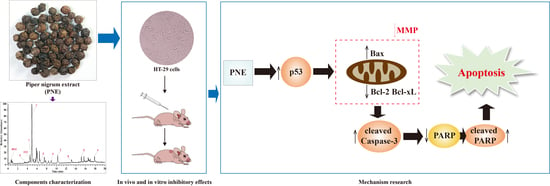
Piper nigrum Extract Inhibits the Growth of Human Colorectal Cancer HT-29 Cells by Inducing p53-Mediated Apoptosis
Abstract
:1. Introduction
2. Results
2.1. Qualitative Analysis of PNE
2.2. PNE Inhibited the Proliferation of HT-29 Cells
2.3. PNE Inhibited Colony Formation in HT-29 Cells
2.4. PNE Induced Apoptosis in HT-29 Cells
2.5. PNE Reduced the Mitochondrial Membrane Potential (MMP) of HT-29 Cells
2.6. PNE Inhibited Tumor Growth In Vivo
2.7. PNE Regulated the Expression of p53 and Its Downstream Target Proteins
3. Discussion
4. Materials and Methods
4.1. Chemical Reagent
4.2. Plant Material
4.3. Preparation and UPLC-Q-Exactive Plus MS analysis of PNE
4.4. Cell Culture
4.5. Cell Proliferation Inhibition Assay
4.6. Colony Formation Assay
4.7. Cell Apoptosis Assay
4.8. Measurement of MMP
4.9. Animals
4.10. Establishment of the Xenograft Model and Treatment
4.11. Hematoxylin–Eosin (HE) Staining
4.12. IHC Assay
4.13. WB Assay
4.14. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 5-FU | 5-fluorouracil |
| AOD | Average optical density |
| CRC | Colorectal cancer |
| HE | Hematoxylin–eosin |
| IHC | Immunohistochemistry |
| MMP | Mitochondrial membrane potential |
| OD | Optical density |
| PNE | Piper nigrum extract |
| TV | Tumor volume |
| WB | Western blot |
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Dekker, E.; Tanis, P.J.; Vleugels, J.L.A.; Kasi, P.M.; Wallace, M.B. Colorectal cancer. Lancet 2019, 394, 1467–1480. [Google Scholar] [CrossRef]
- Jung, E.; Choi, J.; Kim, J.-S.; Han, T.-S. MicroRNA-Based Therapeutics for Drug-Resistant Colorectal Cancer. Pharmaceuticals 2021, 14, 136. [Google Scholar] [CrossRef]
- Asma, S.T.; Acaroz, U.; Imre, K.; Morar, A.; Shah, S.R.A.; Hussain, S.Z.; Arslan-Acaroz, D.; Demirbas, H.; Hajrulai-Musliu, Z.; Istanbullugil, F.R.; et al. Natural Products/Bioactive Compounds as a Source of Anticancer Drugs. Cancers 2022, 14, 6203. [Google Scholar] [CrossRef] [PubMed]
- Meghwal, M.; Goswami, T.K. Piper nigrum and piperine: An update. Phytother. Res. 2013, 27, 1121–1130. [Google Scholar] [CrossRef] [PubMed]
- Takooree, H.; Aumeeruddy, M.Z.; Rengasamy, K.R.R.; Venugopala, K.N.; Jeewon, R.; Zengin, G.; Mahomoodally, M.F. A systematic review on black pepper (Piper nigrum L.): From folk uses to pharmacological applications. Crit. Rev. Food Sci. Nutr. 2019, 59, S210–S243. [Google Scholar] [CrossRef]
- Yaffe, P.B.; Power Coombs, M.R.; Doucette, C.D.; Walsh, M.; Hoskin, D.W. Piperine, an alkaloid from black pepper, inhibits growth of human colon cancer cells via G1 arrest and apoptosis triggered by endoplasmic reticulum stress. Mol. Carcinog. 2015, 54, 1070–1085. [Google Scholar] [CrossRef]
- Zeng, Y.; Yang, Y. Piperine depresses the migration progression via downregulating the Akt/mTOR/MMP-9 signaling pathway in DU145 cells. Mol. Med. Rep. 2018, 17, 6363–6370. [Google Scholar] [CrossRef]
- Deng, Y.; Sriwiriyajan, S.; Tedasen, A.; Hiransai, P.; Graidist, P. Anti-cancer effects of Piper nigrum via inducing multiple molecular signaling in vivo and in vitro. J. Ethnopharmacol. 2016, 188, 87–95. [Google Scholar] [CrossRef]
- Sriwiriyajan, S.; Tedasen, A.; Lailerd, N.; Boonyaphiphat, P.; Nitiruangjarat, A.; Deng, Y.; Graidist, P. Anticancer and Cancer Prevention Effects of Piperine-Free Piper nigrum Extract on N-nitrosomethylurea-Induced Mammary Tumorigenesis in Rats. Cancer Prev. Res. 2016, 9, 74–82. [Google Scholar] [CrossRef]
- Sriwiriyajan, S.; Sukpondma, Y.; Srisawat, T.; Madla, S.; Graidist, P. (−)-Kusunokinin and piperloguminine from Piper nigrum: An alternative option to treat breast cancer. Biomed. Pharmacother. 2017, 92, 732–743. [Google Scholar] [CrossRef] [PubMed]
- Ee, G.C.; Lim, C.M.; Rahmani, M.; Shaari, K.; Bong, C.F. Pellitorine, a potential anti-cancer lead compound against HL6 and MCT-7 cell lines and microbial transformation of piperine from Piper Nigrum. Molecules 2010, 15, 2398–2404. [Google Scholar] [CrossRef] [PubMed]
- Wong, R.S.Y. Apoptosis in cancer: From pathogenesis to treatment. J. Exp. Clin. Cancer Res. 2011, 30, 87. [Google Scholar] [CrossRef]
- Soussi, T.; Béroud, C. Assessing TP53 status in human tumours to evaluate clinical outcome. Nat. Rev. Cancer 2001, 1, 233–240. [Google Scholar] [CrossRef] [PubMed]
- Prashant, A.; Rangaswamy, C.; Yadav, A.K.; Reddy, V.; Sowmya, M.N.; Madhunapantula, S. In vitro anticancer activity of ethanolic extracts of Piper nigrum against colorectal carcinoma cell lines. Int. J. Appl. Basic. Med. Res. 2017, 7, 67–72. [Google Scholar] [CrossRef]
- de Souza Grinevicius, V.M.; Kviecinski, M.R.; Santos Mota, N.S.; Ourique, F.; Porfirio Will Castro, L.S.; Andreguetti, R.R.; Gomes Correia, J.F.; Filho, D.W.; Pich, C.T.; Pedrosa, R.C. Piper nigrum ethanolic extract rich in piperamides causes ROS overproduction, oxidative damage in DNA leading to cell cycle arrest and apoptosis in cancer cells. J. Ethnopharmacol. 2016, 189, 139–147. [Google Scholar] [CrossRef]
- Viet Phong, N.; Thi Nguyet Anh, D.; Yeong Chae, H.; Young Yang, S.; Jeong Kwon, M.; Sun Min, B.; Ah Kim, J. Anti-inflammatory activity and cytotoxicity against ovarian cancer cell lines by amide alkaloids and piperic esters isolated from Piper longum fruits: In vitro assessments and molecular docking simulation. Bioorg. Chem. 2022, 128, 106072. [Google Scholar] [CrossRef]
- Yun, Y.S.; Noda, S.; Takahashi, S.; Takahashi, Y.; Inoue, H. Piperine-like alkamides from Piper nigrum induce BDNF promoter and promote neurite outgrowth in Neuro-2a cells. J. Nat. Med. 2018, 72, 238–245. [Google Scholar] [CrossRef]
- Kim, C.R.; Kim, H.S.; Choi, S.J.; Kim, J.K.; Gim, M.C.; Kim, Y.J.; Shin, D.H. Erucamide from Radish Leaves Has an Inhibitory Effect Against Acetylcholinesterase and Prevents Memory Deficit Induced by Trimethyltin. J. Med. Food 2018, 21, 769–776. [Google Scholar] [CrossRef]
- Ku, S.K.; Lee, I.C.; Kim, J.A.; Bae, J.S. Anti-septic effects of pellitorine in HMGB1-induced inflammatory responses in vitro and in vivo. Inflammation 2014, 37, 338–348. [Google Scholar] [CrossRef]
- Abd-Allah, E.R.; Amin, S.; El Ghareeb, A.E.W.; Badawy, M.A. Effect of Rythmol (propafenone HCl) administration during pregnancy in Wistar rats. J. Biochem. Mol. Toxicol. 2022, 36, e23085. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Hu, X.; Xu, R.; Ba, Y.; Chen, X.; Wang, X.; Cao, B.; Wu, X. Amide alkaloids characterization and neuroprotective properties of Piper nigrum L.: A comparative study with fruits, pericarp, stalks and leaves. Food Chem. 2022, 368, 130832. [Google Scholar] [CrossRef] [PubMed]
- Reynoso-Moreno, I.; Najar-Guerrero, I.; Escareño, N.; Flores-Soto, M.E.; Gertsch, J.; Viveros-Paredes, J.M. An Endocannabinoid Uptake Inhibitor from Black Pepper Exerts Pronounced Anti-Inflammatory Effects in Mice. J. Agric. Food Chem. 2017, 65, 9435–9442. [Google Scholar] [CrossRef]
- Gao, X.; Wang, C.; Chen, Z.; Chen, Y.; Santhanam, R.K.; Xue, Z.; Ma, Q.; Guo, Q.; Liu, W.; Zhang, M.; et al. Effects of N-trans-feruloyltyramine isolated from laba garlic on antioxidant, cytotoxic activities and H2O2-induced oxidative damage in HepG2 and L02 cells. Food Chem. Toxicol. 2019, 130, 130–141. [Google Scholar] [CrossRef]
- Mohamadi, N.; Sharififar, F.; Pournamdari, M.; Ansari, M. A Review on Biosynthesis, Analytical Techniques, and Pharmacological Activities of Trigonelline as a Plant Alkaloid. J. Diet. Suppl. 2018, 15, 207–222. [Google Scholar] [CrossRef]
- Arumugam, M.K.; Paal, M.C.; Donohue, T.M., Jr.; Ganesan, M.; Osna, N.A.; Kharbanda, K.K. Beneficial Effects of Betaine: A Comprehensive Review. Biology 2021, 10, 456. [Google Scholar] [CrossRef]
- Shim, G.; Han, S.E.; Yu, Y.H.; Lee, S.; Lee, H.Y.; Kim, K.; Kwon, I.C.; Park, T.G.; Kim, Y.B.; Choi, Y.S.; et al. Trilysinoyl oleylamide-based cationic liposomes for systemic co-delivery of siRNA and an anticancer drug. J. Control. Release 2011, 155, 60–66. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Díaz, C.; Juárez-Oropeza, M.A.; Mascher, D.; Pavón, N.; Regla, I.; Paredes-Carbajal, M.C. Effects of Oleamide on the Vasomotor Responses in the Rat. Cannabis Cannabinoid Res. 2020, 5, 42–50. [Google Scholar] [CrossRef]
- Qi, H.-S.; Liu, P.; Gao, S.-Q.; Diao, Z.-Y.; Yang, L.-L.; Xu, J.; Qu, X.; Han, E.-J. Inhibitory effect of piperlonguminine/ dihydropiperlonguminine on the production of amyloid beta and APP in SK-N-SH cells. Chin. J. Physiol. 2009, 52, 160–168. [Google Scholar] [CrossRef]
- Xu, R.; Zhao, W.; Yu, L.; Chen, Q.; Hu, X.; Ba, Y.; Chen, X.; Wang, X.; Wu, X. A selective and sensitive UFLC-MS/MS method for the simultaneous determination of five alkaloids from Piper longum L. and its application in the pharmacokinetic study of 6-OHDA-induced Parkinson’s disease rats. RSC Adv. 2019, 9, 37082–37091. [Google Scholar] [CrossRef]
- Hou, X.-F.; Pan, H.; Xu, L.-H.; Zha, Q.-B.; He, X.-H.; Ouyang, D.-Y. Piperine Suppresses the Expression of CXCL8 in Lipopolysaccharide-Activated SW480 and HT-29 Cells via Downregulating the Mitogen-Activated Protein Kinase Pathways. Inflammation 2015, 38, 1093–1102. [Google Scholar] [CrossRef]
- Shaheer, K.; Somashekarappa, H.M.; Lakshmanan, M.D. Piperine sensitizes radiation-resistant cancer cells towards radiation and promotes intrinsic pathway of apoptosis. J. Food Sci. 2020, 85, 4070–4079. [Google Scholar] [CrossRef] [PubMed]
- Turrini, E.; Sestili, P.; Fimognari, C. Overview of the Anticancer Potential of the “King of Spices” Piper nigrum and Its Main Constituent Piperine. Toxins 2020, 12, 747. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.M.; Su, X.L. Anticancer effect of ursolic acid via mitochondria-dependent pathways. Oncol. Lett. 2019, 17, 4761–4767. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Xu, Z.; Huang, Z.; Tang, Y.; Yang, D.; Huang, J.; He, L.; Liu, M.; Chen, Z.; Teng, Y. CPI-613 rewires lipid metabolism to enhance pancreatic cancer apoptosis via the AMPK-ACC signaling. J. Exp. Clin. Cancer Res. 2020, 39, 73. [Google Scholar] [CrossRef] [PubMed]
- Day, C.P.; Merlino, G.; Van Dyke, T. Preclinical mouse cancer models: A maze of opportunities and challenges. Cell 2015, 163, 39–53. [Google Scholar] [CrossRef]
- Vodenkova, S.; Buchler, T.; Cervena, K.; Veskrnova, V.; Vodicka, P.; Vymetalkova, V. 5-fluorouracil and other fluoropyrimidines in colorectal cancer: Past, present and future. Pharmacol. Ther. 2020, 206, 107447. [Google Scholar] [CrossRef]
- Kroemer, G.; El-Deiry, W.S.; Golstein, P.; Peter, M.E.; Vaux, D.; Vandenabeele, P.; Zhivotovsky, B.; Blagosklonny, M.V.; Malorni, W.; Knight, R.A.; et al. Classification of cell death: Recommendations of the Nomenclature Committee on Cell Death. Cell Death Differ. 2005, 12 (Suppl. 2), 1463–1467. [Google Scholar] [CrossRef]
- Wei, H.; Qu, L.; Dai, S.; Li, Y.; Wang, H.; Feng, Y.; Chen, X.; Jiang, L.; Guo, M.; Li, J.; et al. Structural insight into the molecular mechanism of p53-mediated mitochondrial apoptosis. Nat. Commun. 2021, 12, 2280. [Google Scholar] [CrossRef]
- Gross, A.; McDonnell, J.M.; Korsmeyer, S.J. BCL-2 family members and the mitochondria in apoptosis. Genes Dev. 1999, 13, 1899–1911. [Google Scholar] [CrossRef]
- Oliver, F.J.; de la Rubia, G.; Rolli, V.; Ruiz-Ruiz, M.C.; de Murcia, G.; Murcia, J.M. Importance of poly(ADP-ribose) polymerase and its cleavage in apoptosis. Lesson from an uncleavable mutant. J. Biol. Chem. 1998, 273, 33533–33539. [Google Scholar] [CrossRef] [PubMed]
- Fohlen, A.; Bordji, K.; Assenat, E.; Gongora, C.; Bazille, C.; Boulonnais, J.; Naveau, M.; Breuil, C.; Pérès, E.A.; Bernaudin, M.; et al. Anticancer Drugs for Intra-Arterial Treatment of Colorectal Cancer Liver Metastases: In-Vitro Screening after Short Exposure Time. Pharmaceuticals 2021, 14, 639. [Google Scholar] [CrossRef] [PubMed]







| No. | tR * (Min) | Chemical Formula | Experimental Mass (m/z) [M + H]+ | Theoretical Mass (m/z) [M + H]+ | Error (ppm) | MS/MS Fragment Ions | Compound Identification | Peak Area% | Biological Activity |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 4.89 | C17H19NO3 | 286.1447 | 286.1438 | 3.15 | 201.0552, 171.0445, 143.0496, 135.0445, 112.0763, 84.0815 | Piperine | 23.05 | Antitumor, antioxidant, cardioprotective, anti-inflammatory, neuroprotective [5] |
| 2 | 10.23 | C21H29NO3 | 344.2231 | 344.2220 | 3.20 | 314.2103, 222.1859, 135.0445, 112.0763, 84.0816 | Piperolein B | 3.33 | Antitumor, anti-inflammatory [17] |
| 3 | 4.50 | C16H17NO3 | 272.1289 | 272.1281 | 2.94 | 201.0552, 143.0496, 137.0840, 135.0445, 98.0608, 70.0660 | Piperyline | 2.46 | Neuroprotective [18] |
| 4 | 17.86 | C22H43NO | 338.3427 | 338.3417 | 2.96 | 321.3160, 303.3051, 170.1543 | Erucamide | 2.19 | Neuroprotective [19] |
| 5 | 6.47 | C14H25NO | 224.2015 | 224.2009 | 2.68 | 168.1389, 151.1122 | Pellitorine | 1.34 | Antitumor, anti-inflammatory [12,20] |
| 6 | 8.90 | C21H27NO3 | 342.2074 | 342.2064 | 2.92 | 135.0445 | Propafenone | 1.23 | Cardioprotective [21] |
| 7 | 4.83 | C17H21NO3 | 288.1602 | 288.1594 | 2.78 | 175.0756, 138.0918, 135.0445, 112.0764 | Piperanine | 1.22 | Neuroprotective [22] |
| 8 | 13.11 | C24H33NO3 | 384.2543 | 384.2533 | 2.60 | 283.1703, 161.0602, 135.0445 | Guineensine | 1.06 | Neuroprotective, anti-inflammatory [23] |
| 9 | 3.44 | C18H19NO4 | 314.1395 | 314.1387 | 2.55 | 177.0551, 149.0599, 145.0288, 121.0653 | N-trans-Feruloyltyramine | 0.43 | Antitumor, antioxidant, anti-inflammatory [24] |
| 10 | 0.69 | C7H7NO2 | 138.0554 | 138.0550 | 2.90 | 94.0658 | Trigonelline | 0.20 | Antitumor, cardioprotective, neuroprotective [25] |
| 11 | 0.69 | C5H11NO2 | 118.0868 | 118.0863 | 4.23 | 101.0601 | Betaine | 0.08 | Antitumor, antioxidant, anti-inflammatory, neuroprotective [26] |
| 12 | 15.07 | C18H35NO | 282.2798 | 282.2791 | 2.48 | 265.2531, 247.2425 | Oleamide | 0.05 | Antitumor, neuroprotective [27,28] |
| 13 | 4.57 | C16H21NO3 | 276.1604 | 276.1594 | 3.62 | 175.0760, 135.0445 | 4, 5-Dihydropiperlonguminine | 0.01 | Antitumor, neuroprotective [29,30] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, R.; Zhao, J.; Wei, P.; Tang, M.; Ma, Z.; Zhao, Y.; Du, L.; Wan, L. Piper nigrum Extract Inhibits the Growth of Human Colorectal Cancer HT-29 Cells by Inducing p53-Mediated Apoptosis. Pharmaceuticals 2023, 16, 1325. https://doi.org/10.3390/ph16091325
Wu R, Zhao J, Wei P, Tang M, Ma Z, Zhao Y, Du L, Wan L. Piper nigrum Extract Inhibits the Growth of Human Colorectal Cancer HT-29 Cells by Inducing p53-Mediated Apoptosis. Pharmaceuticals. 2023; 16(9):1325. https://doi.org/10.3390/ph16091325
Chicago/Turabian StyleWu, Rui, Jiajia Zhao, Panhong Wei, Minghai Tang, Ziyan Ma, Yunyan Zhao, Leilei Du, and Li Wan. 2023. "Piper nigrum Extract Inhibits the Growth of Human Colorectal Cancer HT-29 Cells by Inducing p53-Mediated Apoptosis" Pharmaceuticals 16, no. 9: 1325. https://doi.org/10.3390/ph16091325
APA StyleWu, R., Zhao, J., Wei, P., Tang, M., Ma, Z., Zhao, Y., Du, L., & Wan, L. (2023). Piper nigrum Extract Inhibits the Growth of Human Colorectal Cancer HT-29 Cells by Inducing p53-Mediated Apoptosis. Pharmaceuticals, 16(9), 1325. https://doi.org/10.3390/ph16091325