Overload and Iron Deficiency in Chronic Obstructive Pulmonary Disease. Am. J. Respir. Crit. Care Med. 2017, 196, 1103–1112. [Google Scholar] [CrossRef] [Green Version]
- Iheanacho, I.; Zhang, S.; King, D.; Rizzo, M.; Ismaila, A.S. Economic Burden of Chronic Obstructive Pulmonary Disease (COPD): A Systematic Literature Review. Int. J. Chron. Obstruct. Pulmon. Dis. 2020, 15, 439–460. [Google Scholar] [CrossRef] [Green Version]
- Riley, C.M.; Sciurba, F.C. Diagnosis and Outpatient Management of Chronic Obstructive Pulmonary Disease: A Review. JAMA 2019, 321, 786. [Google Scholar] [CrossRef]
- Gela, A.; Bhongir, R.K.V.; Mori, M.; Keenan, P.; Mörgelin, M.; Erjefält, J.S.; Herwald, H.; Egesten, A.; Kasetty, G. Osteopontin That Is Elevated in the Airways during COPD Impairs the Antibacterial Activity of Common Innate Antibiotics. PLoS ONE 2016, 11, e0146192. [Google Scholar] [CrossRef]
- Parameswaran, G.I.; Sethi, S.; Murphy, T.F. Effects of Bacterial Infection on Airway Antimicrobial Peptides and Proteins in COPD. Chest 2011, 140, 611–617. [Google Scholar] [CrossRef]
- Ficker, J. Physiologie und Pathophysiologie der bronchialen Sekretion. Pneumologie 2008, 62, S11–S13. [Google Scholar] [CrossRef]
- Thompson, A.B.; Bohling, T.; Payvandi, F.; Rennard, S.I. Lower Respiratory Tract Lactoferrin and Lysozyme Arise Primarily in the Airways and Are Elevated in Association with Chronic Bronchitis. J. Lab. Clin. Med. 1990, 115, 148–158. [Google Scholar]
- Gohy, S.T.; Hupin, C.; Pilette, C.; Ladjemi, M.Z. Chronic Inflammatory Airway Diseases: The Central Role of the Epithelium Revisited. Clin. Exp. Allergy 2016, 46, 529–542. [Google Scholar] [CrossRef]
- Vargas Buonfiglio, L.G.; Borcherding, J.A.; Frommelt, M.; Parker, G.J.; Duchman, B.; Vanegas Calderón, O.G.; Fernandez-Ruiz, R.; Noriega, J.E.; Stone, E.A.; Gerke, A.K.; et al. Airway Surface Liquid from Smokers Promotes Bacterial Growth and Biofilm Formation via Iron-Lactoferrin Imbalance. Respir. Res. 2018, 19, 42. [Google Scholar] [CrossRef]
- Schoonbrood, D.F.M.; Out, T.A.; Lutter, R.; Reimert, C.M.; van Overveld, F.J.; Jansen, H.M. Plasma Protein Leakage and Local Secretion of Proteins Assessed in Sputum in Asthma and COPD. The Effect of Inhaled Corticosteroids. Clin. Chim. Acta 1995, 240, 163–178. [Google Scholar] [CrossRef]
- Chen, H.-L.; Yen, C.-C.; Wang, S.-M.; Tsai, T.-C.; Lai, Z.-L.; Sun, J.-Y.; Lin, W.; Hsu, W.-H.; Chen, C.-M. Aerosolized Bovine Lactoferrin Reduces Lung Injury and Fibrosis in Mice Exposed to Hyperoxia. BioMetals 2014, 27, 1057–1068. [Google Scholar] [CrossRef]
- Terlizzi, M.; Colarusso, C.; Di Maio, U.; Bagnulo, A.; Pinto, A.; Sorrentino, R. Antioxidant and Antimicrobial Properties of Pelargonium Sidoides DC and Lactoferrin Combination. Biosci. Rep. 2020, 40, BSR20203284. [Google Scholar] [CrossRef]
- Bojanowski, C.M.; Lu, S.; Kolls, J.K. Mucosal Immunity in Cystic Fibrosis. J. Immunol. 2021, 207, 2901–2912. [Google Scholar] [CrossRef]
- Lepanto, M.S.; Rosa, L.; Paesano, R.; Valenti, P.; Cutone, A. Lactoferrin in Aseptic and Septic Inflammation. Molecules 2019, 24, 1323. [Google Scholar] [CrossRef] [Green Version]
- Hildebrandt, T.; Zawilska, A.; Trzcionka, A.; Tanasiewicz, M.; Mazurek, H.; Świętochowska, E. Estimation of Proinflammatory Factors in the Saliva of Adult Patients with Cystic Fibrosis and Dental Caries. Medicina (Mex.) 2020, 56, 612. [Google Scholar] [CrossRef]
- Sagel, S.D.; Sontag, M.K.; Accurso, F.J. Relationship between Antimicrobial Proteins and Airway Inflammation and Infection in Cystic Fibrosis: Antimicrobial Proteins in CF. Pediatr. Pulmonol. 2009, 44, 402–409. [Google Scholar] [CrossRef]
- Rogan, M.P.; Taggart, C.C.; Greene, C.M.; Murphy, P.G.; O’Neill, S.J.; McElvaney, N.G. Loss of Microbicidal Activity and Increased Formation of Biofilm Due to Decreased Lactoferrin Activity in Patients with Cystic Fibrosis. J. Infect. Dis. 2004, 190, 1245–1253. [Google Scholar] [CrossRef]
- Valenti, P.; Frioni, A.; Rossi, A.; Ranucci, S.; De Fino, I.; Cutone, A.; Rosa, L.; Bragonzi, A.; Berlutti, F. Aerosolized Bovine Lactoferrin Reduces Neutrophils and Pro-Inflammatory Cytokines in Mouse Models of Pseudomonas Aeruginosa Lung Infections. Biochem. Cell Biol. 2017, 95, 41–47. [Google Scholar] [CrossRef] [Green Version]
- Cutone, A.; Lepanto, M.S.; Rosa, L.; Scotti, M.J.; Rossi, A.; Ranucci, S.; De Fino, I.; Bragonzi, A.; Valenti, P.; Musci, G.; et al. Aerosolized Bovine Lactoferrin Counteracts Infection, Inflammation and Iron Dysbalance in A Cystic Fibrosis Mouse Model of Pseudomonas Aeruginosa Chronic Lung Infection. Int. J. Mol. Sci. 2019, 20, 2128. [Google Scholar] [CrossRef] [Green Version]
- Berlutti, F.; Superti, F.; Nicoletti, M.; Morea, C.; Frioni, A.; Ammendolia, M.G.; Battistoni, A.; Valenti, P. Bovine Lactoferrin Inhibits the Efficiency of Invasion of Respiratory A549 Cells of Different Iron-Regulated Morphological Forms of Pseudomonas Aeruginosa and Burkholderia Cenocepacia. Int. J. Immunopathol. Pharmacol. 2008, 21, 51–59. [Google Scholar] [CrossRef]
- Frioni, A.; Conte, M.P.; Cutone, A.; Longhi, C.; Musci, G.; di Patti, M.C.B.; Natalizi, T.; Marazzato, M.; Lepanto, M.S.; Puddu, P.; et al. Lactoferrin Differently Modulates the Inflammatory Response in Epithelial Models Mimicking Human Inflammatory and Infectious Diseases. BioMetals 2014, 27, 843–856. [Google Scholar] [CrossRef]
- Moreau-Marquis, S.; Coutermarsh, B.; Stanton, B.A. Combination of Hypothiocyanite and Lactoferrin (ALX-109) Enhances the Ability of Tobramycin and Aztreonam to Eliminate Pseudomonas Aeruginosa Biofilms Growing on Cystic Fibrosis Airway Epithelial Cells. J. Antimicrob. Chemother. 2015, 70, 160–166. [Google Scholar] [CrossRef]
- Tunney, M.M.; Payne, J.E.; McGrath, S.J.; Einarsson, G.G.; Ingram, R.J.; Gilpin, D.F.; Juarez-Perez, V.; Elborn, J.S. Activity of Hypothiocyanite and Lactoferrin (ALX-009) against Respiratory Cystic Fibrosis Pathogens in Sputum. J. Antimicrob. Chemother. 2018, 73, 3391–3397. [Google Scholar] [CrossRef]
- Bakowitz, M.; Bruns, B.; McCunn, M. Acute Lung Injury and the Acute Respiratory Distress Syndrome in the Injured Patient. Scand. J. Trauma Resusc. Emerg. Med. 2012, 20, 54. [Google Scholar] [CrossRef] [Green Version]
- Villar, J.; Ferrando, C.; Tusman, G.; Berra, L.; Rodríguez-Suárez, P.; Suárez-Sipmann, F. Unsuccessful and Successful Clinical Trials in Acute Respiratory Distress Syndrome: Addressing Physiology-Based Gaps. Front. Physiol. 2021, 12, 774025. [Google Scholar] [CrossRef]
- Han, N.; Li, H.; Li, G.; Shen, Y.; Fei, M.; Nan, Y. Effect of Bovine Lactoferrin as a Novel Therapeutic Agent in a Rat Model of Sepsis-Induced Acute Lung Injury. AMB Express 2019, 9, 177. [Google Scholar] [CrossRef]
- Li, X.; Liu, D.; Chen, H.; Pan, X.; Kong, Q.; Pang, Q. Lactoferrin Protects against Lipopolysaccharide-Induced Acute Lung Injury in Mice. Int. Immunopharmacol. 2012, 12, 460–464. [Google Scholar] [CrossRef]
- Ghio, A.J.; Carter, J.D.; Richards, J.H.; Richer, L.D.; Grissom, C.K.; Elstad, M.R. Iron and Iron-Related Proteins in the Lower Respiratory Tract of Patients with Acute Respiratory Distress Syndrome: Crit. Care Med. 2003, 31, 395–400. [Google Scholar] [CrossRef]
- Kaur, G.; Gathwala, G. Efficacy of Bovine Lactoferrin Supplementation in Preventing Late-Onset Sepsis in Low Birth Weight Neonates: A Randomized Placebo-Controlled Clinical Trial. J. Trop. Pediatr. 2015, 61, 370–376. [Google Scholar] [CrossRef] [Green Version]
- Manzoni, P. Bovine Lactoferrin Supplementation for Prevention of Late-Onset Sepsis in Very Low-Birth-Weight NeonatesA Randomized Trial. JAMA 2009, 302, 1421. [Google Scholar] [CrossRef] [Green Version]
- Ochoa, T.; Loli, S.; Mendoza, K.; Carcamo, C.; Bellomo, S.; Cam, L.; Castaneda, A.; Campos, M.; Jacobs, J.; Cossey, V.; et al. Effect of Bovine Lactoferrin on Prevention of Late-Onset Sepsis in Infants <1500 g: A Pooled Analysis of Individual Patient Data from Two Randomized Controlled Trials. Biochem. Cell Biol. 2021, 99, 14–19. [Google Scholar] [CrossRef]
- Pammi, M.; Suresh, G. Enteral Lactoferrin Supplementation for Prevention of Sepsis and Necrotizing Enterocolitis in Preterm Infants. Cochrane Database Syst. Rev. 2020, 3, CD007137. [Google Scholar] [CrossRef] [PubMed]
- Berrington, J.E.; McGuire, W.; Embleton, N.D. ELFIN, the United Kingdom Preterm Lactoferrin Trial: Interpretation and Future Questions. Biochem. Cell Biol. 2021, 99, 1–6. [Google Scholar] [CrossRef]
- Embleton, N.; Berrington, J.; Cummings, S.; Dorling, J.; Ewer, A.; Frau, A.; Juszczak, E.; Kirby, J.; Lamb, C.; Lanyon, C.; et al. Lactoferrin Impact on Gut Microbiota in Preterm Infants with Late-Onset Sepsis or Necrotising Enterocolitis: The MAGPIE Mechanisms of Action Study; Efficacy and Mechanism Evaluation; NIHR Journals Library: Southampton, UK, 2021. [Google Scholar]
- Guntupalli, K.; Dean, N.; Morris, P.E.; Bandi, V.; Margolis, B.; Rivers, E.; Levy, M.; Lodato, R.F.; Ismail, P.M.; Reese, A.; et al. A Phase 2 Randomized, Double-Blind, Placebo–Controlled Study of the Safety and Efficacy of Talactoferrin in Patients With Severe Sepsis *. Crit. Care Med. 2013, 41, 706–716. [Google Scholar] [CrossRef]
- Vincent, J.-L.; Marshall, J.C.; Dellinger, R.P.; Simonson, S.G.; Guntupalli, K.; Levy, M.M.; Singer, M.; Malik, R. Talactoferrin in Severe Sepsis: Results From the Phase II/III Oral TAlactoferrin in Severe SepsIS Trial. Crit. Care Med. 2015, 43, 1832–1838. [Google Scholar] [CrossRef]
- Zimecki, M.; Actor, J.K.; Kruzel, M.L. The Potential for Lactoferrin to Reduce SARS-CoV-2 Induced Cytokine Storm. Int. Immunopharmacol. 2021, 95, 107571. [Google Scholar] [CrossRef]
- MacManus, C.F.; Collins, C.B.; Nguyen, T.T.; Alfano, R.W.; Jedlicka, P.; de Zoeten, E.F. VEN-120, a Recombinant Human Lactoferrin, Promotes a Regulatory T Cell [Treg] Phenotype and Drives Resolution of Inflammation in Distinct Murine Models of Inflammatory Bowel Disease. J. Crohns Colitis 2017, 11, 1101–1112. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Wang, P.; Wang, H.; Luo, Y.; Wan, L.; Jiang, M.; Chu, Y. Lactoferrin for the Treatment of COVID-19 (Review). Exp. Ther. Med. 2020, 20, 1. [Google Scholar] [CrossRef]
- Baveye, S.; Elass, E.; Fernig, D.G.; Blanquart, C.; Mazurier, J.; Legrand, D. Human Lactoferrin Interacts with Soluble CD14 and Inhibits Expression of Endothelial Adhesion Molecules, E-Selectin and ICAM-1, Induced by the CD14-Lipopolysaccharide Complex. Infect. Immun. 2000, 68, 6519–6525. [Google Scholar] [CrossRef] [Green Version]
- Kim, C.W.; Lee, T.H.; Park, K.H.; Choi, S.-Y.; Kim, J. Human Lactoferrin Suppresses TNF-α-Induced Intercellular Adhesion Molecule-1 Expression via Competition with NF-ΚB in Endothelial Cells. FEBS Lett. 2012, 586, 229–234. [Google Scholar] [CrossRef] [Green Version]
- Lee, T. Lactoferrin Inhibits Immune Cell Adhesion via Suppression of Cell Adhesion Molecules Expression in Hypoxia/Reoxygenation Animal Model. J. Immunol. 2018, 200, 42. [Google Scholar] [CrossRef]
- Algahtani, F.D.; Elabbasy, M.T.; Samak, M.A.; Adeboye, A.A.; Yusuf, R.A.; Ghoniem, M.E. The Prospect of Lactoferrin Use as Adjunctive Agent in Management of SARS-CoV-2 Patients: A Randomized Pilot Study. Medicina (Mex.) 2021, 57, 842. [Google Scholar] [CrossRef] [PubMed]
- Iijima, H.; Tomizawa, Y.; Iwasaki, Y.; Sato, K.; Sunaga, N.; Dobashi, K.; Saito, R.; Nakajima, T.; Minna, J.D.; Mori, M. Genetic and Epigenetic Inactivation of LTF Gene at 3p21.3 in Lung Cancers. Int. J. Cancer 2006, 118, 797–801. [Google Scholar] [CrossRef]
- Bezault, J.; Bhimani, R.; Wiprovnick, J.; Furmanski, P. Human Lactoferrin Inhibits Growth of Solid Tumors and Development of Experimental Metastases in Mice. Cancer Res. 1994, 54, 2310–2312. [Google Scholar]
- Wei, L.; Zhang, X.; Wang, J.; Ye, Q.; Zheng, X.; Peng, Q.; Zheng, Y.; Liu, P.; Zhang, X.; Li, Z.; et al. Lactoferrin Deficiency Induces a Pro-Metastatic Tumor Microenvironment through Recruiting Myeloid-Derived Suppressor Cells in Mice. Oncogene 2020, 39, 122–135. [Google Scholar] [CrossRef]
- Digumarti, R.; Wang, Y.; Raman, G.; Doval, D.C.; Advani, S.H.; Julka, P.K.; Parikh, P.M.; Patil, S.; Nag, S.; Madhavan, J.; et al. A Randomized, Double-Blind, Placebo-Controlled, Phase II Study of Oral Talactoferrin in Combination with Carboplatin and Paclitaxel in Previously Untreated Locally Advanced or Metastatic Non-Small Cell Lung Cancer. J. Thorac. Oncol. 2011, 6, 1098–1103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parikh, P.M.; Vaid, A.; Advani, S.H.; Digumarti, R.; Madhavan, J.; Nag, S.; Bapna, A.; Sekhon, J.S.; Patil, S.; Ismail, P.M.; et al. Randomized, Double-Blind, Placebo-Controlled Phase II Study of Single-Agent Oral Talactoferrin in Patients With Locally Advanced or Metastatic Non–Small-Cell Lung Cancer That Progressed After Chemotherapy. J. Clin. Oncol. 2011, 29, 4129–4136. [Google Scholar] [CrossRef] [PubMed]
- Kelly, R.J.; Giaccone, G. The Role of Talactoferrin Alpha in the Treatment of Non-Small Cell Lung Cancer. Expert Opin. Biol. Ther. 2010, 10, 1379–1386. [Google Scholar] [CrossRef]
- Ramalingam, S.S.; Crawford, J.; Chang, A.; Manegold, C.; Perez-Soler, R.; Douillard, J.; Thatcher, N.; Barlesi, F.; Owonikoko, T.K.; Wang, Y.; et al. Fortis-M, a Randomized, Double-Blind, Placebo-Controlled Phase 3 Study of Oral Talactoferrin Alfa with Best Supportive Care in Patients with Advanced Non-Small Cell Lung Cancer Following Two or More Prior Regimens- by the Fortis-M Study Group. Ann. Oncol. 2012, 23, ixe23. [Google Scholar] [CrossRef]
- Tung, Y.-T.; Chen, H.-L.; Yen, C.-C.; Lee, P.-Y.; Tsai, H.-C.; Lin, M.-F.; Chen, C.-M. Bovine Lactoferrin Inhibits Lung Cancer Growth through Suppression of Both Inflammation and Expression of Vascular Endothelial Growth Factor. J. Dairy Sci. 2013, 96, 2095–2106. [Google Scholar] [CrossRef] [Green Version]
- Li, D.; Sakashita, S.; Morishita, Y.; Kano, J.; Shiba, A.; Sato, T.; Noguchi, M. Binding of Lactoferrin to IGBP1 Triggers Apoptosis in a Lung Adenocarcinoma Cell Line. Anticancer. Res. 2011, 31, 529–534. [Google Scholar] [PubMed]
- Li, H.Y.; Li, P.; Yang, H.G.; Wang, Y.Z.; Huang, G.X.; Wang, J.Q.; Zheng, N. Investigation and Comparison of the Anti-Tumor Activities of Lactoferrin, α-Lactalbumin, and β-Lactoglobulin in A549, HT29, HepG2, and MDA231-LM2 Tumor Models. J. Dairy Sci. 2019, 102, 9586–9597. [Google Scholar] [CrossRef] [PubMed]
- Olszewska, P.; Pazdrak, B.; Kruzel, M.L. A Novel Human Recombinant Lactoferrin Inhibits Lung Adenocarcinoma Cell Growth and Migration with No Cytotoxic Effect on Normal Human Epithelial Cells. Arch. Immunol. Ther. Exp. (Warsz.) 2021, 69, 33. [Google Scholar] [CrossRef] [PubMed]
- Abd Elwakil, M.M.; Mabrouk, M.T.; Helmy, M.W.; Abdelfattah, E.-Z.A.; Khiste, S.K.; Elkhodairy, K.A.; Elzoghby, A.O. Inhalable Lactoferrin–Chondroitin Nanocomposites for Combined Delivery of Doxorubicin and Ellagic Acid to Lung Carcinoma. Nanomed 2018, 13, 2015–2035. [Google Scholar] [CrossRef]
- Abdelaziz, H.M.; Elzoghby, A.O.; Helmy, M.W.; Abdelfattah, E.-Z.A.; Fang, J.-Y.; Samaha, M.W.; Freag, M.S. Inhalable Lactoferrin/Chondroitin-Functionalized Monoolein Nanocomposites for Localized Lung Cancer Targeting. ACS Biomater. Sci. Eng. 2020, 6, 1030–1042. [Google Scholar] [CrossRef]
- Rosa, L.; Cutone, A.; Lepanto, M.S.; Scotti, M.J.; Conte, M.P.; Paesano, R.; Valenti, P. Physico-Chemical Properties Influence the Functions and Efficacy of Commercial Bovine Lactoferrins. BioMetals 2018, 31, 301–312. [Google Scholar] [CrossRef]
- Troost, F.J.; Steijns, J.; Saris, W.H.M.; Brummer, R.-J.M. Gastric Digestion of Bovine Lactoferrin In Vivo in Adults. J. Nutr. 2001, 131, 2101–2104. [Google Scholar] [CrossRef]
- Wakabayashi, H.; Yamauchi, K.; Takase, M. Lactoferrin Research, Technology and Applications. Int. Dairy J. 2006, 16, 1241–1251. [Google Scholar] [CrossRef]
- Mann, J.K.; Ndung’u, T. The Potential of Lactoferrin, Ovotransferrin and Lysozyme as Antiviral and Immune-Modulating Agents in COVID-19. Future Virol. 2020, 15, 609–624. [Google Scholar] [CrossRef]
- Bhimani, R.S.; Vendrov, Y.; Furmanski, P. Influence of Lactoferrin Feeding and Injection against Systemic Staphylococcal Infections in Mice. J. Appl. Microbiol. 1999, 86, 135–144. [Google Scholar] [CrossRef] [Green Version]
- Lim, L.Y.; Koh, P.Y.; Somani, S.; Al Robaian, M.; Karim, R.; Yean, Y.L.; Mitchell, J.; Tate, R.J.; Edrada-Ebel, R.; Blatchford, D.R.; et al. Tumor Regression Following Intravenous Administration of Lactoferrin- and Lactoferricin-Bearing Dendriplexes. Nanomed. Nanotechnol. Biol. Med. 2015, 11, 1445–1454. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paesano, R.; Pietropaoli, M.; Berlutti, F.; Valenti, P. Bovine Lactoferrin in Preventing Preterm Delivery Associated with Sterile Inflammation 1 This Article Is Part of Special Issue Entitled Lactoferrin and Has Undergone the Journal’s Usual Peer Review Process. Biochem. Cell Biol. 2012, 90, 468–475. [Google Scholar] [CrossRef] [PubMed]
- López-Machado, A.; Díaz, N.; Cano, A.; Espina, M.; Badía, J.; Baldomà, L.; Calpena, A.C.; Biancardi, M.; Souto, E.B.; García, M.L.; et al. Development of Topical Eye-Drops of Lactoferrin-Loaded Biodegradable Nanoparticles for the Treatment of Anterior Segment Inflammatory Processes. Int. J. Pharm. 2021, 609, 121188. [Google Scholar] [CrossRef] [PubMed]
- Kilic, E.; Novoselova, M.V.; Lim, S.H.; Pyataev, N.A.; Pinyaev, S.I.; Kulikov, O.A.; Sindeeva, O.A.; Mayorova, O.A.; Murney, R.; Antipina, M.N.; et al. Formulation for Oral Delivery of Lactoferrin Based on Bovine Serum Albumin and Tannic Acid Multilayer Microcapsules. Sci. Rep. 2017, 7, 44159. [Google Scholar] [CrossRef]



| Disease/ Reference | Therapy | Advantages | Disadvantages | Possible Use of LF |
|---|---|---|---|---|
| Allergic Rhinosinusitis | ||||
| [15] | Biologics (anti-IgE, anti-interleukin agents) | Reduction of allergy as a main cause of AR | Very high costs | |
| [16] | Antihistamine drugs | Fast blockade of symptoms | Limited efficacy, possible sedation, interactions with other drugs | |
| [16] | Intranasal corticosteroids | Very high safety and efficiency, fast action | Nasal dryness, not for long- and very long-term use | + |
| [16] | Allergen immunotherapy | Elimination of allergy as the main cause of allergy | Costs, possible side effects, long duration of the treatment | + |
| Viral and Allergic Rhinosinusitis | ||||
| Immunomodulators | Amelioration of natural defense mechanisms/function or the organism, boosting the immune system | Lack of response in some patients, not always acknowledged by clinicians | + | |
| Bacterial Rhinosinusitis | ||||
| [17,18] | Antibiotics | Fast resolution of symptoms, eradication of pathogens, prevention of complications | Side effects, in case of overuse, resistance to antibiotics | + |
| [18] | Intranasal corticosteroids combined with antibiotics | Very high safety and efficiency, fast action | Nasal dryness, not for long- and very long-term use | + |
| Influenza | ||||
| [19] | Antivirals (oseltamivir, zanamivir, peramivir, baloxavir and others) | Shorten disease length, prevention of influenza-related compilations | Application after first symptoms, risk of viral drug resistance | |
| Immunomodulation via immunomodulators | Amelioration of natural defense mechanisms/function or the organism, boosting the immune system | Lack of response in some patients, not always acknowledged by clinicians, rather used in prevention than in the acute treatment | + | |
| [20] | Home-based treatments (bed rest, NSAIDs, sufficient hydration) | Sufficient in case of mild infection | Not applicable in case of complications or severe infections | + |
| COVID-19 | ||||
| [21] | Corticosteroids | Reduction of mortality | Secondary infections | |
| [21] | IL-6 receptor antagonist antibody | Reduction of mortality | Not known | |
| [21] | Anticoagulants in hospitalized patients | Reduction of risk of major thrombotic events | Higher risk of major bleeding | |
| [21] | Non-invasive continuous positive airway pressure/ High-flow nasal oxygen | Reduction of the need of invasive ventilation | Increased aerosol generation | |
| [21] | JAK inhibitors | Reduction of mortality | Not known | |
| Asthma | ||||
| [22] | Inhaled corticosteroids (ICS) | Good management of symptoms, better asthma control, reduction of asthma exacerbations, gold standard, usually well tolerated | Non-adherence due to difficulties in administration (wrong inhalation technique) | + |
| [22] | LABA (long-acting beta2-agonist) recommended only in combination with inhaled corticosteroids | When used with ICS: better lung function, reduction of asthma exacerbations, prevention of asthma progression and airway remodeling | Not recommended for monotherapy due to increased risk of fatal asthma exacerbation, possible cardiac side effects like arrhythmias, palpitations, increased risk of hyperglycemia | |
| [23] | LAMA (long-acting inhaled muscarinic antagonist) alone or in combination | Prevention of asthma progression, better asthma control | Different responses in different age groups recommended only in severe asthma | |
| [22] | SABA (short-acting beta2-agonists) (only in combination with inhaled corticosteroids when ICS alone is insufficient) | Immediate relief of bronchospasm | Increased risk of poor asthma outcome when overused | |
| [22] | Leukotriene receptor antagonist (LTRA, e.g., montelukast) | Alternative drugs to ICS when ICS is not tolerated | Less effective than ICS, mental health-associated side effects | |
| [22] | Immunotherapy in case of allergic asthma | Elimination of the causes of asthma, better asthma control, lower number of exacerbations | High costs, sometimes unavailable, not all patients respond to the treatment | + |
| [23] | Monoclonal anti-IL-5 antibody | Reduction of number of exacerbations | High costs | |
| [23] | Monoclonal anti-IL-4Rα | Reduction of asthma exacerbations, better asthma control and outcomes | Reaction at injection side | |
| [23] | Oral corticosteroids only in severe asthma | Immediate improvement of acute asthma exacerbation, better asthma control, reduction of asthma symptoms | Cushing’s syndrome, weight gain, hyperglycemia, diabetes/metabolic syndrome, osteoporosis, insomnia, or sleep disturbances | |
| COPD | ||||
| [24] | Pulmonary rehabilitation | Increased exercise capacity, reduction of hospital readmission, improvement of quality of life | Not known | |
| [25] | Long-term home non-invasive ventilation | Reduction of hypercapnia | Decrease in quality of life, need for specialized equipment | |
| [26] | Acute non-invasive ventilation during exacerbation | Reduction of hospitalizations and length of hospital stay | Cannot be performed at home | |
| [27] | Inhaled corticosteroids (ICS) | Reduction of inflammation | Lack of response to ICS in some patients | + |
| [26] | Oral corticosteroids | Improvement of lung function in ambulatory patients during exacerbations, fewer hospitalizations | Cushing’s syndrome, weight gain, hyperglycemia, diabetes/metabolic syndrome, osteoporosis, insomnia, or sleep disturbances | + |
| [24] | Mucolytics | Reduction of the number of hospitalization and exacerbations | Not known | + |
| [24] | LAMA (long-acting inhaled muscarinic antagonist (e.g., Tiotropium) alone or in combination | Reduction of risk of exacerbations, better lung function | Different responses in different age-groups | |
| [24] | Phosphodiesterase-4 inhibitor (roflumilast) | Reduction of number of exacerbations, better lung function | Diarrhea, nausea, weight loss, psychiatric disturbances including depression, insomnia, or sleep disturbances | |
| [24] | Macrolide antibiotic therapy | Reduction of number of exacerbations, improvement of quality of life | Hearing decrement, risk of ventricular arrythmia, diarrhea | + |
| Cystic fibrosis | ||||
| [28,29] | Antibiotics | Management of bacterial colonization and infections | Risk of antibiotic resistance | + |
| [28,29] | NSAIDs, mostly ibuprofen, in children | Reduction of airway inflammation | At lower doses possible increase of inflammation, bleeding from the GI, but no effects of ibuprofen in adults | |
| [28,29] | Inhalations with hypertonic saline and dornase alpha | Decrease of viscoelasticity of mucus, elimination of mucus | Time-consuming | |
| [28,29] | Physiotherapy | Better lung function | Time-consuming | |
| ARDS/ALI | ||||
| [30] | High-flow nasal cannula (HFNC) | high oxygenation, alveolar recruitment, increased secretion clearance, reduction of dead space | Not known | |
| [31] | Antibiotics in case of bacterial pneumonia | Elimination of one of the causes of ALI/ARDS | Development of multi-drug resistant pathogens | + |
| Lung cancer | ||||
| [32] | Radiotherapy | Decrease of pain, reduction of metastasis | Possible burns due to incidental irradiation of the surrounding tissue | |
| [33] | Chemotherapy | Reduction of the tumor, longer survival | Fatigue, dizziness, increased risk of infections, anemia, bleedings, diarrhea, nausea, weight loss, anxiety, smell, and taste disturbances, hair loss, etc. | + |
| [34] | Surgery | Physical elimination of the tumor | Surgical complications | + |
| [35] | Immunotherapy | Longer survival, fewer side effects compared to other treatment options | High cost | + |
| Cancer/Cancer Cell Line | Study/ Number of Participants | Lactoferrin | Effect/Mechanism | Reference |
|---|---|---|---|---|
| non-small cell lung cancer (NSCLC) | Clinical study (phase II)/n = 110 | Talactoferrin (TLF)—recombinant human LF combined with carboplatin/paclitaxel | Improved patient survival | [193] |
| Clinical study (phase II)/n = 100 | TLF | Improved patient survival | [194] | |
| Clinical study (phase III)/n = 742 | TLF | No improvement | [196] | |
| lung adenocarcinoma cell line—A549 | In vitro | bLF | Reduced proliferation by a decrease in VEGF expression | [197] |
| LF (iron saturated) | Inhibition of cancer cell viability, migration, and apoptosis induction | [199] | ||
| recombinant human LF (rhLF) | Inhibition of cell growth and migration; cell cycle arrest and induction of apoptosis | [200] | ||
| recombinant human LF (rhLF) in combination with etoposide | Repressed cancer cell growth by cell cycle arrest and induction of apoptosis Reduction by 10-fold the etoposide dose by rhLF to achieve the same anticancer effect | [200] | ||
| lung adenocarcinoma cells—PC-14 | In vitro | bLF | bLF formed a complex with immunoglobulin (CD79A) binding protein 1 (IGBP1), which interacted with the catalytic subunit of protein phosphatase 2A to promote cell apoptosis | [198] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaczyńska, K.; Jampolska, M.; Wojciechowski, P.; Sulejczak, D.; Andrzejewski, K.; Zając, D. Potential of Lactoferrin in the Treatment of Lung Diseases. Pharmaceuticals 2023, 16, 192. https://doi.org/10.3390/ph16020192
Kaczyńska K, Jampolska M, Wojciechowski P, Sulejczak D, Andrzejewski K, Zając D. Potential of Lactoferrin in the Treatment of Lung Diseases. Pharmaceuticals. 2023; 16(2):192. https://doi.org/10.3390/ph16020192
Chicago/Turabian StyleKaczyńska, Katarzyna, Monika Jampolska, Piotr Wojciechowski, Dorota Sulejczak, Kryspin Andrzejewski, and Dominika Zając. 2023. "Potential of Lactoferrin in the Treatment of Lung Diseases" Pharmaceuticals 16, no. 2: 192. https://doi.org/10.3390/ph16020192
APA StyleKaczyńska, K., Jampolska, M., Wojciechowski, P., Sulejczak, D., Andrzejewski, K., & Zając, D. (2023). Potential of Lactoferrin in the Treatment of Lung Diseases. Pharmaceuticals, 16(2), 192. https://doi.org/10.3390/ph16020192