Evaluation of Anti-Inflammatory and Anti-Tubercular Activity of 4-Methyl-7-Substituted Coumarin Hybrids and Their Structure Activity Relationships
Abstract
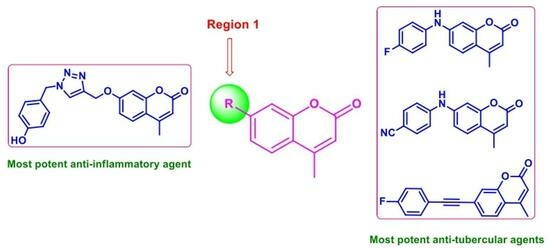
:1. Introduction
2. Results and Discussion
2.1. Chemistry
2.2. The Biological Activity of the Target Compounds
2.2.1. Anti-Inflammatory Activity
2.2.2. Structure–Activity Relationship (SAR) Studies
2.2.3. Anti-Tubercular Activity Studies
2.2.4. SAR Studies
3. Materials and Methods
3.1. General Considerations
3.2. Procedure for Determining In Vitro Anti-Inflammatory Activity: Anti-Denaturation Assay
3.3. Procedure for Determining Anti-Tuberculosis Potential
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ahmed, K.; Choudhary, M.I.; Saleem, R.S.Z. Heterocyclic pyrimidine derivatives as promising antibacterial agents. Eur. J. Med. Chem. 2023, 259, 115701. [Google Scholar] [CrossRef]
- Rahman, S.M.A.; Bhatti, J.S.; Thareja, S.; Monga, V. Current development of 1,2,3-triazole derived potential antimalarial scaffolds: Structure- activity relationship (SAR) and bioactive compounds. Eur. J. Med. Chem. 2023, 259, 115699. [Google Scholar] [CrossRef] [PubMed]
- Tian, G.; Song, Q.; Liu, Z.; Guo, J.; Cao, S.; Long, S. Recent advances in 1,2,3- and 1,2,4-triazole hybrids as antimicrobials and their SAR: A critical review. Eur. J. Med. Chem. 2023, 259, 115603. [Google Scholar] [CrossRef] [PubMed]
- Ravindar, L.; Hasbullah, S.A.; Rakesh, K.P.; Hassan, N.I. Triazole hybrid compounds: A new frontier in malaria treatment. Eur. J. Med. Chem. 2023, 259, 115694. [Google Scholar] [CrossRef]
- El-Shershaby, M.H.; Ghiaty, A.; Bayoumi, A.H.; Ahmed, H.E.A.; El-Zoghbi, M.S.; El-Adl, K.; Abulkhair, H.S. 1,2,4-Triazolo [4,3-c]quinazolines: A bioisosterism-guided approach towards the development of novel PCAF inhibitors with potential anticancer activity. New J. Chem. 2021, 45, 11136–11152. [Google Scholar] [CrossRef]
- Niu, Z.; Wang, Y.; Zhang, S.; Li, Y.; Chen, X.; Wang, S.; Liu, H. Application and synthesis of thiazole ring in clinically approved drugs. Eur. J. Med. Chem. 2023, 250, 115172. [Google Scholar] [CrossRef]
- Zou, Y.; Teng, Y.; Li, J.; Yan, Y. Recent advances in the biosynthesis of coumarin and its derivatives. Green Chem. Eng. 2023, in press. [Google Scholar] [CrossRef]
- Sharma, M.; Vyas, V.K.; Bhatt, S.; Ghate, M.D. Therapeutic potential of 4-substituted coumarins: A conspectus. Eur. J. Med. Chem. 2022, 6, 100086. [Google Scholar] [CrossRef]
- Ghosh, S.; Ghosh, A.; Rajanan, A.; Suresh, A.J.; Raut, P.S.; Kundu, S.; Sahu, B.D. Natural coumarins: Preclinical evidence-based potential candidates to alleviate diabetic nephropathy. Phytomed. Plus 2022, 2, 100379. [Google Scholar] [CrossRef]
- Nasab, N.H.; Azimian, F.; Kruger, H.G.; Kim, S.J. Acetylcoumarin in cyclic and heterocyclic-containing coumarins: Synthesis and biological applications. Tetrahedron. 2022, 129, 133158. [Google Scholar] [CrossRef]
- Hinman, J.W.; Hoeksema, H.; Caron, E.L.; Jackson, W.G. The partial structure of novobiocin (streptonivicin). J. Am. Chem. Soc. 1956, 78, 1072–1074. [Google Scholar] [CrossRef]
- Kawaguchi, H.; Tsukiura, H.; Okanishi, M.; Miyaki, T.; Ohmori, T.; Fujisawa, K.; Koshiyama, H. Studies on coumermycin, a new antibiotic. Production, isolation and characterization of coumermycin A1. J. Antibiot. Ser. A 1965, 18, 1–10. [Google Scholar]
- Singh, A.; Singh, K.; Sharma, A.; Kaur, J.; Kaur, R.; Kaur, J.; Kaur, K.; Chadha, R.; Bedi, P.M.S. Rational utilization of 1,2,3-triazole scaffold in anti-MRSA drug development: Design strategies, Structural insights and Pharmacological outcomes. J. Mol. Struct. 2023, 1259, 136557. [Google Scholar] [CrossRef]
- Deng, C.; Yan, H.; Wang, J.; Liu, K.; Liu, B.; Shi, Y. 1,2,3-Triazole-containing hybrids with potential antibacterial activity against ESKAPE pathogens. Eur. J. Med. Chem. 2022, 244, 114888. [Google Scholar] [CrossRef] [PubMed]
- Gulati, H.K.; Kumar, N.; Sharma, A.; Jyoti; Khanna, A.; Sharma, S.; Salwan, R.; Bedi, P.M.S. A comprehensive review on triazole based conjugates as acetylcholinesterase inhibitors: Design strategies, synthesis, biological activity, structure activity relationships, molecular docking studies. J. Mol. Struct. 2023, 1284, 135354. [Google Scholar] [CrossRef]
- Bozorov, K.; Zhao, J.; Aisa, H.A. 1,2,3-Triazole-containing hybrids as leads in medicinal chemistry: A recent overview. Bioorg. Med. Chem. 2019, 27, 3511–3531. [Google Scholar] [CrossRef]
- Ashok, D.; Gundu, S.; Aamate, V.K.; Devulapally, M.G.; Bathini, R.; Manga, V. Dimers of coumarin-1,2,3-triazole hybrids bearing alkyl spacer: Design, microwave-assisted synthesis, molecular docking and evaluation as antimycobacterial and antimicrobial agents. J. Mol. Struct. 2018, 1157, 312–321. [Google Scholar] [CrossRef]
- Genin, M.J.; Allwine, D.A.; Anderson, D.J.; Barbachyn, M.R.; Emmert, D.E.; Garmon, S.A.; Graber, D.R.; Grega, K.C.; Hester, J.B.; Hutchinson, D.K.; et al. Substituent effects on the antibacterial activity of nitrogen-carbon-linked (azolylphenyl)oxazolidinones with expanded activity against the fastidious gram-negative organisms Haemophilus influenzae and Moraxella catarrhalis. J. Med. Chem. 2000, 43, 953–970. [Google Scholar] [CrossRef]
- Jordao, A.K.; Afonso, P.P.; Ferreira, V.F.; de Souza, M.C.; Almeida, M.C.; Beltrame, C.O.; Paiva, D.P.; Wardell, S.M.; Wardell, J.L.; Tiekink, E.R.; et al. Antiviral evaluation of N-amino-1,2,3-triazoles against Cantagalo virus replication in cell culture. Eur. J. Med. Chem. 2009, 44, 3777–3783. [Google Scholar] [CrossRef]
- Buckle, D.R.; Outred, D.J.; Rockell, C.J.M.; Smith, H.; Spicer, B.A. Studies on v-triazoles. 7. antiallergic 9-oxo-1H,9H-benzopyrano [2,3-d]-v-triazoles. J. Med. Chem. 1983, 26, 251–254. [Google Scholar] [CrossRef]
- Hager, C.; Miethchen, R.; Reinke, H. Organofluorine compounds and fluorinating agents, part 26: New reversed nucleosides-perfluoroalkyl substituted 1,2,3-triazoles linked to D-galactose and D-altrose. J. Fluor Chem. 2000, 104, 135–142. [Google Scholar] [CrossRef]
- Blobaum, A.L.; Marnett, L.J. Structural and functional basis of cyclooxygenase inhibition. J. Med. Chem. 2007, 50, 1425–1441. [Google Scholar] [CrossRef] [PubMed]
- Khan, K.M.; Ambreen, N.; Mughal, U.R.; Jalil, S.; Perveen, S.; Choudhary, M.I. 3-Formylchromones: Potential antiinflammatory agents. Eur. J. Med. Chem. 2010, 45, 4058–4064. [Google Scholar] [CrossRef] [PubMed]
- Glomb, T.; Wiatrak, B.; Gebczak, K.; Gebarowski, T.; Bodetko, D.; Czyznikowska, Z.; Swiatek, P. New 1,3,4-oxadiazole derivatives of pyridothiazine-1,1-dioxide with anti-inflammatory activity. Int. J. Mol. Sci. 2020, 21, 9122. [Google Scholar] [CrossRef]
- Moellering, R.C. Past, present, and future of antimicrobial agents. Am. J. Med. 1995, 99, 11S–18S. [Google Scholar] [CrossRef]
- Singh, P.; Anand, A.; Vipin, K. Recent developments in biological activities of chalcones: A mini review. Eur. J. Med. Chem. 2014, 85, 758–777. [Google Scholar] [CrossRef]
- Norton, P.P. Drug resistance: A growing problem. Drug Discov. Today 2010, 15, 583–586. [Google Scholar]
- Zignol, M.; Dean, A.S.; Falzon, D.; van Gernert, W.; Wright, A.; van Deun, A.; Portaels, F.; Laszlo, A.; Espinal, M.A.; Pablos-Méndez, A.; et al. Twenty years of global surveillance of antituberculosis-drug resistance. N. Engl. J. Med. 2016, 375, 1081–1089. [Google Scholar]
- World Health Organization. Global Tuberculosis Report 2022; World Health Organization: Geneva, Switzerland, 2022. [Google Scholar]
- Olesen, P.H.; Sørensen, A.R.; Ursø, B.; Kurtzhals, P.; Bowler, A.N.; Ehrbar, U.; Hansen, B.F. Synthesis and in vitro characterization of 1-(4-aminofurazan-3-yl)-5-dialkylaminomethyl-1H-[1,2,3]triazole-4-carboxylic acid derivatives. A new class of selective GSK-3 inhibitors. J. Med. Chem. 2003, 46, 3333–3341. [Google Scholar] [CrossRef]
- Meunier, B. Hybrid molecules with a dual mode of action: Dream or reality? Acc. Chem. Res. 2008, 41, 69–77. [Google Scholar] [CrossRef]
- Rajendra, M.A.; Naseem, M.; Joy, M.N.; Sunil, K.; Sajith, A.M.; Howari, F.; Nazzal, Y.; Xavier, C.; Alshammari, M.B.; Haridas, K.R. Application of NMI-TfCl mediated amide bond formation in the synthesis of biologically relevant oxadiazole derivatives employing less basic (hetero)aryl amines. Mol. Divers. 2021, 26, 1761–1767. [Google Scholar] [CrossRef] [PubMed]
- Rishikesan, R.; Karuvalam, R.P.; Muthipeedika, N.J.; Sajith, A.M.; Eeda, K.R.; Pakkath, R.; Haridas, K.R.; Bhaskar, V.; Narasimhamurthy, K.H.; Muralidharan, A. Synthesis of some novel piperidine fused 5-thioxo-1H-1,2,4-triazoles as potential antimicrobial and antitubercular agents. J. Chem. Sci. 2021, 133, 3. [Google Scholar] [CrossRef]
- Joy, M.N.; Bodke, Y.D.; Telkar, S.; Bakulev, V.A. Synthesis of coumarins linked with 1,2,3-triazoles under microwave irradiation and evaluation of their antimicrobial and antioxidant activity. J. Mex. Chem. Soc. 2020, 64, 53–73. [Google Scholar]
- Joy, M.N.; Bodke, Y.D.; Khader, K.K.A.; Padusha, M.S.A.; Sajith, A.M.; Muralidharan, A. A rapid and modified approach for C-7 amination and amidation of 4-methyl-7-nonafluorobutylsulfonyloxy coumarins under microwave irradiation. RSC Adv. 2014, 4, 19766–19777. [Google Scholar] [CrossRef]
- Joy, M.N.; Bodke, Y.D.; Khader, K.K.A.; Sajith, A.M. A rapid approach for the copper, amine, and ligand-free Sonogashira coupling of 4-methyl-7-nonafluorobutylsulfonyloxy coumarins under microwave irradiation. Tetrahedron Lett. 2014, 55, 2355–2361. [Google Scholar] [CrossRef]
- Priya, M.G.R.; Girija, K.; Ravichandran, N. Invitro study of anti-inflammatory and antioxidant activity of 4-(3H)-quinazolinone derivatives. Rasayan J. Chem. 2011, 4, 418–424. [Google Scholar]
- Arya, C.G.; Gondru, R.; Li, Y.; Banothu, J. Coumarin–benzimidazole hybrids: A review of developments in medicinal chemistry. Eur. J. Med. Chem. 2022, 227, 113921. [Google Scholar]
- Neetu, K.T.; Jaya, S.T. Resazurin reduction assays for screening of anti-tubercular compounds against dormant and actively growing Mycobacterium tuberculosis, Mycobacterium bovis BCG and Mycobacterium smegmatis. J. Antimicrob. Chemother. 2007, 60, 288–293. [Google Scholar]
- Sanna, P.; Carta, A.; Nikookar, M.E.R. Synthesis and antitubercular activity of 3-aryl substituted-2-(1H(2H)benzotriazol-1(2)-yl)acrylonitriles. Eur. J. Med. Chem. 2000, 35, 535–543. [Google Scholar] [CrossRef]
- Parker, M.A.; Kurrasch, D.M.; Nichols, D.E. The role of lipophilicity in determining binding affinity and functional activity for 5-HT2A receptor ligands. Bioorg. Med. Chem. 2008, 16, 4661–4669. [Google Scholar] [CrossRef]
- Kulkarni, B.; Manjunatha, K.; Joy, M.N.; Sajith, A.M.; Prashantha, C.N.; Pakkath, R.; Alshammari, M.B. Design, synthesis and molecular docking studies of some 1-(5-(2-fluoro-5-(trifuoromethoxy)phenyl)-1,2,4-oxadiazol-3-yl)piperazine derivatives as potential anti-infammatory agents. Mol. Divers. 2022, 26, 2893–2905. [Google Scholar] [CrossRef] [PubMed]






| Compounds | % Inhibition of Denaturation at Different Concentrations | ||||
|---|---|---|---|---|---|
| 100 µg/mL | 200 µg/mL | 400 µg/mL | 800 µg/mL | 1600 µg/mL | |
| 1 | 24 ± 0.60 | 38 ± 0.59 | 52 ± 1.05 | 64 ± 0.56 | 75 ± 0.18 |
| 3a | 22 ± 0.17 | 34 ± 0.14 | 48 ± 0.48 | 65 ± 1.05 | 80 ± 1.74 |
| 3b | 19 ± 0.45 | 36 ± 0.14 | 55 ± 1.17 | 16 ± 1.14 | 82 ± 0.75 |
| 3c | 20 ± 0.47 | 30 ± 1.02 | 45 ± 0.17 | 60 ± 0.87 | 73 ± 0.55 |
| 3d | 20 ± 0.17 | 42 ± 0.78 | 58 ± 1.51 | 68 ± 1.08 | 75 ± 0.66 |
| 3e | 18 ± 0.11 | 32 ± 0.78 | 56 ± 0.15 | 69 ± 0.63 | 75 ± 1.03 |
| 3f | 40 ± 0.45 | 59 ± 0.45 | 77 ± 1.12 | 87 ± 0.16 | 90 ± 0.17 |
| 3g | 39 ± 0.45 | 60 ± 0.74 | 75 ± 0.64 | 84 ± 0.25 | 88 ± 0.86 |
| 3h | 18 ± 1.12 | 25 ± 1.01 | 36 ± 1.12 | 59 ± 0.23 | 77 ± 1.12 |
| 3i | 22 ± 0.48 | 33 ± 0.15 | 48 ± 0.56 | 58 ± 0.14 | 74 ± 0.45 |
| 3j | 24 ± 1.67 | 38 ± 0.45 | 52 ± 1.12 | 64 ± 1.08 | 75 ± 1.08 |
| 3k | 12 ± 0.56 | 24 ± 1.03 | 32 ± 0.05 | 45 ± 0.56 | 60 ± 1.12 |
| 3l | 10 ± 0.54 | 18 ± 0.56 | 28 ± 1.17 | 40 ± 1.72 | 59 ± 1.17 |
| 3m | 6 ± 0.15 | 18 ± 1.15 | 26 ± 0.78 | 39 ± 1.45 | 58 ± 0.15 |
| 3n | 8 ± 0.46 | 17 ± 0.18 | 28 ± 0.05 | 40 ± 1.03 | 60 ± 0.05 |
| 3o | 44 ± 1.05 | 59 ± 1.16 | 77 ± 0.65 | 87 ± 0.12 | 92 ± 0.45 |
| 3p | 20 ± 1.11 | 25 ± 1.12 | 35 ± 0.14 | 50 ± 0.04 | 70 ± 0.12 |
| 3q | 42 ± 1.05 | 56 ± 0.17 | 80 ± 0.65 | 84 ± 0.02 | 90 ± 1.01 |
| 3r | 18 ± 0.87 | 30 ± 0.64 | 41 ± 1.05 | 56 ± 0.63 | 70 ± 1.30 |
| 3s | 23 ± 0.14 | 38 ± 0.44 | 58 ± 1.09 | 69 ± 0.51 | 72 ± 0.14 |
| 3t | 24 ± 0.56 | 36 ± 0.15 | 45 ± 1.54 | 55 ± 1.14 | 72 ± 0.17 |
| Diclofenac | 41 ± 0.11 | 57 ± 0.46 | 80 ± 1.04 | 86 ± 1.10 | 89 ± 1.12 |
| Compounds | % Inhibition of Denaturation at Different Concentrations | ||||
|---|---|---|---|---|---|
| 100 µg/mL | 200 µg/mL | 400 µg/mL | 800 µg/mL | 1600 µg/mL | |
| 5a | 16 ± 0.55 | 22 ± 1.55 | 27 ± 0.87 | 56 ± 0.48 | 74 ± 0.60 |
| 5b | 3 ± 0.48 | 15 ± 0.46 | 22 ± 0.83 | 40 ± 1.68 | 52 ± 1.33 |
| 5c | 4 ± 0.87 | 14 ± 0.58 | 24 ± 0.60 | 38 ± 0.72 | 54 ± 0.98 |
| 5d | 5 ± 0.57 | 15 ± 0.47 | 20 ± 0.41 | 42 ± 0.97 | 52 ± 0.32 |
| 5e | 18 ± 1.60 | 25 ± 0.74 | 30 ± 0.23 | 50 ± 1.25 | 70 ± 0.74 |
| 5f | 40 ± 0.38 | 60 ± 0.95 | 78 ± 0.65 | 85 ± 0.36 | 90 ± 1.82 |
| 5g | 37 ± 0.45 | 59 ± 0.87 | 80 ± 0.79 | 86 ± 0.77 | 91 ± 0.36 |
| 5h | 14 ± 1.36 | 35 ± 1.55 | 50 ± 0.33 | 64 ± 0.89 | 70 ± 0.22 |
| 5i | 14 ± 0.81 | 28 ± 0.23 | 47 ± 1.60 | 60 ± 1.11 | 72 ± 0.60 |
| 5j | 12 ± 0.90 | 36 ± 0.54 | 52 ± 0.67 | 66 ± 0.49 | 75 ± 1.62 |
| 5k | 10 ± 0.76 | 21 ± 0.79 | 26 ± 0.86 | 38 ± 0.25 | 50 ± 0.55 |
| 5l | 14 ± 0.55 | 35 ± 0.57 | 50 ± 0.74 | 64 ± 0.78 | 70 ± 1.22 |
| 5m | 38 ± 1.05 | 58 ± 0.69 | 72 ± 0.36 | 82 ± 0.93 | 89 ± 0.63 |
| 5n | 18 ± 0.78 | 32 ± 1.32 | 48 ± 0.77 | 58 ± 0.98 | 72 ± 0.75 |
| 5o | 15 ± 0.95 | 30 ± 0.44 | 44 ± 0.99 | 59 ± 0.77 | 68 ± 0.19 |
| Diclofenac | 41 ± 0.70 | 57 ± 0.78 | 80 ± 1.03 | 86 ± 0.85 | 89 ± 0.60 |
| Compounds | % Inhibition of Denaturation at Different Concentrations | ||||
|---|---|---|---|---|---|
| 100 µg/mL | 200 µg/mL | 400 µg/mL | 800 µg/mL | 1600 µg/mL | |
| 6a | 17 ± 0.23 | 36 ± 0.35 | 47 ± 0.80 | 59 ± 0.49 | 70 ± 1.42 |
| 6b | 34 ± 0.57 | 50 ± 0.87 | 60 ± 0.63 | 72 ± 1.71 | 83 ± 0.29 |
| 6c | 37 ± 0.47 | 45 ± 1.23 | 57 ± 0.26 | 68 ± 0.96 | 80 ± 0.12 |
| 6d | 3 ± 0.76 | 17 ± 0.24 | 20 ± 1.04 | 46 ± 0.80 | 54 ± 0.72 |
| 6e | 10 ± 0.88 | 22 ± 0.92 | 35 ± 0.65 | 44 ± 0.36 | 50 ± 1.23 |
| 6f | 12 ± 0.23 | 25 ± 0.83 | 38 ± 0.92 | 48 ± 0.23 | 55 ± 0.70 |
| 6g | 20 ± 0.28 | 30 ± 0.33 | 41 ± 0.90 | 54 ± 0.74 | 65 ± 0.43 |
| 6h | 16 ± 0.64 | 32 ± 0.42 | 47 ± 0.25 | 59 ± 0.93 | 72 ± 0.64 |
| 6i | 14 ± 0.45 | 38 ± 0.61 | 45 ± 0.46 | 58 ± 1.23 | 68 ± 0.35 |
| 6j | 15 ± 0.36 | 25 ± 0.82 | 29 ± 0.39 | 50 ± 1.65 | 65 ± 0.24 |
| 6k | 5 ± 0.97 | 15 ± 0.61 | 22 ± 0.28 | 36 ± 1.01 | 48 ± 0.20 |
| 6l | 29 ± 0.52 | 42 ± 0.29 | 58 ± 0.60 | 70 ± 0.29 | 84 ± 1.67 |
| 6m | 18 ± 0.28 | 30 ± 1.26 | 42 ± 1.25 | 53 ± 0.27 | 64 ± 0.23 |
| 6n | 30 ± 0.82 | 47 ± 0.23 | 56 ± 0.28 | 68 ± 0.35 | 80 ± 1.39 |
| 7a | 3 ± 1.20 | 15 ± 0.36 | 22 ± 0.84 | 38 ± 0.58 | 45 ± 0.25 |
| 7b | 14 ± 0.27 | 28 ± 0.78 | 36 ± 0.42 | 47 ± 0.82 | 56 ± 0.39 |
| 7c | 10 ± 0.50 | 26 ± 0.86 | 40 ± 0.97 | 50 ± 0.09 | 58 ± 0.65 |
| 7d | 37 ± 0.39 | 49 ± 0.87 | 57 ± 1.26 | 69 ± 0.68 | 82 ± 0.89 |
| 7e | 18 ± 1.28 | 26 ± 0.45 | 40 ± 1.05 | 50 ± 0.36 | 57 ± 0.51 |
| 7f | 16 ± 0.24 | 25 ± 1.28 | 36 ± 0.69 | 46 ± 0.47 | 53 ± 0.45 |
| Diclofenac | 41 ± 0.68 | 57 ± 1.62 | 80 ± 1.44 | 86 ± 1.04 | 89 ± 0.23 |
| Preliminary In Vitro Screening Results, MIC (µg/mL) | Second Level Screening Results, MIC (µg/mL) | |||||||
|---|---|---|---|---|---|---|---|---|
| Compound | MTB a | MS b | MF c | % d | MTB | MS | MF | MDR-TB |
| 1 | 10 ± 0.05 | 10 ± 0.05 | >100 | <90 | >5 | >5 | >5 | >50 |
| 3a | >100 | 10 ± 0.05 | >100 | <90 | - | >5 | - | 25 ± 0.54 |
| 3b | >100 | >100 | >100 | 0 | - | - | - | - |
| 3c | >100 | 10 ± 0.07 | >100 | <90 | - | >5 | - | 25 ± 0.18 |
| 3d | >100 | 10 ± 0.07 | >100 | <90 | - | >5 | - | 25 ± 0.46 |
| 3e | >100 | >100 | >100 | 0 | - | - | - | - |
| 3f | 10 ± 0.10 | 10 ± 0.20 | >100 | <90 | >5 | - | >5 | >50 |
| 3g | 10 ± 0.25 | 10 ± 0.30 | >100 | <90 | >5 | - | >5 | >50 |
| 3h | >100 | >100 | >100 | 0 | - | - | - | - |
| 3i | >100 | 10 ± 0.05 | >100 | <90 | - | >5 | - | 25 ± 0.28 |
| 3j | >100 | >100 | >100 | 0 | - | - | - | - |
| 3k | 1 ± 0.15 | 1 ± 0.25 | 10 ± 0.35 | 90 | 0.625 ± 0.07 | 1.25 ± 0.11 | 5 ± 0.57 | 6.25 ± 0.11 |
| 3l | 1 ± 0.19 | 1 ± 0.30 | 10 ± 0.25 | 90 | 1.25 ± 0.40 | 5 ± 0.27 | >5 | 6.25 ± 0.12 |
| 3m | 1 ± 0.38 | 10 ± 0.04 | 1 ± 0.29 | 90 | 0.625 ± 0.34 | >5 | >5 | 12.5 ± 0.22 |
| 3n | 1 ± 0.51 | 1 ± 0.60 | 10 ± 0.23 | 90 | 1.25 ± 0.95 | 5± 0.33 | >5 | 6.25 ± 0.20 |
| 3o | >100 | >100 | >100 | 0 | - | - | - | - |
| 3p | 10 ± 0.51 | 10 ± 0.22 | >100 | <90 | >5 | - | >5 | >50 |
| 3q | >100 | >100 | >100 | 0 | - | - | - | - |
| 3r | >100 | 10 ± 0.31 | >100 | <90 | - | - | - | 25 ± 0.01 |
| 3s | 10 ± 0.52 | 10 ± 0.71 | >100 | <90 | >5 | - | >5 | >50 |
| 3t | >100 | 10 ± 0.15 | >100 | <90 | - | - | - | 25 ± 0.05 |
| Isoniazid | 0.7 ± 0.03 | 50 ± 0.65 | 12.5 ± 0.14 | 95 | 0.7 ± 0.18 | 50 ± 0.08 | 12.5 ± 0.14 | 12.5 ± 0.09 |
| Rifampicin | 0.5 ± 0.65 | 1.5 ± 0.32 | 1.5 ± 0.22 | 95 | 0.5 ± 0.61 | 1.5 ± 0.03 | 1.5 ± 0.28 | 25 ± 0.11 |
| Preliminary In Vitro Screening Results, MIC (µg/mL) | Second Level Screening Results, MIC (µg/mL) | |||||||
|---|---|---|---|---|---|---|---|---|
| Compound | MTB a | MS b | MF c | % d | MTB | MS | MF | MDR-TB |
| 5a | >100 | 10 ± 0.15 | >100 | <90 | - | >5 | - | 25 ± 0.11 |
| 5b | 1 ± 0.45 | 1 ± 0.75 | 10 ± 0.42 | 90 | 0.625 ± 0.17 | 1.25 ± 0.38 | 5 ± 0.51 | 6.25 ± 0.23 |
| 5c | 10 ± 0.05 | 10 ± 0.11 | 10 ± 0.34 | 90 | 1.25 ± 0.41 | 5 ± 0.28 | >5 | 6.25 ± 0.12 |
| 5d | 1 ± 0.17 | 1 ± 0.22 | 10 ± 0.41 | 90 | 0.625 ± 0.24 | >5 | 5 ± 0.05 | 12.5 ± 0.21 |
| 5e | >100 | 10 ± 0.08 | >100 | <90 | - | >5 | - | 25 ± 0.05 |
| 5f | >100 | >100 | >100 | 0 | - | - | - | - |
| 5g | >100 | >100 | >100 | 0 | - | - | - | - |
| 5h | 10 ± 0.51 | 10 ± 0.15 | >100 | <90 | >5 | - | >5 | >50 |
| 5i | 10 ± 0.28 | 10 ± 0.18 | >100 | 0 | >5 | - | >5 | >50 |
| 5j | 10 ± 0.14 | 10 ± 0.85 | >100 | <90 | >5 | - | - | >50 |
| 5k | 10 ± 0.16 | 10 ± 0.39 | >100 | <90 | >5 | >5 | >5 | 25 ± 0.48 |
| 5l | 10 ± 0.33 | 10 ± 0.28 | >100 | <90 | >5 | - | >5 | >50 |
| 5m | >100 | >100 | >100 | 0 | - | - | - | - |
| 5n | 10 ± 0.20 | 10 ± 0.65 | >100 | <90 | >5 | - | >5 | >50 |
| 5o | >100 | 10 ± 0.21 | >100 | <90 | - | >5 | - | 25 ± 0.61 |
| Isoniazid | 0.7 ± 0.10 | 50 ± 0.09 | 12.5 ± 0.34 | 95 | 0.7 ± 0.05 | 50 ± 0.01 | 12.5 ± 0.16 | 12.5 ± 0.38 |
| Rifampicin | 0.5 ± 0.17 | 1.5 ± 0.14 | 1.5 ± 0.11 | 95 | 0.5 ± 0.84 | 1.5 ± 0.54 | 1.5 ± 0.13 | 25 ± 0.74 |
| Preliminary In Vitro Screening Results, MIC (µg/mL) | Second Level Screening Results, MIC (µg/mL) | |||||||
|---|---|---|---|---|---|---|---|---|
| Compound | MTB a | MS b | MF c | % d | MTB | MS | MF | MDR-TB |
| 6a | 10 ± 0.19 | 10 ± 0.17 | >100 | <90 | >5 | - | >5 | >50 |
| 6b | 10 ± 0.45 | 10 ± 0.11 | >100 | <90 | >5 | - | >5 | >50 |
| 6c | >100 | >100 | >100 | 0 | - | - | - | - |
| 6d | 1 ± 0.50 | 1 ± 0.45 | 10 ± 0.31 | 90 | 0.625 ± 0.18 | 1.25 ± 0.74 | 5 ± 0.14 | 6.25 ± 0.26 |
| 6e | 1 ± 0.61 | 1 ± 0.10 | 10 ± 0.34 | 90 | 1.25 ± 0.41 | 5 ± 0.07 | >5 | 6.25 ± 0.16 |
| 6f | 1 ± 0.28 | 1 ± 0.07 | 10 ± 0.31 | 90 | 0.625 ± 0.23 | 1.25 ± 0.14 | 5 ± 0.05 | 6.25 ± 0.22 |
| 6g | 10 ± 0.15 | 10 ± 0.70 | >100 | <90 | >5 | - | >5 | >50 |
| 6h | >100 | >100 | >100 | 0 | - | - | - | - |
| 6i | >100 | 10 ± 0.04 | >100 | <90 | - | >5 | - | 25 ± 0.41 |
| 6j | >100 | >100 | >100 | 0 | - | - | - | - |
| 6k | 1 ± 0.21 | 1 ± 0.44 | 10 ± 0.13 | 90 | 1.25 ± 0.02 | 5 ± 0.21 | >5 | 6.25 ± 0.56 |
| 6l | >100 | >100 | >100 | 0 | - | - | - | - |
| 6m | >100 | >100 | >100 | 0 | - | - | - | - |
| 6n | >100 | 10 ± 0.09 | >100 | <90 | - | >5 | - | 25 ± 0.54 |
| 7a | 10 ± 0.18 | 10 ± 0.21 | >100 | <90 | >5 | >5 | >5 | >50 |
| 7b | 10 ± 0.33 | 10 ± 0.08 | >100 | <90 | >5 | - | >5 | >50 |
| 7c | >100 | 10 ± 0.17 | >100 | <90 | - | >5 | - | 25 ± 0.54 |
| 7d | >100 | >100 | >100 | 0 | - | - | - | - |
| 7e | 10 ± 0.11 | 10 ± 0.51 | >100 | <90 | >5 | >5 | >5 | >50 |
| 7f | 1 ± 0.37 | 1 ± 0.71 | 10 ± 0.15 | 90 | 1.25 ± 0.11 | 5 ± 0.31 | >5 | 6.25 ± 0.26 |
| Isoniazid | 0.7 ± 0.28 | 50 ± 0.04 | 12.5 ± 0.17 | 95 | 0.7 ± 0.16 | 50 ± 0.16 | 12.5 ± 0.05 | 12.5 ± 0.19 |
| Rifampicin | 0.5 ± 0.35 | 1.5 ± 0.18 | 1.5 ± 0.16 | 95 | 0.5 ± 0.28 | 1.5 ± 0.64 | 1.5 ± 0.49 | 25 ± 0.14 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nibin Joy, M.; Guda, M.R.; Zyryanov, G.V. Evaluation of Anti-Inflammatory and Anti-Tubercular Activity of 4-Methyl-7-Substituted Coumarin Hybrids and Their Structure Activity Relationships. Pharmaceuticals 2023, 16, 1326. https://doi.org/10.3390/ph16091326
Nibin Joy M, Guda MR, Zyryanov GV. Evaluation of Anti-Inflammatory and Anti-Tubercular Activity of 4-Methyl-7-Substituted Coumarin Hybrids and Their Structure Activity Relationships. Pharmaceuticals. 2023; 16(9):1326. https://doi.org/10.3390/ph16091326
Chicago/Turabian StyleNibin Joy, Muthipeedika, Mallikarjuna R. Guda, and Grigory V. Zyryanov. 2023. "Evaluation of Anti-Inflammatory and Anti-Tubercular Activity of 4-Methyl-7-Substituted Coumarin Hybrids and Their Structure Activity Relationships" Pharmaceuticals 16, no. 9: 1326. https://doi.org/10.3390/ph16091326
APA StyleNibin Joy, M., Guda, M. R., & Zyryanov, G. V. (2023). Evaluation of Anti-Inflammatory and Anti-Tubercular Activity of 4-Methyl-7-Substituted Coumarin Hybrids and Their Structure Activity Relationships. Pharmaceuticals, 16(9), 1326. https://doi.org/10.3390/ph16091326