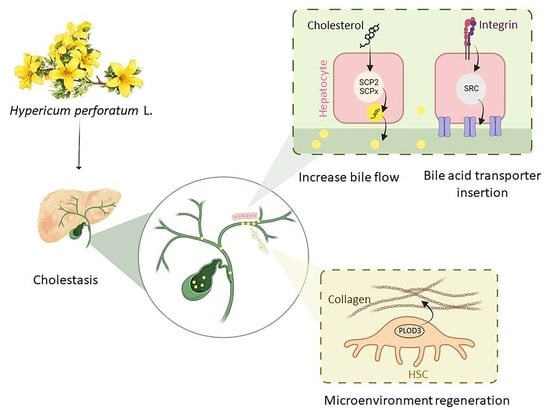
Hypericum perforatum L. and the Underlying Molecular Mechanisms for Its Choleretic, Cholagogue, and Regenerative Properties †
Abstract
:1. Introduction
2. Results and Discussion
2.1. Hypericum Medical Attributes and Its Bioactive Compounds
| Effect | Mechanism | Metabolite | Model of Study |
|---|---|---|---|
| Antidepression | NT * metabolism ↓, Regulation of NT receptors sensitivity, NT synaptic reuptake ↓ | Total extract [29] | In vivo animal model [29] |
| Antioxidative | Free radical scavenger | Total extract [55,56], Rutin, quercetin [57] | In vivo animal model [55,56] In vitro assay and ex vivo rat membrane model [57] |
| Anti-inflammatory | NF-κb ↓, GSH ↑, Ca influx ↓, IL-6 ↓, IL-12 ↓, TNF-α ↓ | Total extract [56], Pseudohypericin, Quercetin, Amentoflavone, Chlorogenic acid [58], Hyperforin [59] | In vivo animal model [56] RAW 264.7 macrophage cell line [58] A549 cells & in vivo animal model [59] |
| Antitumor | Apoptosis ↑ | Hyperforin, Hypericin [60] | In vivo animal model [60] |
| Hypolipidemic Cholesterol ↓ Triglycerides ↓ HDL ↑ | FAS ↓, ACC ↓, PPAR-α ↑ | Total extract [26,61] | In vivo animal model [26] 3T3L1 adipocyte [61] |
| HMG-CoA reductase ↓, Cholesterol excretion via secreted bile acids ↑ | Total extract [62,63,64] Rutin [65] | In vivo animal model [62,63,64,65] | |
| (LP1) ↓, (Dgat1) ↓ | Total extract, Rutin [61] | 3T3L1 adipocyte [61] | |
| (Apo-A1) ↑ | Total extract [66] | In vivo animal model [66] | |
| Hepatoprotective (hepatic steatosis ↓) ALT ↓, AST ↓ | PPAR-γ ↑ | Chlorogenic acid [67] | 3T3L1 preadipocyte [67] |
| AMPK ↑ | Hypericin [61,68], Rutin [69] | L02 and HepG2 cells & in vivo animal models [61,68], HepG2 cells [69] | |
| Free radical scavenger, Neutrophil activation | Quercetin [70], Hyperoside [71] | In vivo animal model [70,71] |
2.1.1. Mechanisms of Antidepressant Activity of H. perforatum
2.1.2. Hepatoprotective and Anti-Atherogenic Activity of Hypericum
2.2. Analysis of Differentially Expressed Genes (DEGs) upon Treatment of HepG2 Cells with H. perforatum Crude Extract
2.3. Identification of Target Genes
2.4. Protein–Protein Interaction (PPI) Network of DEGs
- Epidermal growth factor (EGF)
- Intercellular Adhesion Molecule-1 (ICAM-1)
- Integrin-linked kinase (ILK)
- Secreted phosphoprotein 1 (SPP1)
- Procollagen-Lysine,2-Oxoglutarate 5-Dioxygenase 3 (PLOD3)
- Catalase (CAT)
- Bcl-2-Associated X-protein (BAX)
- Chemokine (C-X-C motif) ligand 2 (CXCL2)
- Enhancer of zeste homolog 2 (EZH2)
- Sterol Carrier Protein 2 (SCP2)
- Cyclin-dependent kinase 6 (CDK6)
- Superkiller Viralicidic Activity 2-Like (SKIV2L)
- NISCH
- Conserved oligomeric Golgi (COG 4,7)
2.5. Interacting Network of Hypericum Metabolites and Their Target Genes
3. Materials and Methods
3.1. Hypericum Bioactive Components and Their Pharmacological Attributes with an Emphasis on Cholestasis Found in Literature
3.2. Potential H. perforatum Target Genes against Cholestasis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, T.; Apte, U. Bile Acid Metabolism and Signaling in Cholestasis, Inflammation, and Cancer. Adv. Pharmacol. 2015, 74, 263–302. [Google Scholar] [PubMed] [Green Version]
- Ding, L.; Yang, L.; Wang, Z.; Huang, W. Bile acid nuclear receptor FXR and digestive system diseases. Acta Pharm. Sin. B 2015, 5, 135–144. [Google Scholar] [CrossRef] [Green Version]
- Cai, S.-Y.; Ouyang, X.; Chen, Y.; Soroka, C.J.; Wang, J.; Mennone, A.; Wang, Y.; Mehal, W.Z.; Jain, D.; Boyer, J.L. Bile acids initiate cholestatic liver injury by triggering a hepatocyte-specific inflammatory response. JCI Insight. 2017, 2, e90780. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, H.; Chen, C.; Ziani, S.; Nelson, L.J.; Ávila, M.A.; Nevzorova, Y.A.; Cubero, F.J. Fibrotic Events in the Progression of Cholestatic Liver Disease. Cells 2021, 10, 1107. [Google Scholar] [CrossRef] [PubMed]
- Saffioti, F.; Gurusamy, K.S.; Eusebi, L.H.; Tsochatzis, E.; Davidson, B.R.; Thorburn, D. Pharmacological interventions for primary biliary cholangitis: An attempted network meta-analysis. Cochrane Database Syst. Rev. 2017, 3, Cd011648. [Google Scholar] [PubMed]
- Rajapaksha, I.G.; Angus, P.W.; Herath, C.B. Current therapies and novel approaches for biliary diseases. World J. Gastrointest. Pathophysiol. 2019, 10, 1–10. [Google Scholar] [CrossRef]
- Di Ciaula, A.; Bonfrate, L.; Baj, J.; Khalil, M.; Garruti, G.; Stellaard, F.; Wang, H.H.; Wang, D.Q.-H.; Portincasa, P. Recent Advances in the Digestive, Metabolic and Therapeutic Effects of Farnesoid X Receptor and Fibroblast Growth Factor 19: From Cholesterol to Bile Acid Signaling. Nutrients 2022, 14, 4950. [Google Scholar] [CrossRef]
- Simental-Mendía, L.E.; Simental-Mendía, M.; Sánchez-García, A.; Banach, M.; Serban, M.-C.; Cicero, A.F.G.; Sahebkar, A. Impact of ursodeoxycholic acid on circulating lipid concentrations: A systematic review and meta-analysis of randomized placebo-controlled trials. Lipids Health Dis. 2019, 18, 88. [Google Scholar] [CrossRef] [Green Version]
- Santiago, P.; Scheinberg, A.R.; Levy, C. Cholestatic liver diseases: New targets, new therapies. Ther. Adv. Gastroenterol. 2018, 11, 1756284818787400. [Google Scholar] [CrossRef] [Green Version]
- de Vries, E.; Beuers, U. Management of cholestatic disease in 2017. Liver Int. 2017, 37 (Suppl. S1), 123–129. [Google Scholar] [CrossRef] [Green Version]
- Khakoo, N.S.; Sultan, S.; Reynolds, J.M.; Levy, C. Efficacy and Safety of Bezafibrate Alone or in Combination with Ursodeoxycholic Acid in Primary Biliary Cholangitis: Systematic Review and Meta-Analysis. Dig. Dis. Sci. 2023, 68, 1559–1573. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Zheng, K.; Chen, Y.; He, C.; Liu, S.; Yang, Y.; Li, M.; Zeng, X.; Wang, L.; Zhang, F. A randomized, controlled trial on fenofibrate in primary biliary cholangitis patients with incomplete response to ursodeoxycholic acid. Ther. Adv. Chronic Dis. 2022, 13, 20406223221114198. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Guo, G.; Li, B.; Zheng, L.; Sun, R.; Wang, X.; Deng, J.; Jia, G.; Zhou, X.; Cui, L.; et al. Prediction and evaluation of high-risk patients with primary biliary cholangitis receiving ursodeoxycholic acid therapy: An early criterion. Hepatol. Int. 2023, 17, 237–248. [Google Scholar] [CrossRef] [PubMed]
- Namisaki, T.; Fujinaga, Y.; Moriya, K.; Yoshiji, H. The association of histological progression with biochemical response to ursodeoxycholic acid in primary biliary cholangitis. Hepatol. Res. 2021, 51, 31–38. [Google Scholar] [CrossRef]
- Chappell, L.C.; Bell, J.L.; Smith, A.; Linsell, L.; Juszczak, E.; Dixon, P.H.; Chambers, J.; Hunter, R.; Dorling, J.; Williamson, C.; et al. Ursodeoxycholic acid versus placebo in women with intrahepatic cholestasis of pregnancy (PITCHES): A randomised controlled trial. Lancet 2019, 394, 849–860. [Google Scholar] [CrossRef] [Green Version]
- Kotb, M.A.; Mosallam, D.; Basanti, C.W.S.; El Sorogy, S.T.M.; Badr, A.M.; Abd El Baky, H.E.H.; Draz, I.H. Ursodeoxycholic acid use is associated with significant risk of morbidity and mortality in infants with cholestasis: A strobe compliant study. Medicine 2020, 99, e18730. [Google Scholar] [CrossRef]
- Agrawal, R.; Majeed, M.; Attar, B.M.; Abu Omar, Y.; Mbachi, C.; Wang, Y.; Flores, E.; Shaqib, S.; Wang, Y.; Udechukwu, V.; et al. Effectiveness of bezafibrate and ursodeoxycholic acid in patients with primary biliary cholangitis: A meta-analysis of randomized controlled trials. Ann. Gastroenterol. 2019, 32, 489–497. [Google Scholar] [CrossRef]
- Goldstein, J.; Levy, C. Novel and emerging therapies for cholestatic liver diseases. Liver Int. 2018, 38, 1520–1535. [Google Scholar] [CrossRef] [Green Version]
- Lu, L. Guidelines for the Management of Cholestatic Liver Diseases (2021). J. Clin. Transl. Hepatol. 2022, 10, 757–769. [Google Scholar] [CrossRef]
- Clichici, S.; David, L.; Moldovan, B.; Baldea, I.; Olteanu, D.; Filip, M.; Nagy, A.; Luca, V.; Crivii, C.; Mircea, P.; et al. Hepatoprotective effects of silymarin coated gold nanoparticles in experimental cholestasis. Mater. Sci. Eng. C Mater Biol. Appl. 2020, 115, 111117. [Google Scholar] [CrossRef]
- Shen, K.; Feng, X.; Pan, H.; Zhang, F.; Xie, H.; Zheng, S. Baicalin Ameliorates Experimental Liver Cholestasis in Mice by Modulation of Oxidative Stress, Inflammation, and NRF2 Transcription Factor. Oxidative Med. Cell. Longev. 2017, 2017, 6169128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, S.; Tan, H.-Y.; Wang, N.; Zhang, Z.-J.; Lao, L.; Wong, C.-W.; Feng, Y. The Role of Oxidative Stress and Antioxidants in Liver Diseases. Int. J. Mol. Sci. 2015, 16, 26087–26124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, L.; Zhang, F.C.; Zhang, X. Therapeutic advances for primary biliary cholangitis: The old and the new. Eur. J. Gastroenterol. Hepatol. 2016, 28, 615–621. [Google Scholar] [CrossRef] [PubMed]
- Mohagheghzadeh, A.; Hemmati, S.; Mehregan, I.; Alfermann, A.W. Linum persicum: Lignans and placement in Linaceae. Phytochem. Rev. 2003, 2, 363–369. [Google Scholar] [CrossRef]
- Rahmatabadi, S.S.; Sadeghian, I.; Ghasemi, Y.; Sakhteman, A.; Hemmati, S. Identification and characterization of a sterically robust phenylalanine ammonia-lyase among 481 natural isoforms through association of in silico and in vitro studies. Enzym. Microb. Technol. 2019, 122, 36–54. [Google Scholar] [CrossRef]
- Pérez-Ramírez, I.F.; Gallegos-Corona, M.A.; González-Dávalos, M.L.; Mora, O.; Rocha-Guzmán, N.E.; Reynoso-Camacho, R. Mechanisms Associated with the Effect of Hypericum perforatum and Smilax cordifolia Aqueous Extracts on Hepatic Steatosis in Obese Rats: A Lipidomic Approach. Eur. J. Lipid Sci. Technol. 2019, 121, 1800403. [Google Scholar] [CrossRef]
- Barnes, J.; Anderson, L.A.; Phillipson, J.D. St John’s wort (Hypericum perforatum L.): A review of its chemistry, pharmacology and clinical properties. J. Pharm. Pharmacol. 2001, 53, 583–600. [Google Scholar] [CrossRef] [Green Version]
- Greeson, J.M.; Sanford, B.; Monti, D.A. St. John’s wort (Hypericum perforatum): A review of the current pharmacological, toxicological, and clinical literature. Psychopharmacology 2001, 153, 402–414. [Google Scholar] [CrossRef]
- Ben-Eliezer, D.; Yechiam, E. Hypericum perforatum as a cognitive enhancer in rodents: A meta-analysis. Sci. Rep. 2016, 6, 35700. [Google Scholar] [CrossRef] [Green Version]
- Romm, A.; Hardy, M.L.; Mills, S. ST. John’s Wort. In Botanical Medicine for Women’s Health; Romm, A., Hardy, M.L., Mills, S., Eds.; Churchill Livingstone: Saint Louis, MO, USA, 2010; pp. 544–546. [Google Scholar]
- Booker, A.; Agapouda, A.; Frommenwiler, D.A.; Scotti, F.; Reich, E.; Heinrich, M. St John’s wort (Hypericum perforatum) products—An assessment of their authenticity and quality. Phytomedicine 2018, 40, 158–164. [Google Scholar] [CrossRef]
- Klemow, K.M.; Bartlow, A.; Crawford, J.; Kocher, N.; Shah, J.; Ritsick, M. Medical Attributes of St. John’s Wort (Hypericum Perforatum), 2nd ed.; CRC Press/Taylor & Francis: Boca Raton, FL, USA, 2011. [Google Scholar]
- Russo, E.; Scicchitano, F.; Whalley, B.J.; Mazzitello, C.; Ciriaco, M.; Esposito, S.; Patanè, M.; Upton, R.; Pugliese, M.; Chimirri, S.; et al. Hypericum perforatum: Pharmacokinetic, Mechanism of Action, Tolerability, and Clinical Drug–Drug Interactions. Phytother. Res. 2014, 28, 643–655. [Google Scholar] [CrossRef]
- Khorasani, M.A. Makhzan al Advieh (The Storehouse of Medicaments). In Research Institute for Islamic and Complementary Medicine; Iran University of Medical Sciences Bavardaran Press: Tehran, Iran, 2001; Volume 351. (In Persian) [Google Scholar]
- Öztürk, Y.; Aydin, S.; Başer, K.H.C.; Kirimer, N.; Kurtar-Öztürk, N. Hepatoprotective activity of Hypericum perforatum L. alcoholic extract in rodents. Phytother. Res. 1992, 6, 44–46. [Google Scholar] [CrossRef]
- Bokelmann, J.M. 67—St. John’s Wort (Hypericum perforatum): Flowering Buds and Tops. In Medicinal Herbs in Primary Care; Bokelmann, J.M., Ed.; Elsevier: Philadelphia, PA, USA, 2021; pp. 569–577. [Google Scholar]
- Zhang, R.; Ji, Y.; Zhang, X.; Kennelly, E.J.; Long, C. Ethnopharmacology of Hypericum species in China: A comprehensive review on ethnobotany, phytochemistry and pharmacology. J. Ethnopharmacol. 2020, 254, 112686. [Google Scholar] [CrossRef]
- Galeotti, N. Hypericum perforatum (St John’s wort) beyond depression: A therapeutic perspective for pain conditions. J. Ethnopharmacol. 2017, 200, 136–146. [Google Scholar] [CrossRef]
- Wölfle, U.; Seelinger, G.; Schempp, C.M. Topical application of St. John’s wort (Hypericum perforatum). Planta Med. 2014, 80, 109–120. [Google Scholar]
- Hammer, K.D.; Birt, D.F. Evidence for Contributions of Interactions of Constituents to the Anti-Inflammatory Activity of Hypericum perforatum. Crit. Rev. Food Sci. Nutr. 2014, 54, 781–789. [Google Scholar] [CrossRef]
- Shrivastava, M.; Dwivedi, L. Therapeutic potential of Hypericum perforatum: A review. Int. J. Pharm. Sci. Res. 2015, 6, 4982–4988. [Google Scholar]
- Hemmati, S.; Behzadipour, Y.; Haddad, M. Decoding the proteome of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) for cell-penetrating peptides involved in pathogenesis or applicable as drug delivery vectors. Infect. Genet Evol. 2020, 85, 104474. [Google Scholar] [CrossRef] [PubMed]
- Behzadipour, Y.; Gholampour, M.; Pirhadi, S.; Seradj, H.; Khoshneviszadeh, M.; Hemmati, S. Viral 3CL(pro) as a Target for Antiviral Intervention Using Milk-Derived Bioactive Peptides. Int. J. Pept. Res. Ther. 2021, 27, 2703–2716. [Google Scholar] [CrossRef]
- Mohamed, F.F.; Anhlan, D.; Schöfbänker, M.; Schreiber, A.; Classen, N.; Hensel, A.; Hempel, G.; Scholz, W.; Kühn, J.; Hrincius, E.R.; et al. Hypericum perforatum and Its Ingredients Hypericin and Pseudohypericin Demonstrate an Antiviral Activity against SARS-CoV-2. Pharmaceuticals 2022, 15, 530. [Google Scholar] [CrossRef] [PubMed]
- Bilia, A.R.; Costa, M.D.C. Medicinal plants and their preparations in the European market: Why has the harmonization failed? The cases of St. John’s wort, valerian, ginkgo, ginseng, and green tea. Phytomedicine 2021, 81, 153421. [Google Scholar] [CrossRef]
- Colombo, F.; Restani, P.; Biella, S.; Di Lorenzo, C. Botanicals in Functional Foods and Food Supplements: Tradition, Efficacy and Regulatory Aspects. Appl. Sci. 2020, 10, 2387. [Google Scholar] [CrossRef]
- Jakubczyk, A.; Kiersnowska, K.; Ömeroğlu, B.; Gawlik-Dziki, U.; Tutaj, K.; Rybczyńska-Tkaczyk, K.; Szydłowska-Tutaj, M.; Złotek, U.; Baraniak, B. The Influence of Hypericum perforatum L. Addition to Wheat Cookies on Their Antioxidant, Anti-Metabolic Syndrome, and Antimicrobial Properties. Foods 2021, 10, 1379. [Google Scholar] [CrossRef]
- Ang, C.Y.W.; Hu, L.; Heinze, T.M.; Cui, Y.; Freeman, J.P.; Kozak, K.; Luo, W.; Liu, F.F.; Mattia, A.; DiNovi, M. Instability of St. John’s Wort (Hypericum perforatum L.) and Degradation of Hyperforin in Aqueous Solutions and Functional Beverage. J. Agric. Food Chem. 2004, 52, 6156–6164. [Google Scholar] [CrossRef]
- Tiwari, P.; Tiwari, P. Probiotic Novel Beverages and Their Applications. Syst. Rev. Pharm. 2011, 2, 41–47. [Google Scholar] [CrossRef] [Green Version]
- Sánchez-Muniz, F.J.; Olivero-David, R.; Triki, M.; Salcedo, L.; González-Muñoz, M.J.; Cofrades, S.; Ruiz-Capillas, C.; Jiménez-Colmenero, F.; Benedi, J. Antioxidant activity of Hypericum perforatum L. extract in enriched n-3 PUFA pork meat systems during chilled storage. Food Res. Int. 2012, 48, 909–915. [Google Scholar] [CrossRef]
- Carrubba, A.; Lazzara, S.; Giovino, A.; Ruberto, G.; Napoli, E. Content variability of bioactive secondary metabolites in Hypericum perforatum L. Phytochem. Lett. 2021, 46, 71–78. [Google Scholar] [CrossRef]
- Hemmati, S.; Schneider, B.; Schmidt, T.J.; Federolf, K.; Alfermann, A.W.; Fuss, E. Justicidin B 7-hydroxylase, a cytochrome P450 monooxygenase from cell cultures of Linum perenne Himmelszelt involved in the biosynthesis of diphyllin. Phytochemistry 2007, 68, 2736–2743. [Google Scholar] [CrossRef] [PubMed]
- Mohagheghzadeh, A.; Dehshahri, S.; Hemmati, S. Accumulation of Lignans by in vitro Cultures of Three Linum Species. Z. Nat. C 2009, 64, 73–76. [Google Scholar] [CrossRef] [PubMed]
- Danova, K.; Motyka, V.; Trendafilova, A.; Dobrev, P.I.; Ivanova, V.; Aneva, I. Evolutionary Aspects of Hypericin Productivity and Endogenous Phytohormone Pools Evidenced in Hypericum Species In Vitro Culture Model. Plants 2022, 11, 2753. [Google Scholar] [CrossRef] [PubMed]
- De Paola, R.; Muià, C.; Mazzon, E.; Genovese, T.; Crisafulli, C.; Menegazzi, M.; Caputi, A.P.; Suzuki, H.; Cuzzocrea, S. Effects of Hypericum perforatum Extract in a Rat Model of Ischemia and Reperfusion Injury. Shock 2005, 24, 255–263. [Google Scholar] [CrossRef]
- Aydin, A.; Sakrak, O.; Yilmaz, T.U.; Kerem, M. The effects of Hypericum perforatum on hepatic ischemia-reperfusion injury in rats. Bratisl. Lek. Listy 2014, 115, 209–215. [Google Scholar] [CrossRef] [Green Version]
- Saija, A.; Scalese, M.; Lanza, M.; Marzullo, D.; Bonina, F.; Castelli, F. Flavonoids as antioxidant agents: Importance of their interaction with biomembranes. Free. Radic. Biol. Med. 1995, 19, 481–486. [Google Scholar] [CrossRef]
- Huang, N.; Rizshsky, L.; Hauck, C.; Nikolau, B.J.; Murphy, P.A.; Birt, D.F. Identification of anti-inflammatory constituents in Hypericum perforatum and Hypericum gentianoides extracts using RAW 264.7 mouse macrophages. Phytochemistry 2011, 72, 2015–2023. [Google Scholar] [CrossRef] [Green Version]
- Koeberle, A.; Rossi, A.; Bauer, J.; Dehm, F.; Verotta, L.; Northoff, H.; Sautebin, L.; Werz, O. Hyperforin, an Anti-Inflammatory Constituent from St. John’s Wort, Inhibits Microsomal Prostaglandin E2 Synthase-1 and Suppresses Prostaglandin E2 Formation in vivo. Front. Pharmacol. 2011, 2, 7. [Google Scholar] [CrossRef] [Green Version]
- Menegazzi, M.; Masiello, P.; Novelli, M. Anti-Tumor Activity of Hypericum perforatum L. and Hyperforin through Modulation of Inflammatory Signaling, ROS Generation and Proton Dynamics. Antioxidants 2020, 10, 18. [Google Scholar] [CrossRef]
- Tokgöz, H.B.; Altan, F. Hypericum perforatum L.: A medicinal plant with potential as a curative agent against obesity-associated complications. Mol. Biol. Rep. 2020, 47, 8679–8686. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Liu, Y.; Tang, Z.; Shi, X.; Song, Z.; Cao, F.; Wei, P.; Li, M.; Li, X.; Jiang, D.; et al. Improvements in estrogen deficiency-induced hypercholesterolemia by Hypericum perforatum L. extract are associated with gut microbiota and related metabolites in ovariectomized (OVX) rats. Biomed. Pharmacother. 2021, 135, 111131. [Google Scholar] [CrossRef] [PubMed]
- Maleki, M.; Moghaddam, M.H.G.; Roghani, M. Effect of Hypericum perforatum aqueous extracts on serum lipids, aminotransferases, and lipid peroxidation in hyperlipidemic rats. Res. Cardiovasc. Med. 2016, 5, e31326. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zou, Y.; Lu, Y.; Wei, D. Hypocholesterolemic effects of a flavonoid-rich extract of Hypericum perforatum L. in rats fed a cholesterol-rich diet. J. Agric. Food Chem. 2005, 53, 2462–2466. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.-L.; Wu, C.-H.; Huang, S.-L.; Yen, G.-C. Phenolic Compounds Rutin and o-Coumaric Acid Ameliorate Obesity Induced by High-Fat Diet in Rats. J. Agric. Food Chem. 2009, 57, 425–431. [Google Scholar] [CrossRef] [PubMed]
- Asgary, S.; Parkhideh, S.; Mahzouni, P.; Solhpour, A.; Madani, H.; Kabiri, N. Effect of hydroalcoholic extract of Hypericum perforatum on selected traditional and novel biochemical factors of cardiovascular diseases and atherosclerotic lesions in hypercholesterolemic rabbits: A comparison between the extract and lovastatin. J. Pharm. Bioallied Sci. 2012, 4, 212–218. [Google Scholar] [CrossRef] [PubMed]
- Peng, S.G.; Pang, Y.L.; Zhu, Q.; Kang, J.H.; Liu, M.X.; Wang, Z. Chlorogenic Acid Functions as a Novel Agonist of PPARγ2 during the Differentiation of Mouse 3T3-L1 Preadipocytes. BioMed Res. Int. 2018, 2018, 8594767. [Google Scholar] [CrossRef] [Green Version]
- Liang, C.; Li, Y.; Bai, M.; Huang, Y.; Yang, H.; Liu, L.; Wang, S.; Yu, C.; Song, Z.; Bao, Y.; et al. Hypericin attenuates nonalcoholic fatty liver disease and abnormal lipid metabolism via the PKA-mediated AMPK signaling pathway in vitro and in vivo. Pharmacol. Res. 2020, 153, 104657. [Google Scholar] [CrossRef]
- Wu, C.-H.; Lin, M.-C.; Wang, H.-C.; Yang, M.-Y.; Jou, M.-J.; Wang, C.-J. Rutin Inhibits Oleic Acid Induced Lipid Accumulation via Reducing Lipogenesis and Oxidative Stress in Hepatocarcinoma Cells. J. Food Sci. 2011, 76, T65–T72. [Google Scholar] [CrossRef]
- Chen, X. Protective effects of quercetin on liver injury induced by ethanol. Pharmacogn. Mag. 2010, 6, 135–141. [Google Scholar] [CrossRef] [Green Version]
- Shi, Y.; Qiu, X.; Dai, M.; Zhang, X.; Jin, G. Hyperoside Attenuates Hepatic Ischemia-Reperfusion Injury by Suppressing Oxidative Stress and Inhibiting Apoptosis in Rats. Transplant. Proc. 2019, 51, 2051–2059. [Google Scholar] [CrossRef]
- Skalkos, D.; Gioti, E.; Stalikas, C.D.; Meyer, H.; Papazoglou, T.G.; Filippidis, G. Photophysical properties of Hypericum perforatum L. extracts—Novel photosensitizers for PDT. J. Photochem. Photobiol. B Biol. 2006, 82, 146–151. [Google Scholar] [CrossRef]
- Velingkar, V.S.; Gupta, G.L.; Hegde, N.B. A current update on phytochemistry, pharmacology and herb–drug interactions of Hypericum perforatum. Phytochem. Rev. 2017, 16, 725–744. [Google Scholar] [CrossRef]
- Oliveira, A.I.; Pinho, C.; Sarmento, B.; Dias, A.C.P. Neuroprotective Activity of Hypericum perforatum and Its Major Components. Front. Plant Sci. 2016, 7, 1004. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simmen, U.; Bobirnac, I.; Ullmer, C.; Lübbert, H.; Büter, K.B.; Schaffner, W.; Schoeffter, P. Antagonist effect of pseudohypericin at CRF1 receptors. Eur. J. Pharmacol. 2003, 458, 251–256. [Google Scholar] [CrossRef]
- Tian, J.; Zhang, F.; Cheng, J.; Guo, S.; Liu, P.; Wang, H. Antidepressant-like activity of adhyperforin, a novel constituent of Hypericum perforatum L. Sci. Rep. 2014, 4, 5632. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Herraiz, T.; Guillén, H. Monoamine Oxidase-A Inhibition and Associated Antioxidant Activity in Plant Extracts with Potential Antidepressant Actions. BioMed Res. Int. 2018, 2018, 4810394. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sell, T.S.; Belkacemi, T.; Flockerzi, V.; Beck, A. Protonophore properties of hyperforin are essential for its pharmacological activity. Sci. Rep. 2014, 4, 7500. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leuner, K.; Kazanski, V.; Muller, M.; Essin, K.; Henke, B.; Gollasch, M.; Harteneck, C.; Müller, W.E. Hyperforin—A key constituent of St. John’s wort specifically activates TRPC6 channels. Faseb J. 2007, 21, 4101–4111. [Google Scholar] [CrossRef] [Green Version]
- Örnolfsson, K.T.; Lund, S.H.; Olafsson, S.; Bergmann, O.M.; Björnsson, E.S. Biochemical response to ursodeoxycholic acid among PBC patients: A nationwide population-based study. Scand. J. Gastroenterol. 2019, 54, 609–616. [Google Scholar] [CrossRef] [PubMed]
- Arokiyaraj, S.; Balamurugan, R.; Augustian, P. Antihyperglycemic effect of Hypericum perforatum ethyl acetate extract on streptozotocin–induced diabetic rats. Asian Pac. J. Trop. Biomed. 2011, 1, 386–390. [Google Scholar]
- Ansari, I.; Roghani, M.; Moghaddam, M.; Ghanem, A.; Mehdizade, N. The Effect of Oral Administration of Hypericum Perforatum on Serum Glucose and Lipids, Hepatic Enzymes and Lipid Peroxidation in Streptozotocin-Induced Diabetic Rats. Galen Med. J. 2017, 6, 319–329. [Google Scholar]
- Duan, J.; Chen, W.; Zhao, Y.; He, L.; Li, E.; Bai, Z.; Wang, Y.; Zhang, C. Flavonoids from Hypericum patulum enhance glucose consumption and attenuate lipid accumulation in HepG2 cells. J. Food Biochem. 2021, 45, e13898. [Google Scholar] [CrossRef] [PubMed]
- Thuy, L.T.T.; Hai, H.; Kawada, N. Role of cytoglobin, a novel radical scavenger, in stellate cell activation and hepatic fibrosis. Clin. Mol. Hepatol. 2020, 26, 280–293. [Google Scholar] [CrossRef] [PubMed]
- Popko, K.; Gorska, E.; Stelmaszczyk-Emmel, A.; Plywaczewski, R.; Stoklosa, A.; Gorecka, D.; Pyrzak, B.; Demkow, U. Proinflammatory cytokines IL-6 and TNF-α and the development of inflammation in obese subjects. Eur. J. Med. Res. 2010, 15 (Suppl. S2), 120–122. [Google Scholar] [CrossRef]
- Lehmann, R.; Franken, H.; Dammeier, S.; Rosenbaum, L.; Kantartzis, K.; Peter, A.; Zell, A.; Adam, P.; Li, J.; Xu, G.; et al. Circulating Lysophosphatidylcholines Are Markers of a Metabolically Benign Nonalcoholic Fatty Liver. Diabetes Care 2013, 36, 2331–2338. [Google Scholar] [CrossRef] [Green Version]
- Mizuno, H.; Taketomi, A.; Nakabayashi, T. Potentially Beneficial Effects of St. John’s Wort (Hypericum perforatum) in Patients with Metabolic Syndrome. OBM Integr. Complement. Med. 2018, 3, 021. [Google Scholar] [CrossRef]
- Tian, J.Y.; Tao, R.Y.; Zhang, X.L.; Liu, Q.; He, Y.B.; Su, Y.L.; Ji, T.F.; Ye, F. Effect of Hypericum perforatum L. extract on insulin resistance and lipid metabolic disorder in high-fat-diet induced obese mice. Phytother. Res. 2015, 29, 86–92. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mariotti, V.; Cadamuro, M.; Spirli, C.; Fiorotto, R.; Strazzabosco, M.; Fabris, L. Animal models of cholestasis: An update on inflammatory cholangiopathies. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2019, 1865, 954–964. [Google Scholar] [CrossRef]
- Saran, C.; Fu, D.; Ho, H.; Klein, A.; Fallon, J.K.; Honkakoski, P.; Brouwer, K.L.R. A novel differentiated HuH-7 cell model to examine bile acid metabolism, transport and cholestatic hepatotoxicity. Sci. Rep. 2022, 12, 14333. [Google Scholar] [CrossRef]
- Sadeghian, I.; Khalvati, B.; Ghasemi, Y.; Hemmati, S. TAT-mediated intracellular delivery of carboxypeptidase G2 protects against methotrexate-induced cell death in HepG2 cells. Toxicol. Appl. Pharmacol. 2018, 346, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Anwer, M. Role of P38 MAPK in Bile Formation and Cholestasis. Gastroenterol. Hepatol. Res. 2018, 3, 22. [Google Scholar]
- Reinehr, R.; Becker, S.; Wettstein, M.; Häussinger, D. Involvement of the Src family kinase yes in bile salt-induced apoptosis. Gastroenterology 2004, 127, 1540–1557. [Google Scholar] [CrossRef]
- Reinehr, R.; Sommerfeld, A.; Häussinger, D. The Src family kinases: Distinct functions of c-Src, Yes, and Fyn in the liver. Biomol. Concepts 2013, 4, 129–142. [Google Scholar] [CrossRef] [PubMed]
- Zerdes, I.; Matikas, A.; Bergh, J.; Rassidakis, G.Z.; Foukakis, T. Genetic, transcriptional and post-translational regulation of the programmed death protein ligand 1 in cancer: Biology and clinical correlations. Oncogene 2018, 37, 4639–4661. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Santamaría, E.; Rodríguez-Ortigosa, C.M.; Uriarte, I.; Latasa, M.U.; Urtasun, R.; Alvarez-Sola, G.; Bárcena-Varela, M.; Colyn, L.; Arcelus, S.; Jiménez, M.; et al. The Epidermal Growth Factor Receptor Ligand Amphiregulin Protects from Cholestatic Liver Injury and Regulates Bile Acids Synthesis. Hepatology 2019, 69, 1632–1647. [Google Scholar] [CrossRef] [PubMed]
- Svinka, J.; Pflügler, S.; Mair, M.; Marschall, H.-U.; Hengstler, J.G.; Stiedl, P.; Poli, V.; Casanova, E.; Timelthaler, G.; Sibilia, M.; et al. Epidermal growth factor signaling protects from cholestatic liver injury and fibrosis. J. Mol. Med. 2017, 95, 109–117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Centuori, S.M.; Gomes, C.J.; Trujillo, J.; Borg, J.; Brownlee, J.; Putnam, C.W.; Martinez, J.D. Deoxycholic acid mediates non-canonical EGFR-MAPK activation through the induction of calcium signaling in colon cancer cells. Biochim. Biophys. Acta 2016, 1861, 663–670. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ye, X.; Zhang, T.; Han, H. PPARα: A potential therapeutic target of cholestasis. Front. Pharmacol. 2022, 13, 916866. [Google Scholar] [CrossRef] [PubMed]
- Sun, F.; Li, X.; Xu, C.; Li, P.; Wu, J.; Sui, J. Effect of compound Yindan decoction on alpha-naphthylisothiocyanate-Induced acute intrahepatic cholestasis in rats. J. Tradit. Chin. Med. 2019, 39, 315–323. [Google Scholar]
- Cai, S.-Y.; Li, M.; Boyer, J.L. The Liver: Biology and Pathobiology. In The Role of Bile Acid-Mediated Inflammation in Cholestatic Liver Injury; Wiley: Oxford, UK, 2020; pp. 728–736. [Google Scholar]
- Nakamura, K.; Kageyama, S.; Kupiec-Weglinski, J.W. The Evolving Role of Neutrophils in Liver Transplant Ischemia-Reperfusion Injury. Curr. Transplant. Rep. 2019, 6, 78–89. [Google Scholar] [CrossRef]
- Bartneck, M.; Wang, J. Therapeutic Targeting of Neutrophil Granulocytes in Inflammatory Liver Disease. Front. Immunol. 2019, 10, 2257. [Google Scholar] [CrossRef]
- Tang, J.; Yan, Z.; Feng, Q.; Yu, L.; Wang, H. The Roles of Neutrophils in the Pathogenesis of Liver Diseases. Front. Immunol. 2021, 12, 625472. [Google Scholar] [CrossRef]
- Michalopoulos, G.K.; Bhushan, B. Liver regeneration: Biological and pathological mechanisms and implications. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 40–55. [Google Scholar] [CrossRef]
- Górska, A.; Mazur, A.J. Integrin-linked kinase (ILK): The known vs. the unknown and perspectives. Cell. Mol. Life Sci. 2022, 79, 100. [Google Scholar] [CrossRef] [PubMed]
- Shafiei, M.S.; Rockey, D.C. The function of integrin-linked kinase in normal and activated stellate cells: Implications for fibrogenesis in wound healing. Lab. Investig. 2012, 92, 305–316. [Google Scholar] [CrossRef] [Green Version]
- Martucci, N.; Michalopoulos, G.K.; Mars, W.M. Integrin Linked Kinase (ILK) and its Role in Liver Pathobiology. Gene Expr. 2021, 20, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Almasabi, S.; Ahmed, A.U.; Boyd, R.; Williams, B.R.G. A Potential Role for Integrin-Linked Kinase in Colorectal Cancer Growth and Progression via Regulating Senescence and Immunity. Front. Genet. 2021, 12, 638558. [Google Scholar] [CrossRef] [PubMed]
- Yim, A.; Smith, C.; Brown, A.M. Osteopontin/secreted phosphoprotein-1 harnesses glial-, immune-, and neuronal cell ligand-receptor interactions to sense and regulate acute and chronic neuroinflammation. Immunol. Rev. 2022, 311, 224–233. [Google Scholar] [CrossRef]
- Bruha, R.; Vitek, L.; Smid, V. Osteopontin—A potential biomarker of advanced liver disease. Ann. Hepatol. 2020, 19, 344–352. [Google Scholar] [CrossRef]
- Shirasaki, T.; Honda, M.; Yamashita, T.; Nio, K.; Shimakami, T.; Shimizu, R.; Nakasyo, S.; Murai, K.; Shirasaki, N.; Okada, H.; et al. The osteopontin-CD44 axis in hepatic cancer stem cells regulates IFN signaling and HCV replication. Sci. Rep. 2018, 8, 13143. [Google Scholar] [CrossRef] [Green Version]
- Meng, F.-G.; Zhang, X.-N.; Liu, S.-X.; Wang, Y.-R.; Zeng, T. Roles of peroxisome proliferator-activated receptor α in the pathogenesis of ethanol-induced liver disease. Chem. Interact. 2020, 327, 109176. [Google Scholar] [CrossRef]
- Song, Z.; Chen, W.; Athavale, D.; Ge, X.; Desert, R.; Das, S.; Han, H.; Nieto, N. Osteopontin Takes Center Stage in Chronic Liver Disease. Hepatology 2021, 73, 1594–1608. [Google Scholar] [CrossRef]
- Vahidnezhad, H.; Youssefian, L.; Saeidian, A.H.; Touati, A.; Pajouhanfar, S.; Baghdadi, T.; Shadmehri, A.A.; Giunta, C.; Kraenzlin, M.; Syx, D.; et al. Mutations in PLOD3, encoding lysyl hydroxylase 3, cause a complex connective tissue disorder including recessive dystrophic epidermolysis bullosa-like blistering phenotype with abnormal anchoring fibrils and type VII collagen deficiency. Matrix Biol. 2019, 81, 91–106. [Google Scholar] [CrossRef] [Green Version]
- Yang, B.; Zhao, Y.; Wang, L.; Zhao, Y.; Wei, L.; Chen, D.; Chen, Z. Identification of PLOD Family Genes as Novel Prognostic Biomarkers for Hepatocellular Carcinoma. Front. Oncol. 2020, 10, 1695. [Google Scholar] [CrossRef]
- Banushi, B.; Forneris, F.; Straatman-Iwanowska, A.; Strange, A.; Lyne, A.-M.; Rogerson, C.; Burden, J.J.; Heywood, W.E.; Hanley, J.; Doykov, I.; et al. Regulation of post-Golgi LH3 trafficking is essential for collagen homeostasis. Nat. Commun. 2016, 7, 12111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Du, H.; Pang, M.; Hou, X.; Yuan, S.; Sun, L. PLOD2 in cancer research. Biomed. Pharmacother. 2017, 90, 670–676. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Lu, Y.; Ji, H.; Li, Y. Anti-inflammatory, anti-oxidative stress and novel therapeutic targets for cholestatic liver injury. Biosci. Trends 2019, 13, 23–31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brzački, V.; Mladenović, B.; Dimić, D.; Jeremić, L.; Živanović, D.; Djukić, D.; Stojanović, N.M.; Sokolović, D.T. Comparison between the effects of selenomethionine and S-adenosylmethionine in preventing cholestasis-induced rat liver damage. Amino Acids 2019, 51, 795–803. [Google Scholar] [CrossRef]
- Zou, Y.P.; Lu, Y.H.; Wei, D.Z. Protective effects of a flavonoid-rich extract of Hypericum perforatum L. against hydrogen peroxide-induced apoptosis in PC12 cells. Phytother. Res. 2010, 24 (Suppl. S1), S6–S10. [Google Scholar] [CrossRef]
- Hemmati, S.; Kazerooni, H.R. Polypharmacological Cell-Penetrating Peptides from Venomous Marine Animals Based on Immunomodulating, Antimicrobial, and Anticancer Properties. Mar. Drugs 2022, 20, 763. [Google Scholar] [CrossRef]
- Nasehi, M.; Torabinejad, S.; Hashemi, M.; Vaseghi, S.; Zarrindast, M.R. Effect of cholestasis and NeuroAid treatment on the expression of Bax, Bcl-2, Pgc-1α and Tfam genes involved in apoptosis and mitochondrial biogenesis in the striatum of male rats. Metab. Brain Dis. 2020, 35, 183–192. [Google Scholar] [CrossRef]
- Brankiewicz, A.; Trzos, S.; Mrożek, M.; Opydo, M.; Szostak, E.; Dziurka, M.; Tuleja, M.; Łoboda, A.; Pocheć, E. Cytotoxic and Antioxidant Activity of Hypericum perforatum L. Extracts against Human Melanoma Cells from Different Stages of Cancer Progression, Cultured under Normoxia and Hypoxia. Molecules 2023, 28, 1509. [Google Scholar] [CrossRef]
- Cai, S.-Y.; Boyer, J.L. Studies on the mechanisms of bile acid initiated hepatic inflammation in cholestatic liver injury. Inflamm. Cell Signal. 2017, 4, e1561. [Google Scholar]
- Subat, S.; Mogushi, K.; Yasen, M.; Kohda, T.; Ishikawa, Y.; Tanaka, H. Identification of genes and pathways, including the CXCL2 axis, altered by DNA methylation in hepatocellular carcinoma. J. Cancer Res. Clin. Oncol. 2019, 145, 675–684. [Google Scholar] [CrossRef]
- Moles, A.; Murphy, L.; Wilson, C.L.; Chakraborty, J.B.; Fox, C.; Park, E.J.; Mann, J.; Oakley, F.; Howarth, R.; Brain, J.; et al. A TLR2/S100A9/CXCL-2 signaling network is necessary for neutrophil recruitment in acute and chronic liver injury in the mouse. J. Hepatol. 2014, 60, 782–791. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Konishi, T.; Schuster, R.M.; Goetzman, H.S.; Caldwell, C.C.; Lentsch, A.B. Cell-specific regulatory effects of CXCR2 on cholestatic liver injury. Am. J. Physiol. Gastrointest. Liver Physiol. 2019, 317, G773–G783. [Google Scholar] [CrossRef]
- Luo, Y.; Kang, J.; Luo, J.; Yan, Z.; Li, S.; Lu, Z.; Song, Y.; Zhang, X.; Yang, J.; Liu, A. Hepatocytic AP-1 and STAT3 contribute to chemotaxis in alphanaphthylisothiocyanate-induced cholestatic liver injury. Toxicol. Lett. 2023, 373, 184–193. [Google Scholar] [CrossRef] [PubMed]
- Manieri, E.; Folgueira, C.; Rodríguez, M.E.; Leiva-Vega, L.; Esteban-Lafuente, L.; Chen, C.; Cubero, F.J.; Barrett, T.; Cavanagh-Kyros, J.; Seruggia, D.; et al. JNK-mediated disruption of bile acid homeostasis promotes intrahepatic cholangiocarcinoma. Proc. Natl. Acad. Sci. USA 2020, 117, 16492–16499. [Google Scholar] [CrossRef] [PubMed]
- Dai, M.; Yang, J.; Luo, Y.; Xu, L.; Zhang, H.; Xu, G.; Liu, A. Therapeutic action against chronic cholestatic liver injury by low-dose fenofibrate involves anti-chemotaxis via JNK-AP1-CCL2/CXCL2 signaling. Pharmacol. Rep. 2020, 72, 935–944. [Google Scholar] [CrossRef]
- Lee, Y.-Y.; Mok, M.T.; Kang, W.; Yang, W.; Tang, W.; Wu, F.; Xu, L.; Yan, M.; Yu, Z.; Lee, S.-D.; et al. Loss of tumor suppressor IGFBP4 drives epigenetic reprogramming in hepatic carcinogenesis. Nucleic Acids Res. 2018, 46, 8832–8847. [Google Scholar] [CrossRef]
- Lee, S.; Woo, D.-C.; Kang, J.; Ra, M.; Kim, K.H.; Lee, S.R.; Choi, D.K.; Lee, H.; Hong, K.B.; Min, S.-H.; et al. The Role of the Histone Methyltransferase EZH2 in Liver Inflammation and Fibrosis in STAM NASH Mice. Biology 2020, 9, 93. [Google Scholar] [CrossRef]
- Lv, Y.-C.; Tang, Y.-Y.; Zhang, P.; Wan, W.; Yao, F.; He, P.-P.; Xie, W.; Mo, Z.-C.; Shi, J.-F.; Wu, J.-F.; et al. Histone Methyltransferase Enhancer of Zeste Homolog 2-Mediated ABCA1 Promoter DNA Methylation Contributes to the Progression of Atherosclerosis. PLoS ONE 2016, 11, e0157265. [Google Scholar] [CrossRef] [Green Version]
- Xu, C.; Li, H.; Tang, C.-K. Sterol carrier protein 2: A promising target in the pathogenesis of atherosclerosis. Genes Dis. 2023, 10, 457–467. [Google Scholar] [CrossRef]
- Xu, C.; Li, H.; Tang, C.-K. Sterol carrier protein 2 in lipid metabolism and non-alcoholic fatty liver disease: Pathophysiology, molecular biology, and potential clinical implications. Metabolism 2022, 131, 155180. [Google Scholar] [CrossRef]
- Vuolo, D.; Do Nascimento, C.C.; D’Almeida, V. Reproduction in Animal Models of Lysosomal Storage Diseases: A Scoping Review. Front. Mol. Biosci. 2021, 8, 773384. [Google Scholar] [CrossRef]
- He, H.; Wang, J.; Yannie, P.J.; Kakiyama, G.; Korzun, W.J.; Ghosh, S. Sterol carrier protein-2 deficiency attenuates diet-induced dyslipidemia and atherosclerosis in mice. J. Biol. Chem. 2018, 293, 9223–9231. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Bie, J.; Ghosh, S. Intracellular cholesterol transport proteins enhance hydrolysis of HDL-CEs and facilitate elimination of cholesterol into bile. J. Lipid Res. 2016, 57, 1712–1719. [Google Scholar] [CrossRef] [Green Version]
- Galano, M.; Venugopal, S.; Papadopoulos, V. Role of STAR and SCP2/SCPx in the Transport of Cholesterol and Other Lipids. Int. J. Mol. Sci. 2022, 23, 12115. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Reizel, Y.; Wang, Y.J.; Lapiro, J.L.; Kren, B.T.; Schug, J.; Rao, S.; Morgan, A.; Herman, A.; Shekels, L.L.; et al. A negative reciprocal regulatory axis between cyclin D1 and HNF4α modulates cell cycle progression and metabolism in the liver. Proc. Natl. Acad. Sci. USA 2020, 117, 17177–17186. [Google Scholar] [CrossRef]
- Topacio, B.R.; Zatulovskiy, E.; Cristea, S.; Xie, S.; Tambo, C.S.; Rubin, S.M.; Sage, J.; Kõivomägi, M.; Skotheim, J.M. Cyclin D-Cdk4,6 Drives Cell-Cycle Progression via the Retinoblastoma Protein’s C-Terminal Helix. Mol. Cell 2019, 74, 758–770.e4. [Google Scholar] [CrossRef]
- Di Ciaula, A.; Wang, D.Q.-H.; Molina-Molina, E.; Baccetto, R.L.; Calamita, G.; Palmieri, V.O.; Portincasa, P. Bile Acids and Cancer: Direct and Environmental-Dependent Effects. Ann. Hepatol. 2017, 16, S87–S105. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Hu, X.; Hu, W.; Sun, X.; Chen, L. Novel TTC37 mutations in a patient with Trichohepatoenteric syndrome: A case report and literature review. Transl. Pediatr. 2022, 11, 1050–1057. [Google Scholar] [CrossRef]
- Poulton, C.; Pathak, G.; Mina, K.; Lassmann, T.; Azmanov, D.; McCormack, E.; Broley, S.; Dreyer, L.; Gration, D.; Taylor, E.; et al. Tricho-hepatic-enteric syndrome (THES) without intractable diarrhoea. Gene 2019, 699, 110–114. [Google Scholar] [CrossRef] [PubMed]
- Morgan, N.V.; Hartley, J.L.; Setchell, K.; Simpson, M.A.; Brown, R.; Tee, L.; Kirkham, S.; Pasha, S.; Trembath, R.C.; Maher, E.R.; et al. A combination of mutations in AKR1D1 and SKIV2L in a family with severe infantile liver disease. Orphanet J. Rare Dis. 2013, 8, 74. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Okpechi, S.C.; Yousefi, H.; Nguyen, K.; Cheng, T.; Alahari, N.V.; Collins-Burow, B.; Burow, M.E.; Alahari, S.K. Role of Nischarin in the pathology of diseases: A special emphasis on breast cancer. Oncogene 2022, 41, 1079–1086. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Chang, C.-H.; Ernsberger, P. Identification of IRAS/Nischarin as an I1-imidazoline receptor in PC12 rat pheochromocytoma cells. J. Neurochem. 2007, 101, 99–108. [Google Scholar] [CrossRef] [PubMed]
- Arnoux, A.; Aubertin, G.; Da Silva, S.; Weiss, M.; Bousquet, P.; Monassier, L.; Niederhoffer, N. Nischarin Is Not the Functional I1 Imidazoline Receptor Involved in Blood Pressure Regulation. J. Cardiovasc. Pharmacol. 2022, 79, 229–234. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Li, X.; Liu, Y.; Chen, H.; Gong, J. Activation of imidazoline I 1 receptor by moxonidine regulates the progression of liver fibrosis in the Nrf2-dependent pathway. Biomed. Pharmacother. 2017, 90, 821–834. [Google Scholar] [CrossRef]
- Blackburn, J.B.; Kudlyk, T.; Pokrovskaya, I.; Lupashin, V.V. More than just sugars: Conserved oligomeric Golgi complex deficiency causes glycosylation-independent cellular defects. Traffic 2018, 19, 463–480. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, Y. Glycosylation Quality Control by the Golgi Structure. J. Mol. Biol. 2016, 428, 3183–3193. [Google Scholar] [CrossRef] [Green Version]
- Nicastro, E.; D’Antiga, L. Genetic Cholestatic Disorders. In Pediatric Hepatology and Liver Transplantation; D’Antiga, L., Ed.; Springer: Cham, Switzerland; Bergamo, Italy, 2019; pp. 227–245. [Google Scholar] [CrossRef]
- Kuhn, M.; von Mering, C.; Campillos, M.; Jensen, L.J.; Bork, P. STITCH: Interaction networks of chemicals and proteins. Nucleic Acids Res. 2008, 36, D684–D688. [Google Scholar] [CrossRef] [Green Version]
- Noor, F.; Rehman, A.; Ashfaq, U.A.; Saleem, M.H.; Okla, M.K.; Al-Hashimi, A.; AbdElgawad, H.; Aslam, S. Integrating Network Pharmacology and Molecular Docking Approaches to Decipher the Multi-Target Pharmacological Mechanism of Abrus precatorius L. Acting on Diabetes. Pharmaceuticals 2022, 15, 414. [Google Scholar] [CrossRef]
- Demetz, E.; Schroll, A.; Auer, K.; Heim, C.; Patsch, J.R.; Eller, P.; Theurl, M.; Theurl, I.; Theurl, M.; Seifert, M.; et al. The Arachidonic Acid Metabolome Serves as a Conserved Regulator of Cholesterol Metabolism. Cell Metab. 2014, 20, 787–798. [Google Scholar] [CrossRef] [Green Version]
- Granados, J.C.; Nigam, A.K.; Bush, K.T.; Jamshidi, N.; Nigam, S.K. A key role for the transporter OAT1 in systemic lipid metabolism. J. Biol. Chem. 2021, 296, 100603. [Google Scholar] [CrossRef]
- Lampou, V.K.; Poller, B.; Huth, F.; Fischer, A.; Kullak-Ublick, G.A.; Arand, M.; Schadt, H.S.; Camenisch, G. Novel insights into bile acid detoxification via CYP, UGT and SULT enzymes. Toxicol. Vitr. 2023, 87, 105533. [Google Scholar] [CrossRef]
- Khambu, B.; Yan, S.; Huda, N.; Yin, X.-M. Role of High-Mobility Group Box-1 in Liver Pathogenesis. Int. J. Mol. Sci. 2019, 20, 5314. [Google Scholar] [CrossRef] [Green Version]
- Schwarz, D.; Kisselev, P.; Schunck, W.H.; Roots, I. Inhibition of 17β-estradiol activation by CYP1A1: Genotype- and regioselective inhibition by St. John’s Wort and several natural polyphenols. Biochim. Biophys Acta 2011, 1814, 168–174. [Google Scholar] [CrossRef]
- Ibrahim, Z.S. Chenodeoxycholic acid increases the induction of CYP1A1 in HepG2 and H4IIE cells. Exp. Ther. Med. 2015, 10, 1976–1982. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, J.; Shang, X.; Yao, B.; Sun, D.; Liu, J.; Zhang, Y.; Wang, H.; Shi, J.; Chen, H.; Shi, T.; et al. The role of CYP1A1/2 in cholesterol ester accumulation provides a new perspective for the treatment of hypercholesterolemia. Acta Pharm. Sin. B 2022, 13, 648–661. [Google Scholar] [CrossRef] [PubMed]
- Bal, S.S.; Leishangthem, G.D.; Sethi, R.S.; Singh, A. P-coumaric acid ameliorates fipronil induced liver injury in mice through attenuation of structural changes, oxidative stress and inflammation. Pestic. Biochem. Physiol. 2022, 180, 104997. [Google Scholar] [CrossRef]
- Rensen, S.S.; Slaats, Y.; Nijhuis, J.; Jans, A.; Bieghs, V.; Driessen, A.; Malle, E.; Greve, J.W.; Buurman, W.A. Increased Hepatic Myeloperoxidase Activity in Obese Subjects with Nonalcoholic Steatohepatitis. Am. J. Pathol. 2009, 175, 1473–1482. [Google Scholar] [CrossRef] [Green Version]
- Roby, C.A.; Anderson, G.D.; Kantor, E.; Dryer, D.A.; Burstein, A.H. St John’s Wort: Effect on CYP3A4 activity. Clin. Pharmacol. Ther. 2000, 67, 451–457. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zhao, K.N.; Chen, C. The role of CYP3A4 in the biotransformation of bile acids and therapeutic implication for cholestasis. Ann. Transl. Med. 2014, 2, 7. [Google Scholar]
- Li, F.; Zhu, W.; Gonzalez, F.J. Potential role of CYP1B1 in the development and treatment of metabolic diseases. Pharmacol. Ther. 2017, 178, 18–30. [Google Scholar] [CrossRef] [PubMed]
- Kahraman, A.; Mott, J.L.; Bronk, S.F.; Werneburg, N.W.; Barreyro, F.J.; Guicciardi, M.E.; Akazawa, Y.; Braley, K.; Craig, R.W.; Gores, G.J. Overexpression of Mcl-1 Attenuates Liver Injury and Fibrosis in the Bile Duct–Ligated Mouse. Dig. Dis. Sci. 2009, 54, 1908–1917. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shen, H.; Yu, H.; Li, Q.-Y.; Wei, Y.-T.; Fu, J.; Dong, H.; Cao, D.; Guo, L.-N.; Chen, L.; Yang, Y.; et al. Hepatocyte-derived VEGFA accelerates the progression of non-alcoholic fatty liver disease to hepatocellular carcinoma via activating hepatic stellate cells. Acta Pharmacol. Sin. 2022, 43, 2917–2928. [Google Scholar] [CrossRef] [PubMed]
- Elpek, G.Ö. Angiogenesis and liver fibrosis. World J. Hepatol. 2015, 7, 377–391. [Google Scholar] [CrossRef] [PubMed]
- Merhi, F.; Tang, R.; Legrand, O.; Nguyen-Khac, F.; Susin, S.A.; Bauvois, B. The antiangiogenic phloroglucinol hyperforin inhibits the secretion of proMMP-2, proMMP-9 and VEGF-A during apoptosis of primary acute myeloid leukemia cells. J. Cancer Metastasis Treat. 2021, 7, 42. [Google Scholar] [CrossRef]
- Palladini, G.; Ferrigno, A.; Richelmi, P.; Perlini, S.; Vairetti, M. Role of matrix metalloproteinases in cholestasis and hepatic ischemia/reperfusion injury: A review. World J. Gastroenterol. 2015, 21, 12114–12124. [Google Scholar] [CrossRef]
- Tong, C.; Li, J.; Lin, W.; Cen, W.; Zhang, W.; Zhu, Z.; Lu, B.; Yu, J. Inhibition of heat shock protein 90 alleviates cholestatic liver injury by decreasing IL-1β and IL-18 expression. Exp. Ther. Med. 2021, 21, 241. [Google Scholar] [CrossRef]
- VanderMolen, K.M.; Naciff, J.M.; Kennedy, K.; Otto-Bruc, A.; Shan, Y.; Wang, X.; Daston, G.P.; Mahony, C. Incorporation of in vitro techniques for botanicals dietary supplement safety assessment—Towards evaluation of developmental and reproductive toxicity (DART). Food Chem. Toxicol. 2020, 144, 111539. [Google Scholar] [CrossRef]
- Manna, S.K.; Golla, S.; Golla, J.P.; Tanaka, N.; Cai, Y.; Takahashi, S.; Krausz, K.W.; Matsubara, T.; Korboukh, I.; Gonzalez, F.J. St. John’s Wort Attenuates Colorectal Carcinogenesis in Mice through Suppression of Inflammatory Signaling. Cancer Prev. Res. 2015, 8, 786–795. [Google Scholar] [CrossRef] [Green Version]
- Hammer, K.D.; Yum, M.-Y.; Dixon, P.M.; Birt, D.F. Identification of JAK–STAT pathways as important for the anti-inflammatory activity of a Hypericum perforatum fraction and bioactive constituents in RAW 264.7 mouse macrophages. Phytochemistry 2010, 71, 716–725. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lancet, D. The GeneCards Suite. In Practical Guide to Life Science Databases; Springer: Singapore, 2022; pp. 27–56. [Google Scholar]
- Dennis, G.; Sherman, B.T.; Hosack, D.A.; Yang, J.; Gao, W.; Lane, H.C.; Lempicki, R.A. DAVID: Database for Annotation, Visualization, and Integrated Discovery. Genome Biol. 2003, 4, R60. [Google Scholar] [CrossRef] [Green Version]
- Szklarczyk, D.; Franceschini, A.; Wyder, S.; Forslund, K.; Heller, D.; Huerta-Cepas, J.; Simonovic, M.; Roth, A.; Santos, A.; Tsafou, K.P.; et al. STRING v10: Protein-Protein Interaction Networks, Integrated Over the Tree of Life. Nucleic Acids Res. 2015, 43, D447–D452. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Doncheva, N.T.; Morris, J.H.; Gorodkin, J.; Jensen, L.J. Cytoscape StringApp: Network Analysis and Visualization of Proteomics Data. J. Proteome Res. 2019, 18, 623–632. [Google Scholar] [CrossRef]
- Chin, C.-H.; Chen, S.-H.; Wu, H.-H.; Ho, C.-W.; Ko, M.-T.; Lin, C.-Y. cytoHubba: Identifying hub objects and sub-networks from complex interactome. BMC Syst. Biol. 2014, 8, S11. [Google Scholar] [CrossRef] [PubMed] [Green Version]









| Category | Term | p-Value | Target Gene |
|---|---|---|---|
| MF | Integrin binding | 6 × 10−7 | NISCH, SRC, SPP1, ILK, ICAM1 |
| Bp | GO:0043406~positive regulation of MAP kinase activity | 4 × 10−4 | SRC, EGF, ILK, EZH2 |
| GO:0033993~response to lipid | 7 × 10−4 | SRC, CAT, SPP1, CXCL2, EZH2, ICAM1 | |
| GO:0009893~positive regulation of metabolic process | 4 × 10−3 | CDK6, SCP2, SRC, EGF, SPP1, BAX, ILK, EZH2, ICAM1 | |
| GO:0014065~phosphatidylinositol 3-kinase signaling | 4 × 10−3 | SRC, EGF, CAT | |
| CC | Endomembrane system | 2 × 10−3 | NISCH, COG7, SCP2, SRC, EGF, COG4, CAT, SPP1, BAX, PLOD3 |
| KEGG pathway | Lipid and atherosclerosis | 3 × 10−3 | SRC, BAX, CXCL2, ICAM1 |
| EGFR tyrosine kinase inhibitor resistance | 5 × 10−3 | SRC, BAX, EGF |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohagheghzadeh, A.; Badr, P.; Mohagheghzadeh, A.; Hemmati, S. Hypericum perforatum L. and the Underlying Molecular Mechanisms for Its Choleretic, Cholagogue, and Regenerative Properties. Pharmaceuticals 2023, 16, 887. https://doi.org/10.3390/ph16060887
Mohagheghzadeh A, Badr P, Mohagheghzadeh A, Hemmati S. Hypericum perforatum L. and the Underlying Molecular Mechanisms for Its Choleretic, Cholagogue, and Regenerative Properties. Pharmaceuticals. 2023; 16(6):887. https://doi.org/10.3390/ph16060887
Chicago/Turabian StyleMohagheghzadeh, Ala, Parmis Badr, Abdolali Mohagheghzadeh, and Shiva Hemmati. 2023. "Hypericum perforatum L. and the Underlying Molecular Mechanisms for Its Choleretic, Cholagogue, and Regenerative Properties" Pharmaceuticals 16, no. 6: 887. https://doi.org/10.3390/ph16060887
APA StyleMohagheghzadeh, A., Badr, P., Mohagheghzadeh, A., & Hemmati, S. (2023). Hypericum perforatum L. and the Underlying Molecular Mechanisms for Its Choleretic, Cholagogue, and Regenerative Properties. Pharmaceuticals, 16(6), 887. https://doi.org/10.3390/ph16060887