Cyclodextrins for the Delivery of Bioactive Compounds from Natural Sources: Medicinal, Food and Cosmetics Applications
Abstract
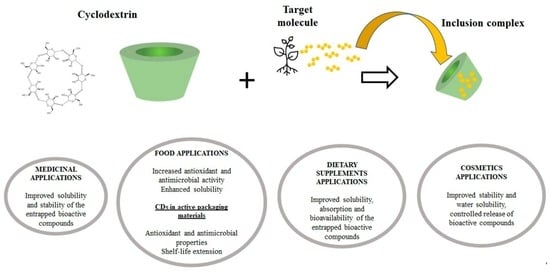
:1. Introduction
2. Medicinal Applications
2.1. Legislation
2.2. Applications
| CD Type | Bioactive Compound | Improved Characteristics | Biological Study | Test Subject of In Vitro/In Vivo Study | Reference |
|---|---|---|---|---|---|
| HPβCD | Ursolic acid | Stability | Antitumor activities | Melanoma cell lines (A375, B16 4A5 and SK-Mel 2) | [17] |
| HPβCD | Saikosaponin-d | Solubility | Antitumor activities | Squamous carcinoma cell line (HSC-1) | [18] |
| β-CD | Betulinic acid | - | Antitumor activities | Breast cancer cell line (MCF7) | [19] |
| HPβCD | Fucoxanthin | Solubility and stability | Antitumor activities | Colorectal carcinoma (CRC) cells (HCT116 and Caco-2) | [20] |
| β-CD and HPβCD | Camptothecin | Stability and solubility | Antitumor activities | Breast cancer (AREc32), lung cancer (H-23), hepatic carcinoma (HepG2), ovarian carcinoma (A2780) and neuroblastoma (SH-SY5Y) cell lines | [21] |
| Luotonin A | Stability and solubility | ||||
| β-CD | Dihydroquercetin | Solubility | Antioxidant and antitumor activities | Hepatocarcinoma cell line (HepG2) | [22] |
| β-CD | Mansonone G | Solubility | Antitumor activities | Lung cancer cells (A549) | [23] |
| β-CD: CDI 1:4 | Oxyresveratrol | Dissolution | Antitumor activities | Prostate (PC-3), colon (HT-29 and HCT-116) cell lines | [24] |
| CD-NSs | Ferulic acid (FA) | Stability | Antitumor activities | Breast cancer cell lines (MCF7 and 4T1) | [25] |
| γ-CD liposomal nanoparticles | Curcumin | Solubility | Antitumor activities | Osteosarcoma (KHOS) and breast cancer (MCF-7) cell lines | [26] |
| HPβCD | Thymoquinone | Solubility | Antiallergic effects | Rat basophilic leukemia cell line (RBL-2H3) | [28] |
| γ-CD | Green propolis supercritical extract (GPSE) | - | Anti-inflammatory activities | Female C57BL/6NRj wild-type mice (liver) | [29] |
| HPβCD | Silybin (silibinin) | Solubility | Restored the gut microbiota and intestinal integrity | Hamsters | [30] |
| β-CD | (−)-linalool | Solubility and stability | Gastroprotective effect | Mice | [31] |
| β-CD, γ-CD | Epigallocatechin gallate | Stability | Antiviral effect | Influenza virus and HCoV-229E | [32] |
| α-CD | Moringin (MOR) | - | Neuroprotection | Neuroblastoma cells (SH-SY5Y) exposed to amyloid beta peptide | [33] |
3. Food Applications
| CD Type | Bioactive Compound/Guest Moiety | IC 1 Preparation Methods | Packaging Material | Food System/Model | Effects in the Final Product | Reference |
|---|---|---|---|---|---|---|
| β-CD | Cinnamaldehyde (CIN) | Mixing and freeze-drying | Non-woven polyethylene terephthalate (PET) | Cold fresh pork | Packaged pork samples with the highest tested CIN concentration were preserved for 11 days under refrigerated storage compared to control samples (7 days). | [50] |
| Methyl-β-CD | Satureja montana L. essential oil (SEO) | Mixing, ultrasonication and freeze-drying | Soy soluble polysaccharide (SSPS) hydrogel | Meat slices | Methyl-β-CD/SEO-SSPS hydrogel effectively reduced S. aureus counts by 3.5 log CFU/g after 7 days of storage at 4 °C. | [51] |
| β-CD | Octyl gallate (OG) | Co-precipitation and freeze-drying | Chitosan film | Fresh fruits vegetables (blueberries and asparagus) | Lower weight loss was reported in coated asparagus samples containing 0.5%, 1.0% and 2.0% β-CD/OG (3.87%, 3.12% and 2.85%, respectively), compared to control (7%) after 25 days storage at 4 °C. TVC 2 was maintained close to the initial 102–103 CFU/g in the coated asparagus samples compared to control (107 CFU/g) after 25 days of storage at 4 °C. | [52] |
| Coated blueberries with films containing 1.0% and 2.0% β-CD/OG presented lower weight loss (2%) compared to control (7%) after 25-day storage at 4 °C. Films containing 2.0% β-CD/OG effectively preserved freshness in blueberries with a 6% rotting rate compared to control (20%). | ||||||
| β-CD | Trans-cinnamaldehyde (TC) and citral (CI) | Co-precipitation and vacuum-drying | Ethylene vinyl alcohol copolymer (EVOH) film | Beef | Shelf-life of EVOH-β-CD-CI and EVOH-β-CD-TC coated samples was extended about 4 days at 4 °C, compared to control and coated samples without ICs. | [53] |
| β-CD | Curcumin (Cur) | Mixing and freeze-drying | κ-Carrageenan (κ-Car) film | Chilled pork | Extension of chilled pork shelf life from 4–5 days to 10 days with application of κ-Car-β-CD-Cur film combined with light treatment, compared to pure κ-Car film and other treatments. | [54] |
| β-CD | Lemongrass essential oil (LEO) | Co-precipitation and drying | Chitosan–gelatin (CS-Gel) coating | Fresh cherry tomatoes | CS/Gel coating with 7% β-CD/LEO presented high antibacterial activity against P. aurantiogriseum in cherry tomatoes artificially during 20 days of cold storage at 8 °C. | [55] |
| α-CD | Benzyl isothiocyanate (BITC) | Mixing, ultrasonication and vacuum freeze-drying | Chitosan (CS) film | Beef | CS-α-CD-BITC-coated beef samples presented lower TVC, TVB-N 3 and TBARS 4 values and higher overall acceptability score, compared to PET- 5 and CS-coated samples after 12 days of refrigerated storage. | [56] |
| CD Type | Bioactive Compound/Guest Moiety | IC 1 Preparation Methods | Food System/Model | Effects in the Final Product | Reference |
|---|---|---|---|---|---|
| β-CD | Cuminaldehyde (CUM) | Ultrasonication, cold nitrogen plasma (CNP) treatment and freeze-drying | Vegetable juices (tomato and cucumber) | CNP-treated ICs decreased the E. coli O157:H7 population from 3.5 log CFU/mL to 2.51 (12 °C) and 1.29 log CFU/mL (4 °C) on cucumber juice, and to 2.58 (12 °C) and 1.33 log CFU/mL (4 °C) on tomato juice after 3 days of storage, compared to control (no added ICs). | [57] |
| β-CD | Ferulic acid (FA) | Crosslinking of β-CD with diphenyl carbonate (nanosponges preparation), agitation and freeze-drying | Pomegranate juice | Highest TPC 2 and antioxidant activity of pomegranate juice treated with FA-CD-NSs 3 containing 500 mg/L FA was reported after 30 days of storage at 4 °C compared to control and samples containing free FA. Total anthocyanins were better stabilized in pomegranate juice treated with FA-CD-NSs containing 250 mg/L FA after 30 days of storage at 4 °C, compared to control and samples containing free FA, through co-pigmentation effect. | [58] |
| β-CD | Clove essential oil (CEO) | β-CD-metal organic frameworks (β-CD-MOFs) preparation through methanol vapor diffusion, mixing and freeze-drying | Chinese bacon (preserved meat product) | The lowest MDA 4 and POV 5 values were reported in Chinese bacon preserved with CEO-β-CD-MOFs in all tested concentrations, after 3 days of preservation and 15 days of fermentation compared to control, samples containing free CEO or BHT 6. | [59] |
| β-CD | Fish oil (FO) | Homogenization for emulsion formation and ultrasonication | Yogurt | FO-IC-treated yogurt presented greater syneresis reduction and lower POV values, but higher DHA 7 and EPA 8 content, after 21 days of storage at 4 °C compared to control and samples containing free FO. | [60] |
| FO-IC-treated yogurt was significantly better accepted regarding sensory characteristics compared to the free-FO-treated one. | |||||
| γ-CD | Resveratrol (RSV) | Mixing, snap-freezing and freeze-drying | Lemon juice | RSV encapsulation in γ-CD improved its solubility in lemon juice by nine times compared to free RSV (43.1% and 4.8% dissolution, respectively) at day 0. Higher RSV content was reported in γ-CD-RSV-treated lemon juice after 28 days of storage under dark conditions (room temperature or 4 °C). | [61] |
| HPβCD | Apple polyphenols (AP) | Mixing and freeze-drying | Lamb | Frozen-stored lamb treated with 1.6 mg/mL AP/HPβCD-ICs presented the lowest carbonyl content (protein oxidation parameter) and improved muscle tissue structure after 40 days of storage compared to control and other tested IC concentrations. | [62] |
| γ-CD | Epigallocatechin-3-gallate (EGCG) | Co-precipitation and freeze-drying | Shrimp surimi products | γ-CD-EGC-treated shrimp surimi products were better preserved regarding lipid oxidation phenomena and browning effects from EGCG oxidation after 5 weeks under refrigerated storage compared to control and free-EGCG-treated samples. | [63] |
| γ-CD | Gingerols (GINs) | Co-precipitation and drying | Yogurt | Lower ΔΕ in γ-CD-GIN-treated yogurt compared to the free-GIN-treated one regarding L*, a* and b* color parameters (control used as reference). | [64] |
| γ-CD-GIN-treated yogurt presented higher ABTS radical scavenging activity compared to control and the free-GIN-treated one. | |||||
| HPβCD | Thymol (Th) | Ultrasonication and freeze-drying | Tomatoes | A 66.55% lower disease incidence from B. cinerea in tomato samples treated with 30 mg/mL HPβCD-Th-ICs compared to control after storage for 3 days at 25 °C. | [65] |
| β-CD and HPβCD | Oxyresveratrol (Ox) | Agitation and spray-drying | Grape juice | Ox-β-CD- and Ox-HPβCD-treated samples, combined with ascorbic acid, presented the lowest L* and ΔΕ value differences (compared to 0 h) after 24 h of storage at room temperature, indicating an anti-browning effect. | [66] |
| β-CD | Rosemary essential oil (REO) | Co-precipitation and drying | Tomato juice | In REO-β-CD-treated tomato juice, the population of S. pastorianus decreased from 5.5 log CFU/100 mL (day 0) to 2 log CFU/100 mL after 15-day storage at 5 °C, and this difference was significantly higher compared to control and free-REO-treated samples. | [67] |
4. Dietary Supplements Applications
| CD Type | Bioactive Compound/Guest Moiety | Targeted Disease/Health Problem | Effects/Key Findings | Reference |
|---|---|---|---|---|
| γ-CD | Vitamins D3 and E | Cystic fibrosis (with pancreatic insufficiency) | Improved bioavailability of fat-soluble vitamins D3 and E | [80] |
| HPβCD | Curcumin and piperine (CUR-PIP) | Diseases of infectious or neurodegenerative etiology | Improved membrane permeability, solubility and antimicrobial and antioxidant activity of CUR-PIP was reported after complexation with HPβCD. The system presented enhanced inhibitory activities against butyrylcholinesterase and acetylcholinesterase. | [81] |
| β-CD | Oregano essential oil (OEO) | Intestinal parasitic infection | Lipid-based nutrient supplement (LNS) containing β-CD-OEO inclusion complexes (27.2 mg/20 g LNS) was stable through the gastrointestinal phases and no differences were reported in sensorial characteristics compared to control. | [82] |
| β-CD | Diosgenin (DIO) | Hyperlipidemia, hyperglycemia, reduced skin thickness | Highly improved oral bioavailability of β-CD-DIO complexes and skin distribution of diosgenin compared to oral administration of free diosgenin. | [83] |
5. Cosmetics Applications
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Atanasov, A.G.; Zotchev, S.B.; Dirsch, V.M.; Supuran, C.T. Natural products in drug discovery: Advances and opportunities. Nat. Rev. Drug Discov. 2021, 20, 200–216. [Google Scholar] [CrossRef]
- González-Manzano, S.; Dueñas, M. Applications of Natural Products in Food. Foods 2021, 10, 300. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.-K. Natural products in cosmetics. Nat. Prod. Bioprospect. 2022, 12, 40. [Google Scholar] [CrossRef] [PubMed]
- Jambhekar, S.S.; Breen, P. Cyclodextrins in pharmaceutical formulations I: Structure and physicochemical properties, formation of complexes, and types of complex. Drug Discov. Today 2016, 21, 356–362. [Google Scholar] [CrossRef] [PubMed]
- Villiers, A. Sur la fermentation de la fecule par l’action du ferment butyrique. Compt. Rend. Acad. Sci. 1891, 112, 435–437. [Google Scholar]
- Villiers, A. Sur le monde d’action du ferment butyrique dans la transformation de le fecule en dextrine. Compt. Rend. Acad. Sci. 1891, 113, 144. [Google Scholar]
- Cramer, F. EINSCHLUSSVERBINDUNGEN VON CYCLODEXTRINEN UND DIE JOD-REAKTION DER STARKE. Angew. Chem. 1951, 63, 487. [Google Scholar]
- Jansook, P.; Ogawa, N.; Loftsson, T. Cyclodextrins: Structure, physicochemical properties and pharmaceutical applications. Int. J. Pharm. 2018, 535, 272–284. [Google Scholar] [CrossRef]
- Pandey, A. Cyclodextrin-based nanoparticles for pharmaceutical applications: A review. Environ. Chem. Lett. 2021, 19, 4297–4310. [Google Scholar] [CrossRef]
- Morin-Crini, N.; Fourmentin, S.; Fenyvesi, É.; Lichtfouse, E.; Torri, G.; Fourmentin, M.; Crini, G. 130 years of cyclodextrin discovery for health, food, agriculture, and the industry: A review. Environ. Chem. Lett. 2021, 19, 2581–2617. [Google Scholar] [CrossRef]
- Chen, H.; Ji, H. Effect of Substitution Degree of 2-Hydroxypropyl-β-Cyclodextrin on the Alkaline Hydrolysis of Cinnamaldehyde to Benzaldehyde. Supramol. Chem. 2014, 26, 796–803. [Google Scholar] [CrossRef]
- Geue, N.; Alcázar, J.J.; Campodónico, P.R. Influence of β-Cyclodextrin Methylation on Host-Guest Complex Stability: A Theoretical Study of Intra- and Intermolecular Interactions as Well as Host Dimer Formation. Molecules 2023, 28, 2625. [Google Scholar] [CrossRef] [PubMed]
- Braga, S.S. Cyclodextrins: Emerging Medicines of the New Millennium. Biomolecules 2019, 9, 801. [Google Scholar] [CrossRef] [PubMed]
- Uekama, K.; Hirayama, F. Inclusion Complexation of Prostaglandin F2α with α- and β-Cyclodextrins in Aqueous Solution. Chem. Pharm. Bull. 1978, 26, 1195–1200. [Google Scholar] [CrossRef]
- Puskás, I.; Szente, L.; Szőcs, L.; Fenyvesi, É. Recent List of Cyclodextrin-Containing Drug Products. Period. Polytech. Chem. Eng. 2023, 67, 11–17. [Google Scholar] [CrossRef]
- European Medicines Agency. Available online: https://www.ema.europa.eu/en/cyclodextrins (accessed on 25 August 2023).
- Oprean, C.; Mioc, M.; Csányi, E.; Ambrus, R.; Bojin, F.; Tatu, C.; Cristea, M.; Ivan, A.; Danciu, C.; Dehelean, C.; et al. Improvement of ursolic and oleanolic acids’ antitumor activity by complexation with hydrophilic cyclodextrins. Biomed. Pharmacother. 2016, 83, 1095–1104. [Google Scholar] [CrossRef]
- Hu, S.C.-S.; Lai, Y.-C.; Lin, C.-L.; Tzeng, W.-S.; Yen, F.-L. Inclusion complex of saikosaponin-d with hydroxypropyl-β-cyclodextrin: Improved physicochemical properties and anti-skin cancer activity. Phytomedicine 2019, 57, 174–182. [Google Scholar] [CrossRef]
- Sun, Y.-F.; Song, C.-K.; Viernstein, H.; Unger, F.; Liang, Z.-S. Apoptosis of human breast cancer cells induced by microencapsulated betulinic acid from sour jujube fruits through the mitochondria transduction pathway. Food Chem. 2013, 138, 1998–2007. [Google Scholar] [CrossRef]
- Sun, X.; Zhu, J.; Liu, C.; Wang, D.; Wang, C.-Y. Fabrication of fucoxanthin/2-hydroxypropyl-β-cyclodextrin inclusion complex assisted by ultrasound procedure to enhance aqueous solubility, stability and antitumor effect of fucoxanthin. Ultrason. Sonochem. 2022, 90, 106215. [Google Scholar] [CrossRef]
- González-Ruiz, V.; Cores, Á.; Martín-Cámara, O.; Orellana, K.; Cervera-Carrascón, V.; Michalska, P.; Olives, A.I.; León, R.; Martín, M.A.; Menéndez, J.C. Enhanced Stability and Bioactivity of Natural Anticancer Topoisomerase I Inhibitors through Cyclodextrin Complexation. Pharmaceutics 2021, 13, 1609. [Google Scholar] [CrossRef]
- Zhang, Y.; Yu, J.; Dong, X.-D.; Ji, H.-Y. Research on Characteristics, Antioxidant and Antitumor Activities of Dihydroquercetin and Its Complexes. Molecules 2017, 23, 20. [Google Scholar] [CrossRef] [PubMed]
- Mahalapbutr, P.; Wonganan, P.; Charoenwongpaiboon, T.; Prousoontorn, M.; Chavasiri, W.; Rungrotmongkol, T. Enhanced Solubility and Anticancer Potential of Mansonone G By β-Cyclodextrin-Based Host-Guest Complexation: A Computational and Experimental Study. Biomolecules 2019, 9, 545. [Google Scholar] [CrossRef] [PubMed]
- Matencio, A.; Dhakar, N.K.; Bessone, F.; Musso, G.; Cavalli, R.; Dianzani, C.; García-Carmona, F.; López-Nicolás, J.M.; Trotta, F. Study of oxyresveratrol complexes with insoluble cyclodextrin based nanosponges: Developing a novel way to obtain their complexation constants and application in an anticancer study. Carbohydr. Polym. 2020, 231, 115763. [Google Scholar] [CrossRef] [PubMed]
- Rezaei, A.; Varshosaz, J.; Fesharaki, M.; Farhang, A.; Jafari, S.M. Improving the solubility and in vitro cytotoxicity (anticancer activity) of ferulic acid by loading it into cyclodextrin nanosponges. Int. J. Nanomed. 2019, 14, 4589–4599. [Google Scholar] [CrossRef] [PubMed]
- Dhule, S.S.; Penfornis, P.; Frazier, T.; Walker, R.; Feldman, J.; Tan, G.; He, J.; Alb, A.; John, V.; Pochampally, R. Curcumin-loaded γ-cyclodextrin liposomal nanoparticles as delivery vehicles for osteosarcoma. Nanomed. Nanotechnol. Biol. Med. 2012, 8, 440–451. [Google Scholar] [CrossRef] [PubMed]
- Jyoti, K.; Bhatia, R.K.; Martis, E.A.F.; Coutinho, E.C.; Jain, U.K.; Chandra, R.; Madan, J. Soluble curcumin amalgamated chitosan microspheres augmented drug delivery and cytotoxicity in colon cancer cells: In vitro and in vivo study. Colloids Surf. B Biointerfaces 2016, 148, 674–683. [Google Scholar] [CrossRef]
- Al-Qubaisi, M.S.; Rasedee, A.; Flaifel, M.H.; Eid, E.E.M.; Hussein-Al-Ali, S.; Alhassan, F.H.; Salih, A.M.; Hussein, M.Z.; Zainal, Z.; Sani, D.; et al. Characterization of thymoquinone/hydroxypropyl-β-cyclodextrin inclusion complex: Application to anti-allergy properties. Eur. J. Pharm. Sci. 2019, 133, 167–182. [Google Scholar] [CrossRef]
- Rimbach, G.; Fischer, A.; Schloesser, A.; Jerz, G.; Ikuta, N.; Ishida, Y.; Matsuzawa, R.; Matsugo, S.; Huebbe, P.; Terao, K. Anti-inflammatory properties of Brazilian green propolis encapsulated in a γ-cyclodextrin complex in mice fed a western-type diet. Int. J. Mol. Sci. 2017, 18, 1141. [Google Scholar] [CrossRef]
- Ren, L.; Ma, X.-L.; Wang, H.-L.; Li, R.; Cui, J.-J.; Yan, P.-J.; Wang, Y.-N.; Yu, X.-Y.; Du, P.; Yu, H.-Y.; et al. Prebiotic-like cyclodextrin assisted silybin on NAFLD through restoring liver and gut homeostasis. J. Control. Release 2022, 348, 825–840. [Google Scholar] [CrossRef]
- da Silva, F.V.; de Barros Fernandes, H.; Oliveira, I.S.; Viana, A.F.S.C.; da Costa, D.S.; Lopes, M.T.P.; de Lira, K.L.; Quintans-Júnior, L.J.; de Sousa, A.A.; de Cássia Meneses Oliveira, R. Beta-cyclodextrin enhanced gastroprotective effect of (−)-linalool, a monoterpene present in rosewood essential oil, in gastric lesion models. Naunyn.-Schmiedeberg’s Arch. Pharmacol. 2016, 389, 1245–1251. [Google Scholar] [CrossRef]
- Matsuura, R.; Kawamura, A.; Matsumoto, Y.; Iida, Y.; Kanayama, M.; Kurokawa, M.; Aida, Y. Epigallocatechin Gallate Stabilized by Cyclodextrin Inactivates Influenza Virus and Human Coronavirus 229E. Microorganisms 2022, 10, 1796. [Google Scholar] [CrossRef] [PubMed]
- Silvestro, S.; Chiricosta, L.; Gugliandolo, A.; Iori, R.; Rollin, P.; Perenzoni, D.; Mattivi, F.; Bramanti, P.; Mazzon, E. The Moringin/α-CD Pretreatment Induces Neuroprotection in an In Vitro Model of Alzheimer’s Disease: A Transcriptomic Study. Curr. Issues Mol. Biol. 2021, 43, 197–214. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Guan, J. Method for Improving Curcumin Stability. Patent No. CN110935033A, 10 December 2019. [Google Scholar]
- Codruţa, S.; Avram, S.; Borcan, F.; Trandafirescu, C.; Dehelean, A.; Danciu, C. Complexes of Triterpenic Acids with Hydrophilic Cyclodextrins, with Optimized Bio-Pharmaceutical Properties. Patent No. RO131432A2, 29 April 2015. [Google Scholar]
- Tomulewicz, M. Combination of Betulin and Cyclodextrin for Use in the Treatment of Alzheimer’s Disease. Patent No. EP3744331A1, 13 May 2020. [Google Scholar]
- Nianzeng, X.; Qinxin, Z.; Feiya, Y.; Dong, C.; Lingquan, M. Pharmaceutical Composition and Application Thereof in Preparation of Products for Treating Prostatitis. Patent No. CN111588716A, 11 November 2020. [Google Scholar]
- Mozuraityte, R.; Kristinova, V.; Rustad, T. Oxidation of Food Components. In Encyclopedia of Food and Health; Caballero, B., Finglas, P.M., Toldrá, F., Eds.; Academic Press: Oxford, UK, 2016; pp. 186–190. ISBN 978-0-12-384953-3. [Google Scholar]
- Ho, S.; Thoo, Y.Y.; Young, D.J.; Siow, L.F. Cyclodextrin encapsulated catechin: Effect of pH, relative humidity and various food models on antioxidant stability. LWT Food Sci. Technol. 2017, 85, 232–239. [Google Scholar] [CrossRef]
- Nguyen, T.A.; Liu, B.; Zhao, J.; Thomas, D.S.; Hook, J.M. An investigation into the supramolecular structure, solubility, stability and antioxidant activity of rutin/cyclodextrin inclusion complex. Food Chem. 2013, 136, 186–192. [Google Scholar] [CrossRef] [PubMed]
- Kfoury, M.; Auezova, L.; Greige-Gerges, H.; Fourmentin, S. Encapsulation in cyclodextrins to widen the applications of essential oils. Environ. Chem. Lett. 2019, 17, 129–143. [Google Scholar] [CrossRef]
- Zhu, G.; Zhu, G.; Xiao, Z. A review of the production of slow-release flavor by formation inclusion complex with cyclodextrins and their derivatives. J. Incl. Phenom. Macrocycl. Chem. 2019, 95, 17–33. [Google Scholar] [CrossRef]
- Sedaghat Doost, A.; Nikbakht Nasrabadi, M.; Kassozi, V.; Nakisozi, H.; Van der Meeren, P. Recent advances in food colloidal delivery systems for essential oils and their main components. Trends Food Sci. Technol. 2020, 99, 474–486. [Google Scholar] [CrossRef]
- Waleczek, K.J.; Marques, H.M.C.; Hempel, B.; Schmidt, P.C. Phase solubility studies of pure (−)-α-bisabolol and camomile essential oil with β-cyclodextrin. Eur. J. Pharm. Biopharm. 2003, 55, 247–251. [Google Scholar] [CrossRef]
- European Commission. Commission regulation (EU) No 231/2012 of 9 March 2012 laying down specifications for food additives listed in Annexes II and III to Regulation (EC) No 1333/2008 of the European Parliament and of the Council. In force. Off. J. Eur. Union 2012, L83, 1–295. [Google Scholar]
- European Parliament and the Council of the European Union. Regulation (EC) No 1333/2008 of the European Parliament and of the Council of 16 December 2008 on food additives. In force. Off. J. Eur. Union 2008, L354, 16–33. [Google Scholar]
- Jansook, P.; Kurkov, S.V.; Loftsson, T. Cyclodextrins as solubilizers: Formation of complex aggregates. J. Pharm. Sci. 2010, 99, 719–729. [Google Scholar] [CrossRef] [PubMed]
- Velázquez-Contreras, F.; Zamora-Ledezma, C.; López-González, I.; Meseguer-Olmo, L.; Núñez-Delicado, E.; Gabaldón, J.A. Cyclodextrins in Polymer-Based Active Food Packaging: A Fresh Look at Nontoxic, Biodegradable, and Sustainable Technology Trends. Polymers 2021, 14, 104. [Google Scholar] [CrossRef] [PubMed]
- Atarés, L.; Chiralt, A. Essential oils as additives in biodegradable films and coatings for active food packaging. Trends Food Sci. Technol. 2016, 48, 51–62. [Google Scholar] [CrossRef]
- Zhou, Z.; Liu, Y.; Liu, Z.; Fan, L.; Dong, T.; Jin, Y.; Saldaña, M.D.A.; Sun, W. Sustained-release antibacterial pads based on nonwovens polyethylene terephthalate modified by β-cyclodextrin embedded with cinnamaldehyde for cold fresh pork preservation. Food Packag. Shelf Life 2020, 26, 100554. [Google Scholar] [CrossRef]
- Cui, H.; Wang, Y.; Li, C.; Chen, X.; Lin, L. Antibacterial efficacy of Satureja montana L. essential oil encapsulated in methyl-β-cyclodextrin/soy soluble polysaccharide hydrogel and its assessment as meat preservative. LWT 2021, 152, 112427. [Google Scholar] [CrossRef]
- Yang, P.; Shi, Y.; Li, D.; Chen, R.; Zheng, M.; Ma, K.; Xu, N.; Dong, L.; Li, T. Antimicrobial and Mechanical Properties of β-Cyclodextrin Inclusion with Octyl Gallate in Chitosan Films and their Application in Fresh Vegetables. Food Biophys. 2022, 17, 598–611. [Google Scholar] [CrossRef]
- Chen, H.; Li, L.; Ma, Y.; Mcdonald, T.P.; Wang, Y. Development of active packaging film containing bioactive components encapsulated in β-cyclodextrin and its application. Food Hydrocoll. 2019, 90, 360–366. [Google Scholar] [CrossRef]
- Wu, J.; Li, J.; Xu, F.; Zhou, A.; Zeng, S.; Zheng, B.; Lin, S. Effective Preservation of Chilled Pork Using Photodynamic Antibacterial Film Based on Curcumin-β-Cyclodextrin Complex. Polymers 2023, 15, 1023. [Google Scholar] [CrossRef]
- Erceg, T.; Šovljanski, O.; Stupar, A.; Ugarković, J.; Aćimović, M.; Pezo, L.; Tomić, A.; Todosijević, M. A comprehensive approach to chitosan-gelatine edible coating with β-cyclodextrin/lemongrass essential oil inclusion complex—Characterization and food application. Int. J. Biol. Macromol. 2023, 228, 400–410. [Google Scholar] [CrossRef]
- Wu, H.; Ao, X.; Liu, J.; Zhu, J.; Bi, J.; Hou, H.; Hao, H.; Zhang, G. A Bioactive Chitosan-Based Film Enriched with Benzyl Isothiocyanate/α-Cyclodextrin Inclusion Complex and Its Application for Beef Preservation. Foods 2022, 11, 2687. [Google Scholar] [CrossRef]
- Lin, L.; Liao, X.; Li, C.; Abdel-Samie, M.A.; Siva, S.; Cui, H. Cold nitrogen plasma modified cuminaldehyde/β-cyclodextrin inclusion complex and its application in vegetable juices preservation. Food Res. Int. 2021, 141, 110132. [Google Scholar] [CrossRef]
- Amani, F.; Rezaei, A.; Kharazmi, M.S.; Jafari, S.M. Loading ferulic acid into β-cyclodextrin nanosponges; antibacterial activity, controlled release and application in pomegranate juice as a copigment agent. Colloids Surfaces A Physicochem. Eng. Asp. 2022, 649, 129454. [Google Scholar] [CrossRef]
- Wang, Y.; Du, Y.-T.; Xue, W.-Y.; Wang, L.; Li, R.; Jiang, Z.-T.; Tang, S.-H.; Tan, J. Enhanced preservation effects of clove (Syzygium aromaticum) essential oil on the processing of Chinese bacon (preserved meat products) by beta cyclodextrin metal organic frameworks (β-CD-MOFs). Meat Sci. 2023, 195, 108998. [Google Scholar] [CrossRef]
- Ghorbanzade, T.; Akhavan-Mahdavi, S.; Kharazmi, M.S.; Ibrahim, S.A.; Jafari, S.M. Loading of fish oil into β-cyclodextrin nanocomplexes for the production of a functional yogurt. Food Chem. X 2022, 15, 100406. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.F.R.; Monteiro, M.; Resende, D.; Braga, S.S.; Coimbra, M.A.; Silva, A.M.S.; Cardoso, S.M. Inclusion Complex of Resveratrol with γ-Cyclodextrin as a Functional Ingredient for Lemon Juices. Foods 2020, 10, 16. [Google Scholar] [CrossRef]
- Zhong, Y.; Han, P.; Sun, S.; An, N.; Ren, X.; Lu, S.; Wang, Q.; Dong, J. Effects of apple polyphenols and hydroxypropyl-β-cyclodextrin inclusion complexes on the oxidation of myofibrillar proteins and microstructures in lamb during frozen storage. Food Chem. 2022, 375, 131874. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Guo, C.; Li, D.; Zhou, D.; Liu, D.; Zhu, B. Nanoprecipitates of γ-cyclodextrin/epigallocatechin-3-gallate inclusion complexes as efficient antioxidants for preservation of shrimp surimi products: Synthesis, performance and mechanism. J. Sci. Food Agric. 2023, 103, 3129–3138. [Google Scholar] [CrossRef]
- Pais, J.M.; Pereira, B.; Paz, F.A.A.; Cardoso, S.M.; Braga, S.S. Solid γ-Cyclodextrin Inclusion Compound with Gingerols, a Multi-Component Guest: Preparation, Properties and Application in Yogurt. Biomolecules 2020, 10, 344. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Cao, J.; Wang, Y.; Chen, J.; Huang, L.; Zhang, H.; Wu, J.; Sun, C. Ultrasound-mediated molecular self-assemble of thymol with 2-hydroxypropyl-β-cyclodextrin for fruit preservation. Food Chem. 2021, 363, 130327. [Google Scholar] [CrossRef]
- He, J.; Guo, F.; Lin, L.; Chen, H.; Chen, J.; Cheng, Y.; Zheng, Z.-P. Investigating the oxyresveratrol β-cyclodextrin and 2-hydroxypropyl-β-cyclodextrin complexes: The effects on oxyresveratrol solution, stability, and antibrowning ability on fresh grape juice. LWT 2019, 100, 263–270. [Google Scholar] [CrossRef]
- Garcia-Sotelo, D.; Silva-Espinoza, B.; Perez-Tello, M.; Olivas, I.; Alvarez-Parrilla, E.; González-Aguilar, G.A.; Ayala-Zavala, J.F. Antimicrobial activity and thermal stability of rosemary essential oil:β−cyclodextrin capsules applied in tomato juice. LWT 2019, 111, 837–845. [Google Scholar] [CrossRef]
- Upreti, M.; Prakash, I.; Chen, Y.L. Solubility Enhanced Terpene Glycoside(s). Patent No. US20110195161, 8 February 2011. [Google Scholar]
- Smit, R. Extension of Beverage Shelf-Stability by Solute-Ligand Complexes. Patent No. EP2312958, 27 July 2009. [Google Scholar]
- Bogkhani, N.; Gebreselassi, P. Encapsulated Compositions and Methods of Preparation. Patent No. US20060068059, 30 September 2004. [Google Scholar]
- Bogkhani, N.; Gebreselassi, P. Encapsulated Compositions and Its Production Method. Patent No. RU2366292C2, 30 September 2005. [Google Scholar]
- Gebreselassi, P.; Luo Shiukh, D.; Bogkhani, N. Stain Removing Chewing Gum Composition. Patent No. US20050008732, 11 July 2003. [Google Scholar]
- Snabe, T.; Thompson, B.W.; Mallory, S.L. Natamycin-Cyclodextrin Complexes for Use in Foodstuff, Process for Their Manufacture and Use Thereof. Patent No. US20120196003, 28 January 2011. [Google Scholar]
- Mortensen, B.; Jansson, S.T.K. Complexes of Cyclodextrins and Carotenoids. Patent No. AU2003258890, 4 July 2003. [Google Scholar]
- Wüpper, S.; Lüersen, K.; Rimbach, G. Cyclodextrins, Natural Compounds, and Plant Bioactives-A Nutritional Perspective. Biomolecules 2021, 11, 401. [Google Scholar] [CrossRef]
- Prosek, M.; Smidovnik, A.; Fir, M.; Strazisar, M.; Golc Wondra, A.; Andrensek, S.; Zmitek, J. Water-Soluble Coenzyme Q10 Inclusion Complex with Beta-Cyclodextrin, Process of Preparing, and Use Thereof. Patent No. EP1755683, 10 May 2005. [Google Scholar]
- Bartlett, M.; Mastaloudis, A.; Smitdt, C.; Poole, S.J. Nanosized Carotenoid Cyclodextrin Complexes. Patent No. US20070191307, 4 October 2006. [Google Scholar]
- Arigony Souto, A. Process for the Preparation of a Water-Soluble Complex Having Resveratrol Compounds; Products Comprising Said Complex; and Uses Thereof. Patent No. US20100204179, 23 July 2008. [Google Scholar]
- Klaveness, J.; Brudeli, B.; Rongved, P. Cyclodextrins for Administering Fatty Acids in Tablets. Patent No. EP2164520, 2 June 2008. [Google Scholar]
- Nowak, J.K.; Sobkowiak, P.; Drzymała-Czyż, S.; Krzyżanowska-Jankowska, P.; Sapiejka, E.; Skorupa, W.; Pogorzelski, A.; Nowicka, A.; Wojsyk-Banaszak, I.; Kurek, S.; et al. Fat-Soluble Vitamin Supplementation Using Liposomes, Cyclodextrins, or Medium-Chain Triglycerides in Cystic Fibrosis: A Randomized Controlled Trial. Nutrients 2021, 13, 4554. [Google Scholar] [CrossRef] [PubMed]
- Gaur, S.; Lopez, E.C.; Ojha, A.; Andrade, J.E. Functionalization of Lipid-Based Nutrient Supplement with β-Cyclodextrin Inclusions of Oregano Essential Oil. J. Food Sci. 2018, 83, 1748–1756. [Google Scholar] [CrossRef] [PubMed]
- Stasiłowicz, A.; Tykarska, E.; Lewandowska, K.; Kozak, M.; Miklaszewski, A.; Kobus-Cisowska, J.; Szymanowska, D.; Plech, T.; Jenczyk, J.; Cielecka-Piontek, J. Hydroxypropyl-β-cyclodextrin as an effective carrier of curcumin—Piperine nutraceutical system with improved enzyme inhibition properties. J. Enzyme Inhib. Med. Chem. 2020, 35, 1811–1821. [Google Scholar] [CrossRef]
- Okawara, M.; Tokudome, Y.; Todo, H.; Sugibayashi, K.; Hashimoto, F. Enhancement of diosgenin distribution in the skin by cyclodextrin complexation following oral administration. Biol. Pharm. Bull. 2013, 36, 36–40. [Google Scholar] [CrossRef]
- Ferreira, L.; Mascarenhas-Melo, F.; Rabaça, S.; Mathur, A.; Sharma, A.; Giram, P.S.; Pawar, K.D.; Rahdar, A.; Raza, F.; Veiga, F.; et al. Cyclodextrin-based dermatological formulations: Dermopharmaceutical and cosmetic applications. Colloids Surf. B Biointerfaces 2023, 221, 113012. [Google Scholar] [CrossRef]
- Hans-Jurgen, B.; Eckhard, S. Applications of cyclodextrins in cosmetic products: A review. J. Cosmet. Sci. 2002, 53, 185–192. [Google Scholar]
- Santos, A.C.; Morais, F.; Simões, A.; Pereira, I.; Sequeira, J.A.D.; Pereira-Silva, M.; Veiga, F.; Ribeiro, A. Nanotechnology for the development of new cosmetic formulations. Expert Opin. Drug Deliv. 2019, 16, 313–330. [Google Scholar] [CrossRef]
- Crini, G.; Fenyvesi, É.; Szente, L. Outstanding contribution of Professor József Szejtli to cyclodextrin applications in foods, cosmetics, drugs, chromatography and biotechnology: A review. Environ. Chem. Lett. 2021, 19, 2619–2641. [Google Scholar] [CrossRef]
- Pereira, A.G.; Carpena, M.; Oliveira, P.G.; Mejuto, J.C.; Prieto, M.A.; Gandara, J.S. Main applications of cyclodextrins in the food industry as the compounds of choice to form host–guest complexes. Int. J. Mol. Sci. 2021, 22, 1339. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Kaler, A.; Singh, V.; Patil, R.; Banerjee, U.C. Cyclodextrins and Biotechnological Applications. In Cyclodextrins in Pharmaceutics, Cosmetics, and Biomedicine: Current and Future Industrial Applications; John Wiley & Sons: Hoboken, NJ, USA, 2011; pp. 275–285. [Google Scholar]
- Braga, S.S.; Pais, J. Getting under the skin: Cyclodextrin inclusion for the controlled delivery of active substances to the dermis. In Design of Nanostructures for Versatile Therapeutic Applications, 1st ed.; Grumezescu, A.M., Ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2018; pp. 407–449. [Google Scholar]
- Suvarna, V.; Bore, B.; Bhawar, C.; Mallya, R. Complexation of phytochemicals with cyclodextrins and their derivatives—An update. Biomed. Pharmacother. 2022, 149, 112862. [Google Scholar] [CrossRef] [PubMed]
- Numanoǧlu, U.; Şen, T.; Tarimci, N.; Kartal, M.; Koo, O.M.Y.; Önyüksel, H. Use of cyclodextrins as a cosmetic delivery system for fragrance materials: Linalool and benzyl acetate. AAPS PharmSciTech 2007, 8, 34–42. [Google Scholar] [CrossRef] [PubMed]
- Aytac, Z.; Yildiz, Z.I.; Kayaci-Senirmak, F.; Tekinay, T.; Uyar, T. Electrospinning of cyclodextrin/linalool-inclusion complex nanofibers: Fast-dissolving nanofibrous web with prolonged release and antibacterial activity. Food Chem. 2017, 231, 192–201. [Google Scholar] [CrossRef]
- Xiao, Z.; Zhang, Y.; Niu, Y.; Ke, Q.; Kou, X. Cyclodextrins as carriers for volatile aroma compounds: A review. Carbohydr. Polym. 2021, 269, 118292. [Google Scholar] [CrossRef]
- Arana-Sánchez, A.; Estarrón-Espinosa, M.; Obledo-Vázquez, E.N.; Padilla-Camberos, E.; Silva-Vázquez, R.; Lugo-Cervantes, E. Antimicrobial and antioxidant activities of Mexican oregano essential oils (Lippia graveolens H. B. K.) with different composition when microencapsulated inβ-cyclodextrin. Lett. Appl. Microbiol. 2010, 50, 585–590. [Google Scholar] [CrossRef]
- Ruksiriwanich, W.; Sirithunyalug, J.; Khantham, C.; Leksomboon, K.; Jantrawut, P. Skin penetration and stability enhancement of Celastrus paniculatus seed oil by 2-hydroxypropyl-β-cyclodextrin inclusion complex for cosmeceutical applications. Sci. Pharm. 2018, 86, 33. [Google Scholar] [CrossRef]
- Marijan, M.; Tomić, D.; Strawa, J.W.; Jakupović, L.; Inić, S.; Jug, M.; Tomczyk, M.; Zovko Končić, M. Optimization of Cyclodextrin-Assisted Extraction of Phenolics from Helichrysum italicum for Preparation of Extracts with Anti-Elastase and Anti-Collagenase Properties. Metabolites 2023, 13, 257. [Google Scholar] [CrossRef]
- Wang, W.; Yang, Y.; Tang, K. Selective Extraction of Glabridin from Glycyrrhiza Glabra Crude Extract by sulfobutylether -β-CD in a Ternary Solvent System. Process Biochem. 2023, 129, 1–10. [Google Scholar] [CrossRef]
- Kalouta, K.; Eleni, P.; Boukouvalas, C.; Vassilatou, K.; Krokida, M. Dynamic mechanical analysis of novel cosmeceutical facial creams containing nano-encapsulated natural plant and fruit extracts. J. Cosmet. Dermatol. 2020, 19, 1146–1154. [Google Scholar] [CrossRef]
- Spanidi, E.; Athanasopoulou, S.; Liakopoulou, A.; Chaidou, A.; Hatziantoniou, S.; Gardikis, K. Royal Jelly Components Encapsulation in a Controlled Release System—Skin Functionality, and Biochemical Activity for Skin Applications. Pharmaceuticals 2022, 15, 907. [Google Scholar] [CrossRef]
- Dias, P.H.; Scopel, M.; Martiny, S.; Bianchi, S.E.; Bassani, V.L.; Zuanazzi, J.A.S. Hydroxypropyl-β-cyclodextrin-containing hydrogel enhances skin formononetin permeation/retention. J. Pharm. Pharmacol. 2018, 70, 865–873. [Google Scholar] [CrossRef] [PubMed]
- Spanidi, E.; Karapetsas, A.; Voulgaridou, G.P.; Letsiou, S.; Aligiannis, N.; Tsochantaridis, I.; Kynigopoulos, S.; Lambropoulou, M.; Mourtzinos, I.; Pappa, A.; et al. A new controlled release system for propolis polyphenols and its biochemical activity for skin applications. Plants 2021, 10, 420. [Google Scholar] [CrossRef] [PubMed]
- Dalla, E.; Koumentakou, I.; Bikiaris, N.; Balla, E.; Lykidou, S.; Nikolaidis, N. Formulation, Characterization and Evaluation of Innovative O/W Emulsions Containing Curcumin Derivatives with Enhanced Antioxidant Properties. Antioxidants 2022, 11, 2271. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Liu, G.; Wang, W.; Liu, R.; Liao, L.; Cheng, N.; Li, W.; Zhang, W.; Ding, D. Cyclodextrin-modified CeO2 nanoparticles as a multifunctional nanozyme for combinational therapy of psoriasis. Int. J. Nanomed. 2020, 15, 2515–2527. [Google Scholar] [CrossRef]
- Kumar, S.; Singh, K.K.; Rao, R. Enhanced anti-psoriatic efficacy and regulation of oxidative stress of a novel topical babchi oil (Psoralea corylifolia) cyclodextrin-based nanogel in a mouse tail model. J. Microencapsul. 2019, 36, 140–155. [Google Scholar] [CrossRef]
- Wathoni, N.; Motoyama, K.; Higashi, T.; Okajima, M.; Kaneko, T.; Arima, H. Enhancement of curcumin wound healing ability by complexation with 2-hydroxypropyl-γ-cyclodextrin in sacran hydrogel film. Int. J. Biol. Macromol. 2017, 98, 268–276. [Google Scholar] [CrossRef]
- Pipkin, J.D.; Rajewski, R.; Mainous, B. Compositions Containing Silymarin and Sulfoalkyl Ether Cyclodextrin and Methods of Using the Same. Patent No. EP3270941, 18 March 2016. [Google Scholar]
- Deng, Y.; Pan, Z.; Bernard, A.-L.S.; Wu, S. Aqueous Compositions with Mangiferin for Cosmetic Applications. Patent No. US20190159989, 30 November 2011. [Google Scholar]
- Mourtzinos, I.; Koutsianas, N.; Dragani, P.; Patera, A. Extraction And Formation of Inclusion Complexes of Propolis Active Components with Hydroxypropyl—Beta—Cyclodextrin. Patent No. EP2646041, 30 November 2011. [Google Scholar]
- Gardikis, K.; Koutsianas, N.; Patera, A.; Dragani, P.; Tsoukalas, A.-I.; Letsiou, S. Method for Preparing a Stable Controlled Release Propolis Colloidal Dispersion System for Various Uses. Patent No. EP3380081, 23 November 2015. [Google Scholar]
- Gardikis, K. Preparation Method of a Colloidal System of Stabilisation and Contolled Release of Royal Jelly Components for Various Uses. Patent No. EP4117693, 29 October 2020. [Google Scholar]
- Loftsson, T.; Duchêne, D. Cyclodextrins and their pharmaceutical applications. Int. J. Pharm. 2007, 329, 1–11. [Google Scholar] [CrossRef]
- Bonini, M.; Rossi, S.; Karlsson, G.; Almgren, M.; Nostro, P.L.; Baglioni, P. Self-assembly of β-cyclodextrin in water. Part 1: Cryo-TEM and dynamic and static light scattering. Langmuir 2006, 22, 1478–1484. [Google Scholar] [CrossRef]
- Okamoto, H.; Komatsu, H.; Hashida, M.; Sezaki, H. Effects of β-cyclodextrin and di-O-methyl-β-cyclodextrin on the percutaneous absorption of butylparaben, indomethacin and sulfanilic acid. Int. J. Pharm. 1986, 30, 35–45. [Google Scholar] [CrossRef]
- Loftsson, T.; Vogensen, S.B.; Brewster, M.E.; Konrádsdóttir, F. Effects of cyclodextrins on drug delivery through biological membranes. J. Pharm. Sci. 2007, 96, 2532–2546. [Google Scholar] [CrossRef] [PubMed]
- Andreia, A.; Helena, R.; Sandra, S. Carrier-Mediated Dermal Delivery: Applications in the Prevention and Treatment of Skin Disorders, 1st ed.; Jenny Stanford Publishing: Singapore, 2017; ISBN 9789814745581. [Google Scholar]
- Piel, G.; Moutard, S.; Uhoda, E.; Pilard, F.; Piérard, G.E.; Perly, B.; Delattre, L.; Evrard, B. Skin compatibility of cyclodextrins and their derivatives: A comparative assessment using a corneoxenometry bioassay. Eur. J. Pharm. Biopharm. 2004, 57, 479–482. [Google Scholar] [CrossRef] [PubMed]

| CD Type | Bioactive Compound/Guest Moiety | System/Model | Effects/Key Findings | Reference |
|---|---|---|---|---|
| β-CDs and HPβCD | Linalool | Gel formulations | Increased the water solubility, improved handling of raw materials (liquid fragrance material to be turned into powder). Controlled release of bioactive compounds and their stability within the formula. | [92] |
| HPβCD | Celastrus paniculatus seed oil (CPSO) | Serum and gel base | Physical stability of formulations containing CPSO with HPβCD after 3 months of storage, with the percentage of oleic acid maintained above 80% of the initial amount. Higher skin penetration of oleic acid is shown compared to other formulations. The formula exhibits appropriate viscosities for use in cosmetic products. | [96] |
| β-CDs | Tea tree oil | Cosmeceutical facial creams | Stable formulas mainly in rheological properties | [99] |
| HPβCD | Royal jelly | Cosmeceutical facial creams | Encapsulates the sensitive components of royal jelly (10-HDA), eliminating its stability disadvantages while at the same time allowing time-controlled release which could prove useful for skin applications as indicated by the in-cell experiments | [100] |
| HPβCD and MβCD | Isoflavones (formononetin and biochanin A) | Hydrogel formulations | Significant increase in the penetration of isoflavones (formononetin and biochanin A) into the epidermis and dermis with the use of HPβCD in hydrogels, with potential use in cosmetic formulations for the prevention of skin aging | [101] |
| HPβCD | Propolis | Cosmeceutical facial creams | Encapsulation of propolis polyphenols in a physicochemically stable system with a controlled release rate. It maintains the antioxidant, anti-mutagenic and anti-aging properties of propolis polyphenols at levels similar to a methanolic extract | [102] |
| β-CD | Curcumin | Semisolid Oil in Water (O/W) emulsions | Formulas containing curcumin and β-CD showed greater antioxidant capacity and improved viscosity as well as stability, but presented a low rate of antimicrobial activity | [103] |
| β-CDs | Ceria NPs (CeNPs), natural antioxidant enzymes | Increase biocompatibility, water solubility and antioxidant capacity, anti-psoriatic effects. | [104] | |
| β-CD | Babchi Oil—BO | Nanostructure gel | Reduce oxidative stress, anti-psoriatic effects | [105] |
| HPγCD | Curcumin | Hydrogel film | Wound healing | [106] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Christaki, S.; Spanidi, E.; Panagiotidou, E.; Athanasopoulou, S.; Kyriakoudi, A.; Mourtzinos, I.; Gardikis, K. Cyclodextrins for the Delivery of Bioactive Compounds from Natural Sources: Medicinal, Food and Cosmetics Applications. Pharmaceuticals 2023, 16, 1274. https://doi.org/10.3390/ph16091274
Christaki S, Spanidi E, Panagiotidou E, Athanasopoulou S, Kyriakoudi A, Mourtzinos I, Gardikis K. Cyclodextrins for the Delivery of Bioactive Compounds from Natural Sources: Medicinal, Food and Cosmetics Applications. Pharmaceuticals. 2023; 16(9):1274. https://doi.org/10.3390/ph16091274
Chicago/Turabian StyleChristaki, Stamatia, Eleni Spanidi, Eleni Panagiotidou, Sophia Athanasopoulou, Anastasia Kyriakoudi, Ioannis Mourtzinos, and Konstantinos Gardikis. 2023. "Cyclodextrins for the Delivery of Bioactive Compounds from Natural Sources: Medicinal, Food and Cosmetics Applications" Pharmaceuticals 16, no. 9: 1274. https://doi.org/10.3390/ph16091274
APA StyleChristaki, S., Spanidi, E., Panagiotidou, E., Athanasopoulou, S., Kyriakoudi, A., Mourtzinos, I., & Gardikis, K. (2023). Cyclodextrins for the Delivery of Bioactive Compounds from Natural Sources: Medicinal, Food and Cosmetics Applications. Pharmaceuticals, 16(9), 1274. https://doi.org/10.3390/ph16091274