The Potential of Alternative Therapies and Vaccine Candidates against Helicobacter pylori
Abstract
:1. Introduction
2. Results
2.1. Literature Assessment
2.2. Summary of Studies on Alternative Therapies and Vaccines Conducted Prior to 2018
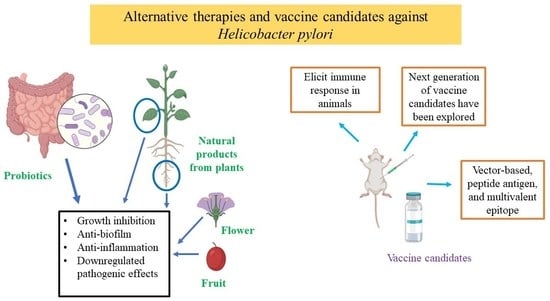
2.3. Antibacterial Activity of Probiotics against H. pylori
2.4. Antibacterial Activity of Natural Products from Plants and Nanoparticles against H. pylori
2.5. Progress on H. pylori Vaccine
3. Discussion
4. Materials and Methods
4.1. Literature Search
4.2. Inclusion and Exclusion Criteria
4.3. Data Extraction and Synthesis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Alexander, S.M.; Retnakumar, R.J.; Chouhan, D.; Devi, T.N.B.; Dharmaseelan, S.; Devadas, K.; Thapa, N.; Tamang, J.P.; Lamtha, S.C.; Chattopadhyay, S. Helicobacter pylori in human stomach: The inconsistencies in clinical outcomes and the probable causes. Front. Microbiol. 2021, 12, 713955. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Ford, A.C.; Yuan, Y.; Moayyedi, P. Helicobacter pylori eradication therapy to prevent gastric cancer: Systematic review and meta-analysis. Gut 2020, 69, 2113–2121. [Google Scholar] [CrossRef]
- Goderska, K.; Agudo Pena, S.; Alarcon, T. Helicobacter pylori treatment: Antibiotics or probiotics. Appl. Microbiol. Biotechnol. 2018, 102, 1–7. [Google Scholar] [CrossRef]
- Savoldi, A.; Carrara, E.; Graham, D.Y.; Conti, M.; Tacconelli, E. Prevalence of antibiotic resistance in Helicobacter pylori: A systematic review and meta-analysis in World Health Organization regions. Gastroenterology 2018, 155, 1372–1382.e17. [Google Scholar] [CrossRef] [Green Version]
- Tacconelli, E.; Carrara, E.; Savoldi, A.; Harbarth, S.; Mendelson, M.; Monnet, D.L.; Pulcini, C.; Kahlmeter, G.; Kluytmans, J.; Carmeli, Y.; et al. Discovery, research, and development of new antibiotics: The WHO priority list of antibiotic-resistant bacteria and tuberculosis. Lancet Infect. Dis. 2018, 18, 318–327. [Google Scholar] [CrossRef]
- Sukri, A.; Lopes, B.S.; Hanafiah, A. The emergence of multidrug-resistant Helicobacter pylori in Southeast Asia: A systematic review on the trends and intervention strategies using antimicrobial peptides. Antibiotics 2021, 10, 1061. [Google Scholar] [CrossRef]
- Chassagne, F.; Samarakoon, T.; Porras, G.; Lyles, J.T.; Dettweiler, M.; Marquez, L.; Salam, A.M.; Shabih, S.; Farrokhi, D.R.; Quave, C.L. A systematic review of plants with antibacterial activities: A taxonomic and phylogenetic perspective. Front. Pharmacol. 2021, 11, 586548. [Google Scholar] [CrossRef]
- Bagchi, T. Traditional food & modern lifestyle: Impact of probiotics. Indian J. Med. Res. 2014, 140, 333–335. [Google Scholar]
- Wang, L.; Hu, C.; Shao, L. The antimicrobial activity of nanoparticles: Present situation and prospects for the future. Int. J. Nanomed. 2017, 12, 1227–1249. [Google Scholar] [CrossRef] [Green Version]
- Zeng, M.; Mao, X.H.; Li, J.X.; Tong, W.D.; Wang, B.; Zhang, Y.J.; Guo, G.; Zhao, Z.J.; Li, L.; Wu, D.L.; et al. Efficacy, safety, and immunogenicity of an oral recombinant Helicobacter pylori vaccine in children in China: A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2015, 386, 1457–1464. [Google Scholar] [CrossRef]
- Rosania, R.; Minenna, M.F.; Giorgio, F.; Facciorusso, A.; De Francesco, V.; Hassan, C.; Panella, C.; Ierardi, E. Probiotic multistrain treatment may eradicate Helicobacter pylori from the stomach of dyspeptics: A placebo-controlled pilot study. Inflamm. Allergy Drug Targets 2012, 11, 244–249. [Google Scholar] [CrossRef]
- Saracino, I.M.; Pavoni, M.; Saccomanno, L.; Fiorini, G.; Pesci, V.; Foschi, C.; Piccirilli, G.; Bernardini, G.; Holton, J.; Figura, N.; et al. Antimicrobial efficacy of five probiotic strains against Helicobacter pylori. Antibiotics 2020, 9, 244. [Google Scholar] [CrossRef]
- Chen, Y.H.; Tsai, W.H.; Wu, H.Y.; Chen, C.Y.; Yeh, W.L.; Chen, Y.H.; Hsu, H.Y.; Chen, W.W.; Chen, Y.W.; Chang, W.W.; et al. Probiotic Lactobacillus spp. act against Helicobacter pylori-induced inflammation. J. Clin. Med. 2019, 8, 90. [Google Scholar] [CrossRef] [Green Version]
- Yuan, Z.; Xiao, S.; Li, S.; Suo, B.; Wang, Y.; Meng, L.; Liu, Z.; Yin, Z.; Xue, Y.; Zhou, L. The impact of Helicobacter pylori infection, eradication therapy, and probiotics intervention on gastric microbiota in young adults. Helicobacter 2021, 26, e12848. [Google Scholar] [CrossRef]
- Taghizadeh, S.; Falsafi, T.; Kermanshahi, R.K.; Ramezani, R. Antagonistic and Immunomodulant effects of two probiotic strains of Lactobacillus on clinical strains of Helicobacter pylori. Galen. Med. J. 2020, 9, e1794. [Google Scholar] [CrossRef]
- Yarmohammadi, M.; Yadegar, A.; Ebrahimi, M.T.; Zali, M.R. Effects of a potential probiotic strain Lactobacillus gasseri ATCC 33323 on Helicobacter pylori-induced inflammatory response and gene expression in coinfected gastric epithelial cells. Probiotics Antimicrob. Proteins 2021, 13, 751–764. [Google Scholar] [CrossRef]
- Lin, C.C.; Huang, W.C.; Su, C.H.; Lin, W.D.; Wu, W.T.; Yu, B.; Hsu, Y.M. Effects of multi-strain probiotics on immune responses and metabolic balance in Helicobacter pylori-infected mice. Nutrients 2020, 12, 2476. [Google Scholar] [CrossRef]
- Maleki-Kakelar, H.; Dehghani, J.; Barzegari, A.; Barar, J.; Shirmohamadi, M.; Sadeghi, J.; Omidi, Y. Lactobacillus plantarum induces apoptosis in gastric cancer cells via modulation of signaling pathways in Helicobacter pylori. BioImpacts 2020, 10, 65–72. [Google Scholar] [CrossRef] [Green Version]
- He, C.; Peng, C.; Xu, X.; Li, N.; Ouyang, Y.; Zhu, Y.; Lu, N. Probiotics mitigate Helicobacter pylori-induced gastric inflammation and premalignant lesions in INS-GAS mice with the modulation of gastrointestinal microbiota. Helicobacter 2022, 27, e12898. [Google Scholar] [CrossRef]
- Lai, C.H.; Lin, T.L.; Huang, M.Z.; Li, S.W.; Wu, H.Y.; Chiu, Y.F.; Yang, C.Y.; Chiu, C.H.; Lai, H.C. Gut commensal Parabacteroides goldsteinii MTS01 alters gut microbiota composition and reduces cholesterol to mitigate Helicobacter pylori-Induced Pathogenesis. Front. Immunol. 2022, 13, 916848. [Google Scholar] [CrossRef]
- Eftekhari, M.; Shams Ardekani, M.R.; Amin, M.; Mansourian, M.; Saeedi, M.; Akbarzadeh, T.; Khanavi, M. Anti-Helicobacter pylori compounds from Oliveria decumbens Vent. through urease inhibitory in-vitro and in-silico studies. Iran. J. Pharm. Res. 2021, 20, 476–489. [Google Scholar]
- Palacios-Espinosa, J.F.; Núñez-Aragón, P.N.; Gomez-Chang, E.; Linares, E.; Bye, R.; Romero, I. Anti-Helicobacter pylori activity of Artemisia ludoviciana subsp. mexicana and two of its bioactive components, estafiatin and eupatilin. Molecules 2021, 26, 3654. [Google Scholar] [CrossRef]
- Lee, H.A.; Hong, S.; Yoo, J.H.; Chung, Y.; Kim, O. Anti-Helicobacter pylori activity and inhibition of gastritis by Allium hookeri extract. Lab. Anim. Res. 2018, 34, 75–79. [Google Scholar] [CrossRef] [Green Version]
- Stenger Moura, F.C.; Cechinel-Filho, V.; Greco, F.A.; Venzon, L.; Meurer, M.C.; França, T.C.D.S.; Longo, B.; Somensi, L.B.; Mariano, L.N.B.; Cruz, A.B.; et al. Taxifolin and gastro-adhesive microparticles containing taxifolin promotes gastric healing in vivo, inhibits Helicobacter pylori in vitro and proton pump reversibly in silico. Chem. Biol. Interact. 2021, 339, 109445. [Google Scholar] [CrossRef]
- Lu, Q.; Li, C.; Wu, G. Insight into the inhibitory effects of Zanthoxylum nitidum against Helicobacter pylori urease and jack bean urease: Kinetics and mechanism. J. Ethnopharmacol. 2020, 249, 112419. [Google Scholar] [CrossRef]
- Ngnameko, C.R.; Marchetti, L.; Zambelli, B.; Quotadamo, A.; Roncarati, D.; Bertelli, D.; Njayou, F.N.; Smith, S.I.; Moundipa, P.F.; Costi, M.P.; et al. New insights into bioactive compounds from the medicinal plant Spathodea campanulata P. Beauv. and their activity against Helicobacter pylori. Antibiotics 2020, 9, 258. [Google Scholar]
- Park, H.S.; Wijerathne, C.U.B.; Jeong, H.Y.; Seo, C.S.; Ha, H.; Kwun, H.J. Gastroprotective effects of Hwanglyeonhaedok-tang against Helicobacter pylori-induced gastric cell injury. J. Ethnopharmacol. 2018, 216, 239–250. [Google Scholar] [CrossRef]
- Ngan, L.T.M.; Tan, M.T.; Hoang, N.V.M.; Thanh, D.T.; Linh, N.T.T.; Hoa, T.T.H.; Nuong, N.T.M.; Hieu, T.T. Antibacterial activity of Hibiscus rosa-sinensis L. red flower against antibiotic-resistant strains of Helicobacter pylori and identification of the flower constituents. Brazil. J. Med. Biol. Res. 2021, 54, e10889. [Google Scholar] [CrossRef]
- Park, B.; Lim, J.W.; Kim, H. Lycopene treatment inhibits activation of Jak1/Stat3 and Wnt/β-catenin signaling and attenuates hyperproliferation in gastric epithelial cells. Nutr. Res. 2019, 70, 70–81. [Google Scholar] [CrossRef]
- Salinas Ibáñez, Á.G.; Vallés, D.; Adaro, M.; Barberis, S.; Vega, A.E. Antimicrobial effect of a proteolytic enzyme from the fruits of Solanum granuloso-leprosum (Dunal) against Helicobacter pylori. Front. Nutr. 2021, 8, 699955. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.F.; Dai, J.F.; Meng, L.N.; Lu, B. Curcuma wenyujin Y. H. Chen et C. Ling n-butyl alcohol extract inhibits AGS cell Helicobacter pylori CagA+VacA+ promoted invasiveness by down-regulating caudal type homeobox transcription factor and claudin-2 expression. Chin. J. Integr. Med. 2020, 26, 122–129. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, S.L.; Zhang, J.Y.; Song, X.N.; Zhang, Z.Y.; Li, J.F.; Li, S. Anti-ulcer and anti-Helicobacter pylori potentials of the ethyl acetate fraction of Physalis alkekengi L. var. franchetii (Solanaceae) in rodent. J. Ethnopharmacol. 2018, 211, 197–206. [Google Scholar] [CrossRef]
- Fahmy, N.M.; Al-Sayed, E.; Michel, H.E.; El-Shazly, M.; Singab, A.N.B. Gastroprotective effects of Erythrina speciosa (Fabaceae) leaves cultivated in Egypt against ethanol-induced gastric ulcer in rats. J. Ethnopharmacol. 2020, 248, 112297. [Google Scholar] [CrossRef]
- Lee, Y.I.; Kim, J.S.; Cho, J.S.; Kim, H.K.; Hussain, A. Standardized combined plant extract, RUG-com, reduces bacterial levels and suppresses acute and chronic inflammation in Balb/c mice infected with CagA+ Helicobacter pylori. Prev. Nutr. Food Sci. 2019, 24, 426–433. [Google Scholar] [CrossRef] [PubMed]
- Jung, D.H.; Park, M.H.; Kim, C.J.; Lee, J.Y.; Keum, C.Y.; Kim, I.S.; Yun, C.H.; Kim, S.K.; Kim, W.H.; Lee, Y.C. Effect of β-caryophyllene from cloves extract on Helicobacter pylori eradication in mouse model. Nutrients 2020, 12, 1000. [Google Scholar] [CrossRef] [Green Version]
- Li, R.J.; Qin, C.; Huang, G.R.; Liao, L.J.; Mo, X.Q.; Huang, Y.Q. Phillygenin inhibits Helicobacter pylori by preventing biofilm formation and inducing ATP leakage. Front. Microbiol. 2022, 13, 863624. [Google Scholar] [CrossRef] [PubMed]
- Eftekhari, M.; Shams Ardekani, M.R.; Amin, M.; Attar, F.; Akbarzadeh, T.; Safavi, M.; Karimpour-Razkenari, E.; Amini, M.; Isman, M.; Khanavi, M. Oliveria decumbens, a bioactive essential oil: Chemical composition and biological activities. Iran. J. Pharm. Res. 2019, 18, 412–421. [Google Scholar]
- Brito, S.A.; de Almeida, C.L.F.; de Santana, T.I.; da Silva Oliveira, A.R.; do Nascimento Figueiredo, J.C.B.; Souza, I.T.; de Almeida, L.L.; da Silva, M.V.; Borges, A.S.; de Medeiros, J.W.; et al. Antiulcer activity and potential mechanism of action of the leaves of Spondias mombin L. Oxid. Med. Cell Longev. 2018, 2018, 1731459. [Google Scholar] [CrossRef] [Green Version]
- Prazeres, L.D.K.T.; Aragão, T.P.; Brito, S.A.; Almeida, C.L.F.; Silva, A.D.; de Paula, M.M.F.; Farias, J.S.; Vieira, L.D.; Damasceno, B.P.G.L.; Rolim, L.A.; et al. Antioxidant and antiulcerogenic activity of the dry extract of pods of Libidibia ferrea Mart. ex Tul. (Fabaceae). Oxid. Med. Cell Longev. 2019, 2019, 1983137. [Google Scholar] [CrossRef] [Green Version]
- Wylie, M.R.; Windham, I.H.; Blum, F.C.; Wu, H.; Merrell, D.S. In vitro antibacterial activity of nimbolide against Helicobacter pylori. J. Ethnopharmacol. 2022, 285, 114828. [Google Scholar] [CrossRef] [PubMed]
- Peng, C.; Sang, S.; Shen, X.; Zhang, W.; Yan, J.; Chen, P.; Jiang, C.; Yuan, Y.; Zhu, W.; Yao, M. In vitro anti-Helicobacter pylori activity of Syzygium aromaticum and the preliminary mechanism of action. J. Ethnopharmacol. 2022, 288, 114995. [Google Scholar] [CrossRef]
- Sabry, M.M.; El-Fishawy, A.M.; El-Rashedy, A.A.; El Gedaily, R.A. Phytochemical investigation of Cordia africana Lam. stem bark: Molecular simulation approach. Molecules 2022, 27, 4039. [Google Scholar] [CrossRef]
- Almeida, G.V.B.; Arunachalam, K.; Balogun, S.O.; Pavan, E.; Ascêncio, S.D.; Soares, I.M.; Zanatta, A.C.; Vilegas, W.; Macho, A.; Oliveira Martins, D.T. Chemical characterization and evaluation of gastric antiulcer properties of the hydroethanolic extract of the stem bark of Virola elongata (Benth.) Warb. J. Ethnopharmacol. 2019, 231, 113–124. [Google Scholar] [CrossRef]
- So, H.M.; Yu, J.S.; Khan, Z.; Subedi, L.; Ko, Y.J.; Lee, I.K.; Park, W.S.; Chung, S.J.; Ahn, M.J.; Kim, S.Y.; et al. Chemical constituents of the root bark of Ulmus davidiana var. japonica and their potential biological activities. Bioorg. Chem. 2019, 91, 103145. [Google Scholar]
- Sreeja, P.S.; Arunachalam, K.; Saikumar, S.; Kasipandi, M.; Dhivya, S.; Murugan, R.; Parimelazhagan, T. Gastroprotective effect and mode of action of methanol extract of Sphenodesme involucrata var. paniculata (C.B. Clarke) Munir (Lamiaceae) leaves on experimental gastric ulcer models. Biomed. Pharmacother. 2018, 97, 1109–1118. [Google Scholar]
- de Cássia Dos Santos, R.; Bonamin, F.; Périco, L.L.; Rodrigues, V.P.; Zanatta, A.C.; Rodrigues, C.M.; Sannomiya, M.; Dos Santos Ramos, M.A.; Bonifácio, B.V.; Bauab, T.M.; et al. Juss partitions promote gastroprotection against peptic ulcers and improve healing through antioxidant and anti-inflammatory activities. Biomed. Pharmacother. 2019, 111, 1112–1123. [Google Scholar]
- Abdel-Baki, P.M.; El-Sherei, M.M.; Khaleel, A.E.; Abdel-Aziz, M.M.; Okba, M.M. Irigenin, a novel lead from Iris confusa for management of Helicobacter pylori infection with selective COX-2 and HpIMPDH inhibitory potential. Sci. Rep. 2022, 12, 11457. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.S.; Lim, J.W.; Kim, H. Korean red ginseng extract inhibits IL-8 expression via Nrf2 activation in Helicobacter pylori-infected gastric epithelial cells. Nutrients 2022, 14, 1044. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Mumtaz, S.; Li, C.H.; Hussain, I.; Rotello, V.M. Combatting antibiotic-resistant bacteria using nanomaterials. Chem. Soc. Rev. 2019, 48, 415–427. [Google Scholar] [CrossRef] [PubMed]
- Gopalakrishnan, V.; Masanam, E.; Ramkumar, V.S.; Baskaraligam, V.; Selvaraj, G. Influence of N-acylhomoserine lactonase silver nanoparticles on the quorum sensing system of Helicobacter pylori: A potential strategy to combat biofilm formation. J. Basic Microbiol. 2020, 60, 207–215. [Google Scholar] [CrossRef]
- Cen, Q.; Gao, T.; Ren, Y.; Lu, X.; Lei, H. Immune evaluation of a Saccharomyces cerevisiae-based oral vaccine against Helicobacter pylori in mice. Helicobacter 2021, 26, e12772. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Ma, J.; Ji, Q.; Liu, Q. Evaluation of an attenuated Listeria monocytogenes as a vaccine vector to control Helicobacter pylori infection. Immunol. Lett. 2021, 238, 68–74. [Google Scholar] [CrossRef] [PubMed]
- Xie, W.; Zhao, W.; Zou, Z.; Kong, L.; Yang, L. Oral multivalent epitope vaccine, based on UreB, HpaA, CAT, and LTB, for prevention and treatment of Helicobacter pylori infection in C57BL/6 mice. Helicobacter 2021, 26, e12807. [Google Scholar] [CrossRef]
- Liu, Q.; Li, X.; Zhang, Y.; Song, Z.; Li, R.; Ruan, H.; Huang, X. Orally-administered outer-membrane vesicles from Helicobacter pylori reduce H. pylori infection via Th2-biased immune responses in mice. Pathog. Dis. 2019, 77, ftz050. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peng, X.; Zhang, R.; Duan, G.; Wang, C.; Sun, N.; Zhang, L.; Chen, S.; Fan, Q.; Xi, Y. Production and delivery of Helicobacter pylori NapA in Lactococcus lactis and its protective efficacy and immune modulatory activity. Sci. Rep. 2018, 8, 6435. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Espinosa-Ramos, D.; Caballero-Hernández, D.; Gomez-Flores, R.; Trejo-Chávez, A.; Pérez-Limón, L.J.; de la Garza-Ramos, M.A.; Tamez-Guerra, R.; Tamez-Guerra, P.; Rodriguez-Padilla, C. Immunization with a synthetic Helicobacter pylori peptide induces secretory IgA antibodies and protects mice against infection. Can. J. Infect. Dis. Med. Microbiol. 2019, 2019, 8595487. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pan, X.; Ke, H.; Niu, X.; Li, S.; Lv, J.; Pan, L. Protection against Helicobacter pylori infection in BALB/c mouse model by oral administration of multivalent epitope-based vaccine of cholera toxin b subunit-HUUC. Front. Immunol. 2018, 9, 1003. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramirez, J.; Guarner, F.; Bustos Fernandez, L.; Maruy, A.; Sdepanian, V.L.; Cohen, H. Antibiotics as major disruptors of gut microbiota. Front. Cell Infect. Microbiol. 2020, 10, 572912. [Google Scholar] [CrossRef]
- Srinarong, C.; Siramolpiwat, S.; Wongcha-um, A.; Mahachai, V.; Vilaichone, R.K. Improved eradication rate of standard triple therapy by adding bismuth and probiotic supplement for Helicobacter pylori treatment in Thailand. Asian Pac. J. Cancer Prev. 2014, 15, 9909–9913. [Google Scholar] [CrossRef] [Green Version]
- Tongtawee, T.; Dechsukhum, C.; Leeanansaksiri, W.; Kaewpitoon, S.; Kaewpitoon, N.; Loyd, R.A.; Matrakool, L.; Panpimanmas, S. Effect of pretreatment with Lactobacillus delbrueckii and Streptococcus thermophillus on tailored triple therapy for Helicobacter pylori eradication: A prospective randomized controlled clinical trial. Asian Pac. J. Cancer Prev. 2015, 16, 4885–4890. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alemayehu, C.; Mitchell, G.; Nikles, J. Barriers for conducting clinical trials in developing countries—A systematic review. Int. J. Equity Health 2018, 17, 37. [Google Scholar] [CrossRef]
- Hooi, J.K.Y.; Lai, W.Y.; Ng, W.K.; Suen, M.M.Y.; Underwood, F.E.; Tanyingoh, D.; Malfertheiner, P.; Graham, D.Y.; Wong, V.W.S.; Wu, J.C.Y.; et al. Global prevalence of Helicobacter pylori infection: Systematic review and meta-analysis. Gastroenterology 2017, 153, 420–429. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lü, M.; Yu, S.; Deng, J.; Yan, Q.; Yang, C.; Xia, G.; Zhou, X. Efficacy of probiotic supplementation therapy for Helicobacter pylori eradication: A meta-analysis of randomized controlled trials. PLoS ONE 2016, 11, e0163743. [Google Scholar] [CrossRef] [Green Version]
- CLSI. Methods for Antimicrobial Dilution and Disk Susceptibility Testing of Infrequently Isolated or Fastidious Bacteria, 3rd ed.; CLSI guideline M45; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2016. [Google Scholar]
- Sharmin, S.; Rahaman, M.M.; Sarkar, C.; Atolani, O.; Islam, M.T.; Adeyemi, O.S. Nanoparticles as antimicrobial and antiviral agents: A literature-based perspective study. Heliyon 2021, 7, e06456. [Google Scholar] [CrossRef]
- Staroń, A.; Długosz, O. Antimicrobial properties of nanoparticles in the context of advantages and potential risks of their use. J. Environ. Sci. Health A Tox. Hazard. Subst. Environ. Eng. 2021, 56, 680–693. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L. Multi-epitope vaccines: A promising strategy against tumors and viral infections. Cell Mol. Immunol. 2018, 15, 182–184. [Google Scholar] [CrossRef] [Green Version]
- Yurina, V. Live bacterial vectors-a promising DNA vaccine delivery system. Med. Sci. 2018, 6, 27. [Google Scholar] [CrossRef] [Green Version]
- Larussa, T.; Leone, I.; Suraci, E.; Imeneo, M.; Luzza, F. Helicobacter pylori and T helper cells: Mechanisms of immune escape and tolerance. J. Immunol. Res. 2015, 2015, 981328. [Google Scholar] [CrossRef] [Green Version]

| Authors | Type of Study | Name of Bacteria (Probiotics) | Results |
|---|---|---|---|
| Saracino et al. (2020) [13] | Preclinical | L. casei, L. paracasei, L. acidophilus, B. lactis, and S. thermophilus | Growth inhibition of H. pylori. |
| Chen et al. (2019) [14] | Preclinical | L. rhamnosus and L. acidophilus | Inhibited growth, adhesion, and invasion of H. pylori; reduced H. pylori-induced inflammation (decreased NF-κB activity and IL-8 secretion); downregulated phosphorylation and translocation of CagA; reshaped gut microbiota. |
| Yuan et al. (2021) [15] | Clinical | Probiotics therapy (Bifidobacterium tetravaccine tablets) included B. infantis > 0.5 × 106 CFU/tablet, L. acidophilus > 0.5 × 106 CFU/tablet, E. faecalis > 0.5 × 106 CFU/tablet, B. cereus > 0.5 × 105 CFU/tablet) | Upregulated pathogenic bacteria in gut microbiota after administration of probiotics. |
| Taghizadeh et al. (2020) [16] | Preclinical | L. acidophilus ATCC4356 and L. rhamnosus PTCC1607 | Inhibited bacterial growth and adhesion; stimulated IFN-G. |
| Yarmohammadi et al. (2021) [17] | Preclinical | L. gasseri ATCC 33323 | Downregulated the expression of IL-8 and Bcl2. |
| Lin et al. (2020) [18] | Preclinical | L. fermentum P2 (P2), L. casei L21 (L21), L. rhamnosus JB3 (JB3), or a mixture including the aforementioned three (multi-LAB) for 3 days | Modulated metabolites important in immune response. |
| Maleki-Kakelar et al. (2020) [19] | Preclinical | L. plantarum | Increased cell apoptosis. |
| He et al. (2022) [20] | Preclinical | L. salivarius and L. rhamnosus | Anti-inflammation (downregulated proinflammatory signaling pathways that included NF-κB, TNF, and IL-17; increased the abundance of beneficial bacteria in gut microbiota. |
| Lai et al. (2022) [21] | Preclinical | Parabacteroides goldsteinii MTS01 | Downregulated inflammation through downregulation of COX-2, IL-1β, and TNF-α; decreased pathogenic effect of H. pylori virulence factors. |
| Authors | Name of Vaccine | Type of Vaccine | Vaccine Delivery | Target | Model Used | Type of Immune Response Elicited |
|---|---|---|---|---|---|---|
| Cen et al. (2021) [52] | Saccharomyces cerevisiae-based oral vaccine EBY100/pYD1-UreB, EBY100/pYD1-VacA, or EBY100/pYD1-UreB + EBY100/pYD1-VacA | Vector-based | Oral | Urease and VacA | Mice | Humoral and mucosal immune response. |
| Wang et al. (2021) [53] | L. monocytogenes-based vaccine, a multi-epitope chimeric antigen (MECU) containing multiple B cell epitopes | Vector-based | Oral | 5 B-cell epitopes from FlaA, AlpB, SabA, and HpaA | Mice | Elicited high levels of IFN-γ, IL-4, and IL-17 in splenic lymphocytes; increased IgA and IgG. |
| Xie et al. (2021) [54] | Oral multivalent epitope vaccine | Multivalent epitope | Oral | Three Th cell epitopes and five against B cells | Mice | Increased IFN-γ, IL-4, and IL-17 in lymphocyte supernatants to activate Th1, Th2, and Th17 mixed T-cell immune responses; increased IgA and IgG. |
| Liu et al. (2019) [55] | Outer-membrane vesicles (OMVs) derived from gerbil-adapted H. pylori strain 7.13 | Outer membrane vesicle | Oral | Membrane proteins of H. pylori | Mice | Th2-biased immunity; increased IgA and IgG. |
| Peng et al. (2018) [56] | Neutrophil-activating protein A subunit (NapA) and L. lactis as vector | Vector-based | Oral | Neutrophil-activating protein A subunit | Mice | Polarized Th17 and Th1 responses; increased IgA and IgG. |
| Espinosa-Ramos et al. (2019) [57] | H. pylori 50–52 kDa immunogen-derived peptide antigen with the sequence Met–Val–Thr–Leu–Ile–Asn–Asn–Glu (MVTLINNE) | Peptide antigen | Subcutaneous | Immunogen synthetic peptide | Mice | Induced thymus lymphocytes and significantly induced IL-6. |
| Pan et al. (2018) [58] | Multivalent epitope-based vaccine cholera toxin B subunit (CTB)-HUUC with the intramucosal adjuvant CTB and tandem copies of B-cell epitopes | Multivalent epitope | Oral | 3 B-cell and 9 T-cell epitopes | Mice | H. pylori-specific lymphocyte responses and a mixed CD4+ T-cell immune response; increased IgA and IgG. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sukri, A.; Hanafiah, A.; Patil, S.; Lopes, B.S. The Potential of Alternative Therapies and Vaccine Candidates against Helicobacter pylori. Pharmaceuticals 2023, 16, 552. https://doi.org/10.3390/ph16040552
Sukri A, Hanafiah A, Patil S, Lopes BS. The Potential of Alternative Therapies and Vaccine Candidates against Helicobacter pylori. Pharmaceuticals. 2023; 16(4):552. https://doi.org/10.3390/ph16040552
Chicago/Turabian StyleSukri, Asif, Alfizah Hanafiah, Sandip Patil, and Bruno S. Lopes. 2023. "The Potential of Alternative Therapies and Vaccine Candidates against Helicobacter pylori" Pharmaceuticals 16, no. 4: 552. https://doi.org/10.3390/ph16040552
APA StyleSukri, A., Hanafiah, A., Patil, S., & Lopes, B. S. (2023). The Potential of Alternative Therapies and Vaccine Candidates against Helicobacter pylori. Pharmaceuticals, 16(4), 552. https://doi.org/10.3390/ph16040552