Natural Products Targeting PI3K/AKT in Myocardial Ischemic Reperfusion Injury: A Scoping Review
Abstract
:1. Introduction
2. Results
2.1. Literature Search
2.2. MIRI Models Used
2.3. Source of Plant
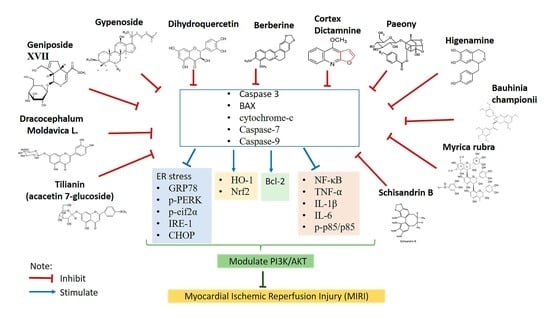
2.4. Effects of Natural Products in MIRI via PI3K/AKT Signaling Pathway
2.4.1. Gypenoside
2.4.2. Gypenoside XVII
2.4.3. Geniposide
2.4.4. Berberine
2.4.5. Dihydroquercetin
2.4.6. Cortex Dictamni
2.4.7. Dracocephalum moldavica
2.4.8. Tilianin
2.4.9. Schisandrin B
2.4.10. Higenamine
2.4.11. Myrica rubra
2.4.12. Total Paeony Glycoside
2.4.13. Bauhinia championii Benth
2.5. Techniques to Detect PI3K/Akt Activation and Its Related Mechanism
2.6. PI3K Inhibitor
3. Discussion
4. Materials and Methods
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brown, J.C.; Gerhardt, T.E.; Kwon, E. Risk Factors for Coronary Artery Disease; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Li, X.; Liu, M.; Sun, R.; Zeng, Y.; Chen, S.; Zhang, P. Protective Approaches against Myocardial Ischemia Reperfusion Injury. Exp. Ther. Med. 2016, 12, 3823–3829. [Google Scholar] [CrossRef]
- Garcia-Dorado, D.; Rodríguez-Sinovas, A.; Ruiz-Meana, M.; Inserte, J. Protection Against Myocardial Ischemia-Reperfusion Injury in Clinical Practice. Rev. Española Cardiol. (Engl. Ed.) 2014, 67, 394–404. [Google Scholar] [CrossRef]
- Dohmen, C.; Bosche, B.; Graf, R.; Reithmeier, T.; Ernestus, R.I.; Brinker, G.; Sobesky, J.; Heiss, W.D. Identification and Clinical Impact of Impaired Cerebrovascular Autoregulation in Patients With Malignant Middle Cerebral Artery Infarction. Stroke 2007, 38, 56–61. [Google Scholar] [CrossRef] [PubMed]
- Malek, M.; Nematbakhsh, M. Renal Ischemia/Reperfusion Injury; from Pathophysiology to Treatment. J. Ren. Inj. Prev. 2015, 4, 20. [Google Scholar] [CrossRef]
- Cowled, P.; Fitridge, R. Pathophysiology of Reperfusion Injury. In Mechanisms of Vascular Disease: A Reference Book for Vascular Specialists; University of Adelaide Press: Adelaide, Australia, 2011; pp. 331–350. [Google Scholar] [CrossRef]
- Heusch, G. The Coronary Circulation as a Target of Cardioprotection. Circ. Res. 2016, 118, 1643–1658. [Google Scholar] [CrossRef]
- Abd Rashid, N.; Mohammed, S.N.F.; Syed Abd Halim, S.A.; Abd Ghafar, N.; Abdul Jalil, N.A. Therapeutic Potential of Honey and Propolis on Ocular Disease. Pharmaceuticals 2022, 15, 1419. [Google Scholar] [CrossRef] [PubMed]
- Adib Ridzuan, N.; Teoh, S.; Abdul Rashid, N.; Othman, F.; Baharum, S.; Hussan, F. Polygonum Minus Ethanolic Extracts Attenuate Cisplatin—Induced Oxidative Stress in the Cerebral Cortex of Rats via Its Antioxidant Properties. Asian Pac. J. Trop. Biomed. 2019, 9, 196–203. [Google Scholar] [CrossRef]
- Abd Rashid, N.; Hussan, F.; Hamid, A.; Adib Ridzuan, N.R.; Teoh, S.L.; Budin, S.B. Preventive Effects of Polygonum Minus Essential Oil on Cisplatin-Induced Hepatotoxicity in Sprague Dawley Rats. Sains Malays. 2019, 48, 1975–1988. [Google Scholar] [CrossRef]
- Abd Rashid, N.; Abd Halim, S.A.S.; Teoh, S.L.; Budin, S.B.; Hussan, F.; Adib Ridzuan, N.R.; Abdul Jalil, N.A. The Role of Natural Antioxidants in Cisplatin-Induced Hepatotoxicity. Biomed. Pharmacother. 2021, 144, 112328. [Google Scholar] [CrossRef]
- Rashid, N.A.; Hussan, F.; Hamid, A.; Ridzuan, N.R.A.; Halim, S.A.S.A.; Jalil, N.A.A.; Najib, N.H.M.; Teoh, S.L.; Budin, S.B. Polygonum Minus Essential Oil Modulates Cisplatin-Induced Hepatotoxicity through Inflammatory and Apoptotic Pathways. EXCLI J. 2020, 19, 1246–1265. [Google Scholar] [CrossRef]
- Li, L.; Zhou, Y.; Li, Y.; Wang, L.; Sun, L.; Zhou, L.; Arai, H.; Qi, Y.; Xu, Y. Aqueous Extract of Cortex Dictamni Protects H9c2 Cardiomyocytes from Hypoxia/Reoxygenation-Induced Oxidative Stress and Apoptosis by PI3K/Akt Signaling Pathway. Biomed. Pharmacother. 2017, 89, 233–244. [Google Scholar] [CrossRef]
- Yu, Y.; Wang, M.; Chen, R.; Sun, X.; Sun, G.; Sun, X. Gypenoside XVII Protects against Myocardial Ischemia and Reperfusion Injury by Inhibiting ER Stress–Induced Mitochondrial Injury. J. Ginseng Res. 2019, 45, 642–653. [Google Scholar] [CrossRef]
- Zhu, Q.W.; Li, Y.G. Berberine Attenuates Myocardial Ischemia Reperfusion Injury by Suppressing the Activation of PI3K/AKT Signaling. Exp. Ther. Med. 2016, 11, 978–984. [Google Scholar] [CrossRef]
- Guo, X.; Cao, W.; Yao, J.; Yuan, Y.; Hong, Y.; Wang, X.; Xing, J. Cardioprotective Effects of Tilianin in Rat Myocardial Ischemia-Reperfusion Injury. Mol. Med. Rep. 2015, 11, 2227–2233. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.-H.; Bu, R.; Wang, Y.-W.; Hu, Y.-C.; Wang, X.-M.; Dong, X.; Zu, W.; Niu, Y.; Zhao, P.-W.; Sun, P.; et al. Validation of Efficacy and Mechanism of Sanwei-Tanxiang Powder in Improving Myocardial Ischemia Reperfusion Injuries. Sci. Rep. 2021, 11, 664. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Shi, X.; Sheng, K.; Han, G.; Li, W.; Zhao, Q.; Jiang, B.; Feng, J.; Li, J.; Gu, Y. PI3K/Akt Signaling Transduction Pathway, Erythropoiesis and Glycolysis in Hypoxia (Review). Mol. Med. Rep. 2019, 19, 783–791. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.X.; Pan, H.; Ouyang, D.Y.; He, X.H. The Critical Molecular Interconnections in Regulating Apoptosis and Autophagy. Ann. Med. 2015, 47, 305–315. [Google Scholar] [CrossRef]
- Franke, T.F.; Hornik, C.P.; Segev, L.; Shostak, G.A.; Sugimoto, C. PI3K/Akt and Apoptosis: Size Matters. Oncogene 2003, 22, 8983–8998. [Google Scholar] [CrossRef]
- Yu, D.; Xiong, J.; Gao, Y.; Li, J.; Zhu, D.; Shen, X.; Sun, L.; Wang, X. Resveratrol Activates PI3K/AKT to Reduce Myocardial Cell Apoptosis and Mitochondrial Oxidative Damage Caused by Myocardial Ischemia/Reperfusion Injury. Acta Histochem. 2021, 123, 151739. [Google Scholar] [CrossRef]
- Zeng, C.; Jiang, W.; Zheng, R.; He, C.; Li, J.; Xing, J. Cardioprotection of Tilianin Ameliorates Myocardial Ischemia-Reperfusion Injury: Role of the Apoptotic Signaling Pathway. PLoS ONE 2018, 13, e0193845. [Google Scholar] [CrossRef]
- Zeng, C.; Jiang, W.; Yang, X.; He, C.; Wang, W.; Xing, J. Pretreatment with Total Flavonoid Extract from Dracocephalum moldavica, L. Attenuates Ischemia Reperfusion-Induced Apoptosis. Sci. Rep. 2018, 8, 17491. [Google Scholar] [CrossRef]
- Zhao, X.; Xiang, Y.; Cai, C.; Zhou, A.; Zhu, N.; Zeng, C. Schisandrin B Protects against Myocardial Ischemia/Reperfusion Injury via the PI3K/Akt Pathway in Rats. Mol. Med. Rep. 2018, 17, 556–561. [Google Scholar] [CrossRef]
- Jiang, Y.-Q.; Chang, G.; Wang, Y.; Zhang, D.-Y.; Cao, L.; Liu, J. Geniposide Prevents Hypoxia/Reoxygenation-Induced Apoptosis in H9c2 Cells: Improvement of Mitochondrial Dysfunction and Activation of GLP-1R and the PI3K/AKT Signaling Pathway. Cell. Physiol. Biochem. 2016, 39, 407–421. [Google Scholar] [CrossRef]
- Wang, M.; Liu, Y.; Pan, R.L.; Wang, R.Y.; Ding, S.L.; Dong, W.R.; Sun, G.B.; Ye, J.X.; Sun, X.B. Protective Effects of Myrica Rubra Flavonoids against Hypoxia/Reoxygenation-Induced Cardiomyocyte Injury via the Regulation of the PI3K/Akt/GSK3β Pathway. Int. J. Mol. Med. 2019, 43, 2133–2143. [Google Scholar] [CrossRef]
- Shen, P.; Chen, J.; Pan, M. The Protective Effects of Total Paeony Glycoside on Ischemia/Reperfusion Injury in H9C2 Cells via Inhibition of the PI3K/Akt Signaling Pathway. Mol. Med. Rep. 2018, 18, 3332–3340. [Google Scholar] [CrossRef]
- Liao, P.; Sun, G.; Zhang, C.; Wang, M.; Sun, Y.; Zhou, Y.; Sun, X.; Jian, J. Bauhinia Championii Flavone Attenuates Hypoxia-Reoxygenation Induced Apoptosis in H9c2 Cardiomyocytes by Improving Mitochondrial Dysfunction. Molecules 2016, 21, 1469. [Google Scholar] [CrossRef]
- Yu, H.; Zhang, H.; Zhao, W.; Guo, L.; Li, X.; Li, Y.; Zhang, X.; Sun, Y. Gypenoside Protects against Myocardial Ischemia-Reperfusion Injury by Inhibiting Cardiomyocytes Apoptosis via Inhibition of CHOP Pathway and Activation of PI3K/Akt Pathway In Vivo and In Vitro. Cell. Physiol. Biochem. 2016, 39, 123–136. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Ma, H.; Xue, Y.; Shi, H.; Ma, T.; Cui, X. Berberine Inhibits the Ischemia-Reperfusion Injury Induced Inflammatory Response and Apoptosis of Myocardial Cells through the Phosphoinositide 3-Kinase/RAC-α Serine/Threonine-Protein Kinase and Nuclear Factor-ΚB Signaling Pathways. Exp. Ther. Med. 2018, 15, 1225–1232. [Google Scholar] [CrossRef] [PubMed]
- Shu, Z.; Yang, Y.; Yang, L.; Jiang, H.; Yu, X.; Wang, Y. Cardioprotective Effects of Dihydroquercetin against Ischemia Reperfusion Injury by Inhibiting Oxidative Stress and Endoplasmic Reticulum Stress-Induced Apoptosis: Via the PI3K/Akt Pathway. Food Funct. 2019, 10, 213–215. [Google Scholar] [CrossRef]
- Wu, M.P.; Zhang, Y.S.; Zhou, Q.M.; Xiong, J.; Dong, Y.R.; Yan, C. Higenamine Protects Ischemia/Reperfusion Induced Cardiac Injury and Myocyte Apoptosis through Activation of Β2-AR/PI3K/AKT Signaling Pathway. Pharmacol. Res. 2016, 104, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Qin, R.; Zhang, J.; Li, C.; Zhang, X.; Xiong, A.; Huang, F.; Yin, Z.; Li, K.; Qin, W.; Chen, M.; et al. Protective Effects of Gypenosides against Fatty Liver Disease Induced by High Fat and Cholesterol Diet and Alcohol in Rats. Arch. Pharm. Res. 2012, 35, 1241–1250. [Google Scholar] [CrossRef]
- Zhang, G.-L.; Deng, J.-P.; Wang, B.-H.; Zhao, Z.-W.; Li, J.; Gao, L.; Liu, B.-L.; Xong, J.-R.; Guo, X.-D.; Yan, Z.-Q.; et al. Gypenosides Improve Cognitive Impairment Induced by Chronic Cerebral Hypoperfusion in Rats by Suppressing Oxidative Stress and Astrocytic Activation. Behav. Pharmacol. 2011, 22, 633–644. [Google Scholar] [CrossRef]
- Zhuang, Q.; Cheng, J.; Xia, J.; Ning, M.; Wu, S.; Shen, S.; Shi, Y.; Huang, D.; Dong, Z.; Wan, X. Gypenosides Prevent and Dissolve Cholesterol Gallstones by Modulating the Homeostasis of Cholesterol and Bile Acids. Front. Med. 2022, 9, 818144. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Zhang, H.; Luo, Y.; Zhang, J.; Wang, M.; Liao, P.; Cao, L.; Guo, P.; Sun, G.; Sun, X. Gypenoside XVII Prevents Atherosclerosis by Attenuating Endothelial Apoptosis and Oxidative Stress: Insight into the ERα-Mediated PI3K/Akt Pathway. Int. J. Mol. Sci. 2017, 18, 77. [Google Scholar] [CrossRef]
- Tie, G.; Yan, J.; Yang, Y.; Park, B.D.; Messina, J.A.; Raffai, R.L.; Nowicki, P.T.; Messina, L.M. Oxidized Low-Density Lipoprotein Induces Apoptosis in Endothelial Progenitor Cells by Inactivating the Phosphoinositide 3-Kinase/Akt Pathway. J. Vasc. Res. 2010, 47, 519–530. [Google Scholar] [CrossRef]
- Cui, G.; Qin, X.; Zhang, Y.; Gong, Z.; Ge, B.; Zang, Y.Q. Berberine Differentially Modulates the Activities of ERK, P38 MAPK, and JNK to Suppress Th17 and Th1 T Cell Differentiation in Type 1 Diabetic Mice. J. Biol. Chem. 2009, 284, 28420–28429. [Google Scholar] [CrossRef] [PubMed]
- Sunil, C.; Xu, B. An Insight into the Health-Promoting Effects of Taxifolin (Dihydroquercetin). Phytochemistry 2019, 166, 112066. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.M.; Ko, F.N.; Su, M.J.; Wu, T.S.; Wang, M.L.; Huang, T.F.; Teng, C.M. Vasorelaxing Effect in Rat Thoracic Aorta Caused by Fraxinellone and Dictamine Isolated from the Chinese Herb Dictamnus Dasycarpus Turcz: Comparison with Cromakalim and Ca2+ Channel Blockers. Naunyn. Schmiedebergs Arch. Pharmacol. 1992, 345, 349–355. [Google Scholar] [CrossRef]
- Chen, R.; Su, W.; Li, P.; Huo, L.; Lu, R.; Peng, M. Free Radical-Scavenging and Antioxidant Activity of Skin of Dictamnus Dasycarpus. Asian J. Chem. 2013, 25, 1753–1754. [Google Scholar] [CrossRef]
- Dastmalchi, K.; Damien Dorman, H.J.; Laakso, I.; Hiltunen, R. Chemical Composition and Antioxidative Activity of Moldavian Balm (Dracocephalum moldavica L.) Extracts. LWT—Food Sci. Technol. 2007, 40, 1655–1663. [Google Scholar] [CrossRef]
- García-Díaz, J.A.; Navarrete-Vázquez, G.; García-Jiménez, S.; Hidalgo-Figueroa, S.; Almanza-Pérez, J.C.; Alarcón-Aguilar, F.J.; Gómez-Zamudio, J.; Cruz, M.; Ibarra-Barajas, M.; Estrada-Soto, S. Antidiabetic, Antihyperlipidemic and Anti-Inflammatory Effects of Tilianin in Streptozotocin-Nicotinamide Diabetic Rats. Biomed. Pharmacother. 2016, 83, 667–675. [Google Scholar] [CrossRef]
- Panossian, A.; Wikman, G. Pharmacology of Schisandra Chinensis Bail.: An Overview of Russian Research and Uses in Medicine. J. Ethnopharmacol. 2008, 118, 183–212. [Google Scholar] [CrossRef] [PubMed]
- Chiu, P.Y.; Leung, H.Y.; Siu, A.H.L.; Poon, M.K.T.; Ko, K.M. Schisandrin B Decreases the Sensitivity of Mitochondria to Calcium Ion-Induced Permeability Transition and Protects against Ischemia-Reperfusion Injury in Rat Hearts. Acta Pharmacol. Sin. 2007, 28, 1559–1565. [Google Scholar] [CrossRef] [PubMed]
- Kosuge, T.; Yokota, M. Studies on Cardiac Principle of Aconite Root. Chem. Pharm. Bull. 1976, 24, 176–178. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.-Q.; Chen, H.-W.; Li, J.; Wu, Q.-J. Efficacy, Chemical Constituents, and Pharmacological Actions of Radix Paeoniae Rubra and Radix Paeoniae Alba. Front. Pharmacol. 2020, 11, 1054. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yan, G.; Sun, C.; Li, H.; Fu, Y.; Xu, W. Apoptosis Effects of Dihydrokaempferol Isolated from Bauhinia Championii on Synoviocytes. Evid.-Based Complement. Altern. Med. 2018, 2018, 9806160. [Google Scholar] [CrossRef]
- Bass, J.; Wilkinson, D.; Rankin, D.; Phillips, B.; Szewczyk, N.; Smith, K.; Atherton, P. An Overview of Technical Considerations for Western Blotting Applications to Physiological Research. Scand. J. Med. Sci. Sports 2017, 27, 4–25. [Google Scholar] [CrossRef]
- Bondonno, N.P.; Dalgaard, F.; Kyrø, C.; Murray, K.; Bondonno, C.P.; Lewis, J.R.; Croft, K.D.; Gislason, G.; Scalbert, A.; Cassidy, A.; et al. Flavonoid Intake Is Associated with Lower Mortality in the Danish Diet Cancer and Health Cohort. Nat. Commun. 2019, 10, 3651. [Google Scholar] [CrossRef]
- D’Arcy, M.S. Cell Death: A Review of the Major Forms of Apoptosis, Necrosis and Autophagy. Cell Biol. Int. 2019, 43, 582–592. [Google Scholar] [CrossRef] [PubMed]
- Tzifi, F.; Economopoulou, C.; Gourgiotis, D.; Ardavanis, A.; Papageorgiou, S.; Scorilas, A. The Role of BCL2 Family of Apoptosis Regulator Proteins in Acute and Chronic Leukemias. Adv. Hematol. 2012, 2012, 524308. [Google Scholar] [CrossRef]
- Wu, N.; Zhang, X.; Jia, P.; Jia, D. Hypercholesterolemia Aggravates Myocardial Ischemia Reperfusion Injury via Activating Endoplasmic Reticulum Stress-Mediated Apoptosis. Exp. Mol. Pathol. 2015, 99, 449–454. [Google Scholar] [CrossRef]
- Ahn, H.J.; Kim, K.I.; Kim, G.; Moon, E.; Yang, S.S.; Lee, J.S. Atmospheric-Pressure Plasma Jet Induces Apoptosis Involving Mitochondria via Generation of Free Radicals. PLoS ONE 2011, 6, e28154. [Google Scholar] [CrossRef]
- Du, J.K.; Cong, B.H.; Yu, Q.; Wang, H.; Wang, L.; Wang, C.N.; Tang, X.L.; Lu, J.Q.; Zhu, X.Y.; Ni, X. Upregulation of MicroRNA-22 Contributes to Myocardial Ischemia-Reperfusion Injury by Interfering with the Mitochondrial Function. Free Radic. Biol. Med. 2016, 96, 406–417. [Google Scholar] [CrossRef]
- Yuan, Y.; Guo, Q.; Ye, Z.; Pingping, X.; Wang, N.; Song, Z. Ischemic Postconditioning Protects Brain from Ischemia/Reperfusion Injury by Attenuating Endoplasmic Reticulum Stress-Induced Apoptosis through PI3K-Akt Pathway. Brain Res. 2011, 1367, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Lu, M.; Wang, P.; Chen, X. Trichostatin A Ameliorates Myocardial Ischemia/Reperfusion Injury Through Inhibition of Endoplasmic Reticulum Stress-Induced Apoptosis. Arch. Med. Res. 2012, 43, 190–196. [Google Scholar] [CrossRef] [PubMed]
- Choy, K.W.; Murugan, D.; Mustafa, M.R. Natural Products Targeting ER Stress Pathway for the Treatment of Cardiovascular Diseases. Pharmacol. Res. 2018, 132, 119–129. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Zhang, J.; Strom, J.; Lee, S.; Chen, Q.M. Myocardial Ischemic Reperfusion Induces de Novo Nrf2 Protein Translation. Biochim. Biophys. Acta—Mol. Basis Dis. 2014, 1842, 1638–1647. [Google Scholar] [CrossRef] [PubMed]
- Surh, Y.J.; Kundu, J.K.; Na, H.K. Nrf2 as a Master Redox Switch in Turning on the Cellular Signaling Involved in the Induction of Cytoprotective Genes by Some Chemopreventive Phytochemicals. Planta Med. 2008, 74, 1526–1539. [Google Scholar] [CrossRef]
- Eggler, A.L.; Gay, K.A.; Mesecar, A.D. Molecular Mechanisms of Natural Products in Chemoprevention: Induction of Cytoprotective Enzymes by Nrf2. Mol. Nutr. Food Res. 2008, 52, S84–S94. [Google Scholar] [CrossRef]
- Ighodaro, O.M.; Akinloye, O.A. First Line Defence Antioxidants-Superoxide Dismutase (SOD), Catalase (CAT) and Glutathione Peroxidase (GPX): Their Fundamental Role in the Entire Antioxidant Defence Grid. Alex. J. Med. 2018, 54, 287–293. [Google Scholar] [CrossRef]
- Ho, E.; Karimi Galougahi, K.; Liu, C.C.; Bhindi, R.; Figtree, G.A. Biological Markers of Oxidative Stress: Applications to Cardiovascular Research and Practice. Redox Biol. 2013, 1, 483–491. [Google Scholar] [CrossRef]
- Liu, Z.Z.; Kong, J.B.; Li, F.Z.; Ma, L.L.; Liu, S.Q.; Wang, L.X. Ischemic Postconditioning Decreases Matrix Metalloproteinase-2 Expression during Ischemiareperfusion of Myocardium in a Rabbit Model: A Preliminary Report. Exp. Clin. Cardiol. 2013, 18, 99. [Google Scholar]
- Shrimali, D.; Shanmugam, M.K.; Kumar, A.P.; Zhang, J.; Tan, B.K.H.; Ahn, K.S.; Sethi, G. Targeted Abrogation of Diverse Signal Transduction Cascades by Emodin for the Treatment of Inflammatory Disorders and Cancer. Cancer Lett. 2013, 341, 139–149. [Google Scholar] [CrossRef] [PubMed]
- Haupt, M.; Zechmeister, B.; Bosche, B.; Lieschke, S.; Zheng, X.; Zhang, L.; Venkataramani, V.; Jin, F.; Hein, K.; Weber, M.S.; et al. Lithium Enhances Post-Stroke Blood-Brain Barrier Integrity, Activates the MAPK/ERK1/2 Pathway and Alters Immune Cell Migration in Mice. Neuropharmacology 2020, 181, 108357. [Google Scholar] [CrossRef]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.J.; Horsley, T.; Weeks, L.; et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Explanation. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef]
- Zheng, Q.N.; Wei, X.H.; Pan, C.S.; Li, Q.; Liu, Y.Y.; Fan, J.Y.; Han, J.Y. QiShenYiQi Pills® Ameliorates Ischemia/Reperfusion-Induced Myocardial Fibrosis Involving RP S19-Mediated TGFβ1/Smads Signaling Pathway. Pharmacol. Res. 2019, 146, 104272. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.X.; Wang, P.; Wang, N.N.; Li, S.D.; Yang, M. hui Tongxinluo Ameliorates Myocardial Ischemia-Reperfusion Injury Mainly via Activating Parkin-Mediated Mitophagy and Downregulating Ubiquitin-Proteasome System. Chin. J. Integr. Med. 2021, 27, 542–550. [Google Scholar] [CrossRef]
- Li, X.D.; Yang, Y.J.; Geng, Y.J.; Jin, C.; Hu, F.A.; Zhao, J.L.; Zhang, H.T.; Cheng, Y.T.; Qian, H.Y.; Wang, L.L.; et al. Tongxinluo Reduces Myocardial No-Reflow and Ischemia-Reperfusion Injury by Stimulating the Phosphorylation of ENOS via the PKA Pathway. Am. J. Physiol. Heart Circ. Physiol. 2010, 299, H1255–H1261. [Google Scholar] [CrossRef] [PubMed]
- Davidson, S.M.; Ferdinandy, P.; Andreadou, I.; Bøtker, H.E.; Heusch, G.; Ibáñez, B.; Ovize, M.; Schulz, R.; Yellon, D.M.; Hausenloy, D.J.; et al. Multitarget Strategies to Reduce Myocardial Ischemia/Reperfusion Injury: JACC Review Topic of the Week. J. Am. Coll. Cardiol. 2019, 73, 89–99. [Google Scholar] [CrossRef] [PubMed]



| Study Characteristics | Count n (%) | |
|---|---|---|
| Year of publication | 2016 | 5 (35.7%) |
| 2017 | 3 (21.4%) | |
| 2018 | 3 (21.4%) | |
| 2019 | 3 (21.4%) | |
| Country of publication | China | 14 (100%) |
| Source of plant | Part of the plant, method of extraction and the solvents used were mentioned. | 5 (35.7%) |
| Processed plants were purchased. | 5 (35.7%) | |
| Not mentioned source of plant | 4 (28.6%) | |
| MIRI model used | In vitro | 5 (35.7%) |
| In vivo | 4 (28.6%) | |
| Mixed in vitro and ex vivo | 2 (14.3%) | |
| Mixed in vitro and in vivo | 2 (14.3%) | |
| In vivo, in vitro, ex vivo | 1 (7.1%) | |
| MIRI Model Used | Brief Explanation of Method to Inflict MIRI | Duration of Ischemic/Reperfusion (I/R) |
|---|---|---|
| In vitro | Hypoxia-reperfusion (H/R) injury was elicited in H9c2 cardiomyocytes. To induce hypoxia, the cells were incubated in an anaerobic box with or without the media changed to non-glucose DMEM. To mimic reperfusion, the cells were moved to the regular incubator with or without the media being replaced with high-glucose media. Duration of ischemia and reperfusion varies according to the studies. | 4 h of hypoxia, 24 h of reperfusion [29] |
| 6 h of hypoxia and 12 h of reoxygenation [14] | ||
| H/R time: 4/2, 6/3, 12/4, 14/5, 16/6, 22/10 h [25] | ||
| 6 h of ischemia, 16 h of reperfusion [31] | ||
| 8 h of hypoxia followed by 4 h of reoxygenation [13] | ||
| 6 h of ischemia, followed by reoxygenation [26] | ||
| 10 h of ischemia, followed by 2 h of reperfusion [27] | ||
| 6 h of hypoxia, followed by 12 h of reoxygenation [28] | ||
| In vivo | Left anterior descending coronary arteries (LAD) of MIRI rat model were ligated temporarily to induce ischemia, followed by a period of reperfusion by releasing the ligation. Duration of ischemia and reperfusion varies according to the studies. | LAD were reversibly occluded for 45 min, followed by 3 h of reperfusion [29] |
| LAD was occluded for 30 min followed by reperfusion for 4 h [15] | ||
| 45 min of ischemia followed by 4 h of reperfusion [22] | ||
| Ischemia for 45 min, followed by 12 h of reperfusion [23] | ||
| 45 min ischemia, followed by 24 h of reperfusion [24] | ||
| Ligation LAD for 30 min followed by 24 h of reperfusion [32]. | ||
| Cold ischemia was performed to induce ischemia followed by reperfusion. | Cold ischemia for 18 h, followed by 8 h of reperfusion [30] | |
| Ex vivo | Langendorff perfusion of rat hearts was used to induce global ischemia and reperfusion. | Global ischemia for 40 min and reperfusion for 60 min [14]. |
| 45 min of ischemia, 1 h reperfusion [31] | ||
| Ischemic state was induced for 30 min by no-flow followed by 30 min of reperfusion [32]. |
| Natural Product | Source | Dose and Duration | References |
|---|---|---|---|
| Gypenoside (GP) | Gynostema pentaphyllum (Thunb.) Makino | For in vivo Short term: GP 50, 100, 200 mg/kg 1 h before MIRI. Long term: GP 200 mg/kg 1 h before MIRI; 24 and 72 h post-MIRI. | [29] |
| For in vitro GP 5µM, 10µM, 20µM incubated before OGD/R | |||
| Gypenoside XVII (GP-17) | Antioxidant phytoestrogen of the gypenoside group. | For in vitro GP-17 1.25, 2.5, 5, 10, 20, 40, 100 µM incubated 24 h before H/R injury. | [14] |
| For ex vivo GP-17 5, 10, 20 µM were dissolved in Krebs–Henseleit (KH) buffer | |||
| Geniposide (glycoside) | An extract from Gardenia jasminoides J. Ellis | For in vitro Geniposide 2.5, 5, 10, 20, 40, 80, 160, 320 µM for 30 min before H/R | [25] |
| Berberine | An alkaloid derivative. The source of plant was not mentioned | For in vivo 10 mg/kg berberine OD for 30 days | [30] |
| For in vitro 10 mg/mL berberine for 24 h | |||
| Berberine | The source of plant was not mentioned | For in vivo Berberine 25, 50, 100 mg/kg for 14 days | [15] |
| Dihydroquercetin (DHQ) | Belongs to the flavanonol subclass in the flavonoids. The source of plant was not mentioned | For in vitro DHQ 2.5, 5, 10, 20, 40, 80 µM incubated for 12 h before H/R | [31] |
| Aqueous extract of Cortex Dictamni (AECD) | A species of Dictamnus dasycarpus Turcz. This plant was aqueously extracted. | For in vitro AECD 0.39, 1.56, 6.25, 25, 100 µg/mL were pretreated with the cells for 24 h. | [13] |
| Tilianin (is a flavonoid antioxidant) | Tilianin is a powerful flavonoid from dried Dracocephalum moldavica L. (DML). Its dried powder was extracted in aqueous ethanol. | For in vivo Tilianin 2.5, 5, 10 mg/kg for 14 days | [22] |
| Total flavonoid extract from Dracocephalum moldavica L. (TFDM) | The plant was extracted in 40% ethanol andHPLC was preformed | For in vivo TFDM 3, 10, 30 mg/kg for 2 weeks | [23] |
| Schisandrin B. (Sch B) | Natural monomer extracted from Schisandra chinensis (Turcz.) Baill. | For in vivo Sch. B 60 mg/kg for 15 days | [24] |
| Higenamine | An alkaloid derivative. The source of plant was not mentioned | For in vivo Higenamine 10 mg/kg 2 h before surgery | [32] |
| For in vitro Higenamine 30, 60, 120 µM | |||
| For ex vivo Higenamine 100µM | |||
| Myrica rubra flavonoids (MRF) | The bark of Myrica rubra was extracted with methanol using reflux extraction. Myrica rubra flavonoid was then chemically identified at the Institute of Medicinal Plant Development. | For in vitro MRF 6.25µg/mL was incubated for 12 h before H/R injury | [26] |
| Total paeony glycoside (TPG) | TPG powder was obtained from Haoxuan Biological Technology Co., Ltd. (Xi’an, China). | For in vitro TPG 10, 40, 160 µg/mL post I/R injury | [27] |
| Bauhinia championii flavone | Dried Bauhinia championii (Benth.) Benth. was extracted with ethanol. | For in vitro BCF 3.125 µg/mL | [28] |
| Natural Products | Regulation of PI3K/Akt Signaling | Administration of PI3K Inhibitor | Parameters Used to Measure Cardioprotective Mechanism of NPs on Cell Survival | References | ||||
|---|---|---|---|---|---|---|---|---|
| Apoptosis | Mitochondrial Injury/Cytochrome C/Caspase-3,9 | Bcl-2/Bax | Endoplasmic Reticulum Stress Marker | Oxidative Stress/Antioxidant | ||||
| Gypenoside (GP) | ↑p-Akt/Akt, p-GSK3β/GSK3β | √ | √ (TUNEL) | × | √ | √ | × | [29] |
| Gypenoside XVII (GP-17) | ↑p-PI3K/PI3K, p-Akt/Akt ratios | √ | √ (Annexin V/PI) | √ | √ | √ | √ | [14] |
| Geniposide (glycoside) | ↑p-Akt/Akt | √ | √ (Annexin-V/PI) | √ | √ | × | √ | [25] |
| Berberine | ↑expression of PI3K, Akt, p-Akt | √ | √ | √ | √ | × | × | [30] |
| Berberine | ↓p-Akt/Akt ratio ↓p-p85/p85 | × | × | × | × | × | × | [15] |
| Dihydroquercetin (DHQ) | ↑p-PI3K/PI3K and p-AKT/AKT ratios | √ | × | √ | √ | √ | √ | [31] |
| Aqueous extract of Cortex Dictamni (AECD) | ↑p-Akt, p-GSK-3β | √ | √ (TUNEL, Annexin/PI) | √ | √ | × | √ | [13] |
| Tilianin (is a flavonoid antioxidant) | ↑p-Akt, p-PI3K, p-Akt/Akt, p-PI3K/PI3K | √ | √ (TUNEL) | √ | √ | × | √ | [22] |
| Total flavonoid extract from Dracocephalum moldavica L. (TFDM) | ↑protein expression of p-PI3K, p-Akt ↑p-PI3K/PI3K, p-Akt/Akt ratios ↑p-GSK-3β, p-ERK 1/2 | √ | √ (TUNEL) | √ | √ | × | √ | [23] |
| Schisandrin B. (Sch B) | ↑p-Akt | √ | √ (TUNEL) | √ | √ | × | × | [24] |
| Higenamine | ↑p-Akt, p-Akt/Akt ratio | √ | √ | √ | × | × | × | [32] |
| Myrica rubra flavonoids (MRF) | ↑p-Akt/Akt, p-GSK-3β/GSK-3β | √ | √ (Annexin V/PI) | √ | √ | × | √ | [26] |
| Total paeony glycoside (TPG) | ↓p-PI3K, p-Akt | × | √ (Annexin V-FITC/PI) | √ | √ | × | √ | [27] |
| Bauhinia championii flavone | ↑p-Akt/Akt, p-PI3K | √ | √ (Annexin V-FITC/PI) | √ | √ | × | √ | [28] |
| Eligibility Criteria | Criteria |
|---|---|
| For title selection | 1. Title in English 2. Year of publication from January 2016 to January 2021 3. Title reflects on MIRI |
| For abstract selection | 1. Abstract reflects that the article is an original article. 2. Abstract provides evidence of a robust study design. 3. Abstract highlights at least one measurable outcome that reflects PI3K/Akt signaling pathway. 4. Abstract clearly mentions the usage of MIRI model. The abstract reflects the use of natural product as intervention. |
| For full text of article selection | 1. The article is available as a full-text article. 2.The article provides well-designed research methodology and/or intervention. 3. The article measures the outcome that related to PI3K/Akt. 4. The article uses natural product as an intervention. 5. The natural product used as intervention was not a combination of multiple natural products. The article explicitly explains the usage of MIRI model. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Syed Abd Halim, S.A.; Abd Rashid, N.; Woon, C.K.; Abdul Jalil, N.A. Natural Products Targeting PI3K/AKT in Myocardial Ischemic Reperfusion Injury: A Scoping Review. Pharmaceuticals 2023, 16, 739. https://doi.org/10.3390/ph16050739
Syed Abd Halim SA, Abd Rashid N, Woon CK, Abdul Jalil NA. Natural Products Targeting PI3K/AKT in Myocardial Ischemic Reperfusion Injury: A Scoping Review. Pharmaceuticals. 2023; 16(5):739. https://doi.org/10.3390/ph16050739
Chicago/Turabian StyleSyed Abd Halim, Syarifah Aisyah, Norhashima Abd Rashid, Choy Ker Woon, and Nahdia Afiifah Abdul Jalil. 2023. "Natural Products Targeting PI3K/AKT in Myocardial Ischemic Reperfusion Injury: A Scoping Review" Pharmaceuticals 16, no. 5: 739. https://doi.org/10.3390/ph16050739
APA StyleSyed Abd Halim, S. A., Abd Rashid, N., Woon, C. K., & Abdul Jalil, N. A. (2023). Natural Products Targeting PI3K/AKT in Myocardial Ischemic Reperfusion Injury: A Scoping Review. Pharmaceuticals, 16(5), 739. https://doi.org/10.3390/ph16050739