Effects of Preoperative Chronic Steroid Use on Postoperative Outcomes in Orthopedic Surgery: A Systematic Review and Meta-Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Search Strategies
2.2. Study Selection and Eligibility Criteria
2.3. Data Extraction and Risk of Bias Assessment
2.4. Statistical Analysis
3. Results
3.1. Literature Retrieval and Summary of Included Articles
3.2. Definition of Preoperative Chronic Steroid Use
3.3. Postoperative Outcomes of Interest
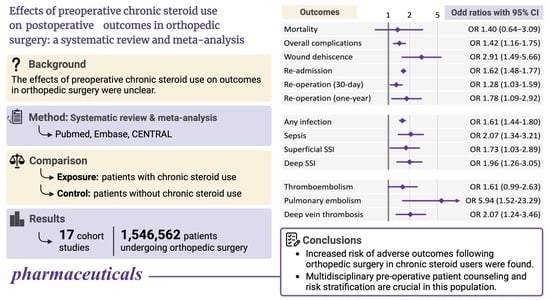
3.4. Primary Outcome: Mortality
3.5. Overall Complications
3.6. Wound Dehiscence
3.7. Re-Admission
3.8. Re-Operation
3.9. Infectious Complications
3.10. Thromboembolism
3.11. Subgroup Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fardet, L.; Petersen, I.; Nazareth, I. Prevalence of long-term oral glucocorticoid prescriptions in the UK over the past 20 years. Rheumatology 2011, 50, 1982–1990. [Google Scholar] [CrossRef] [PubMed]
- Stanbury, R.M.; Graham, E.M. Systemic corticosteroid therapy—Side effects and their management. Br. J. Ophthalmol. 1998, 82, 704–708. [Google Scholar] [CrossRef] [PubMed]
- Ng, M.K.; Celermajer, D.S. Glucocorticoid treatment and cardiovascular disease. Heart 2004, 90, 829–830. [Google Scholar] [CrossRef] [PubMed]
- Poetker, D.M.; Reh, D.D. A comprehensive review of the adverse effects of systemic corticosteroids. Otolaryngol. Clin. N. Am. 2010, 43, 753–768. [Google Scholar] [CrossRef]
- Schäcke, H.; Döcke, W.-D.; Asadullah, K. Mechanisms involved in the side effects of glucocorticoids. Pharmacol. Ther. 2002, 96, 23–43. [Google Scholar] [CrossRef]
- Anstead, G.M. Steroids, retinoids, and wound healing. Adv. Wound Care 1998, 11, 277–285. [Google Scholar]
- Wang, A.S.; Armstrong, E.J.; Armstrong, A.W. Corticosteroids and wound healing: Clinical considerations in the perioperative period. Am. J. Surg. 2013, 206, 410–417. [Google Scholar] [CrossRef]
- Farmer, R.P.; Herbert, B.; Cuellar, D.O.; Hao, J.; Stahel, P.F.; Yasui, R.; Hak, D.J.; Mauffrey, C. Osteoporosis and the orthopaedic surgeon: Basic concepts for successful co-management of patients’ bone health. Int. Orthop. 2014, 38, 1731–1738. [Google Scholar] [CrossRef]
- Chouairi, F.; Torabi, S.J.; Mercier, M.R.; Gabrick, K.S.; Alperovich, M. Chronic steroid use as an independent risk factor for perioperative complications. Surgery 2019, 165, 990–995. [Google Scholar] [CrossRef]
- Turan, A.; Dalton, J.E.; Turner, P.L.; Sessler, D.I.; Kurz, A.; Saager, L. Preoperative prolonged steroid use is not associated with intraoperative blood transfusion in noncardiac surgical patients. Anesthesiology 2010, 113, 285–291. [Google Scholar] [CrossRef]
- Ismael, H.; Horst, M.; Farooq, M.; Jordon, J.; Patton, J.H.; Rubinfeld, I.S. Adverse effects of preoperative steroid use on surgical outcomes. Am. J. Surg. 2011, 201, 305–308; discussion 308–309. [Google Scholar] [CrossRef] [PubMed]
- Kebaish, K.J.; Galivanche, A.R.; Varthi, A.G.; Ottesen, T.D.; Rubin, L.E.; Grauer, J.N. Long-term Corticosteroid Use Independently Correlates with Complications after Posterior Lumbar Spine Surgery. Orthopedics 2021, 44, 172–179. [Google Scholar] [CrossRef] [PubMed]
- Aziz, K.T.; Best, M.J.; Ren, M.; Nayar, S.K.; Timothy Kreulen, R.; Gupta, H.O.; Srikumaran, U. The impact of chronic steroid use on early postoperative complications in shoulder surgery. Phys. Sportsmed. 2021, 49, 223–228. [Google Scholar] [CrossRef] [PubMed]
- Tihista, M.; Gu, A.; Wei, C.; Weinreb, J.H.; Rao, R.D. The impact of long-term corticosteroid use on acute postoperative complications following lumbar decompression surgery. J. Clin. Orthop. Trauma 2020, 11, 921–927. [Google Scholar] [CrossRef] [PubMed]
- Fassihi, S.C.; Gu, A.; Perim, D.A.; Wei, C.; Stake, S.; Thakkar, S.; Unger, A.S.; Ast, M.P.; Sculco, P.K. Chronic preoperative corticosteroid use is not associated with surgical site infection following revision total knee arthroplasty. J. Orthop. 2020, 20, 173–176. [Google Scholar] [CrossRef]
- Stroup, D.F.; Berlin, J.A.; Morton, S.C.; Olkin, I.; Williamson, G.D.; Rennie, D.; Moher, D.; Becker, B.J.; Sipe, T.A.; Thacker, S.B.; et al. Meta-analysis of Observational Studies in Epidemiology: A Proposal for Reporting. JAMA 2000, 283, 2008–2012. [Google Scholar] [CrossRef]
- Wells, G.A.; Shea, B.; O’Connell, D.; Peterson, J.; Welch, V.; Losos, M.; Tugwell, P. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses. Available online: https://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed on 22 August 2022).
- Kuo, L.T.; Shao, S.C.; Chi, C.C. Ten essential steps for performing a systematic review: A quick tutorial. Dermatol. Sin. 2022, 40, 204–206. [Google Scholar] [CrossRef]
- Boddapati, V.; Fu, M.C.; Su, E.P.; Sculco, P.K.; Bini, S.A.; Mayman, D.J. Preoperative corticosteroid use for medical conditions is associated with increased postoperative infectious complications and readmissions after total hip arthroplasty: A propensity-matched study. Am. J. Orthop. 2018, 47. [Google Scholar] [CrossRef]
- Boylan, M.R.; Perfetti, D.C.; Elmallah, R.K.; Krebs, V.E.; Paulino, C.B.; Mont, M.A. Does chronic corticosteroid use increase risks of readmission, thromboembolism, and revision after THA? Clin. Orthop. Relat. Res. 2016, 474, 744–751. [Google Scholar] [CrossRef]
- Cloney, M.B.; Garcia, R.M.; Smith, Z.A.; Dahdaleh, N.S. The effect of steroids on complications, readmission, and reoperation after posterior lumbar fusion. World Neurosurg. 2018, 110, e526–e533. [Google Scholar] [CrossRef]
- Ifarraguerri, A.M.; Gupta, P.; Quan, T.; Cohen, J.S.; Chen, F.R.; Zeitlin, J.; Manzi, J.E.; Farley, B.; Ramamurti, P.; Tabaie, S. Risks of immunosuppressive therapy in patients undergoing open reduction internal fixation for ankle fractures. J. Foot Ankle Surg. 2023, 62, 802–806. [Google Scholar] [CrossRef] [PubMed]
- Kantar, R.S.; Haddad, A.G.; Tamim, H.; Jamali, F.; Taher, A.T. Venous thromboembolism and preoperative steroid use: Analysis of the NSQIP database to evaluate risk in surgical patients. Eur. J. Intern. Med. 2015, 26, 528–533. [Google Scholar] [CrossRef] [PubMed]
- Kittle, H.; Ormseth, A.; Patetta, M.J.; Sood, A.; Gonzalez, M.H. Chronic corticosteroid use as a risk factor for perioperative complications in patients undergoing total joint arthroplasty. J. Am. Acad. Orthop. Surg. Glob. Res. Rev. 2020, 4, e2000001. [Google Scholar] [CrossRef] [PubMed]
- Lee, R.; Lee, D.; Gowda, N.B.; Iweala, U.; Weinreb, J.H.; Falk, D.P.; Yu, W.; O’Brien, J.R. Increased rates of septic shock, cardiac arrest, and mortality associated with chronic steroid use following anterior cervical discectomy and fusion for cervical stenosis. Int. J. Spine Surg. 2020, 14, 649–656. [Google Scholar] [CrossRef] [PubMed]
- Newton, W.N.; Johnson, C.A.; Daley, D.N.; Daly, C.A. Long-term oral steroid use: A unique risk factor in 4-corner fusion compared with other wrist salvage operations. Hand 2023, 15589447231151257. [Google Scholar] [CrossRef]
- Quan, T.; Chen, F.R.; Howard, P.; Gioia, C.; Pollard, T.; Gu, A.; Tabaie, S. The effect of steroid use on complications after distal radius fracture repair. J. Wrist Surg. 2022, 12, 306–311. [Google Scholar] [CrossRef]
- Roberts, S.; Formanek, B.; Wang, J.C.; Buser, Z. Complication rates after elective lumbar fusion procedures in patients with oral preoperative corticosteroid use. Spine 2021, 46, E187–E189. [Google Scholar] [CrossRef]
- Singla, A.; Qureshi, R.; Chen, D.Q.; Nourbakhsh, A.; Hassanzadeh, H.; Shimer, A.L.; Shen, F.H. Risk of surgical site infection and mortality following lumbar fusion surgery in patients with chronic steroid usage and chronic methicillin-resistant Staphylococcus aureus infection. Spine 2019, 44, E408–E413. [Google Scholar] [CrossRef]
- White, S.J.W.; Carrillo, O.; Cheung, Z.B.; Ranson, W.A.; Cho, S.K. The effects of preoperative steroid therapy on perioperative complications after elective anterior lumbar fusion. World Neurosurg. 2019, 126, e314–e322. [Google Scholar] [CrossRef]
- White, S.J.W.; Ranson, W.A.; Cho, B.; Cheung, Z.B.; Ye, I.; Carrillo, O.; Kim, J.S.; Cho, S.K. The effects of preoperative steroid therapy on perioperative morbidity and mortality after adult spinal deformity surgery. Spine Deform. 2019, 7, 779–787. [Google Scholar] [CrossRef]
- Waljee, A.K.; Rogers, M.A.; Lin, P.; Singal, A.G.; Stein, J.D.; Marks, R.M.; Ayanian, J.Z.; Nallamothu, B.K. Short term use of oral corticosteroids and related harms among adults in the United States: Population based cohort study. BMJ 2017, 357, j1415. [Google Scholar] [CrossRef] [PubMed]
- Cazzola, M.; Page, C.P.; Wedzicha, J.A.; Celli, B.R.; Anzueto, A.; Matera, M.G. Use of thiols and implications for the use of inhaled corticosteroids in the presence of oxidative stress in COPD. Respir. Res. 2023, 24, 194. [Google Scholar] [CrossRef]
- Costantini, D.; Marasco, V.; Møller, A.P. A meta-analysis of glucocorticoids as modulators of oxidative stress in vertebrates. J. Comp. Physiol. B 2011, 181, 447–456. [Google Scholar] [CrossRef] [PubMed]
- Kücükakin, B.; Gögenur, I.; Reiter, R.J.; Rosenberg, J. Oxidative stress in relation to surgery: Is there a role for the antioxidant melatonin? J. Surg. Res. 2009, 152, 338–347. [Google Scholar] [CrossRef]
- Aremu, P.A.; Ajayi, A.M.; Ben-Azu, B.; Orewole, O.T.; Umukoro, S. Spinal and general anesthesia produces differential effects on oxidative stress and inflammatory cytokines in orthopedic patients. Drug Metab. Pers. Ther. 2020, 36, 17–23. [Google Scholar] [CrossRef]
- Senoner, T.; Velik-Salchner, C.; Luckner, G.; Tauber, H. Anesthesia-Induced Oxidative Stress: Are there differences between intravenous and inhaled anesthetics? Oxid. Med. Cell. Longev. 2021, 2021, 8782387. [Google Scholar] [CrossRef] [PubMed]
- Oray, M.; Abu Samra, K.; Ebrahimiadib, N.; Meese, H.; Foster, C.S. Long-term side effects of glucocorticoids. Expert Opin. Drug Saf. 2016, 15, 457–465. [Google Scholar] [CrossRef]
- Stuck, A.E.; Minder, C.E.; Frey, F.J. Risk of infectious complications in patients taking glucocorticosteroids. Rev. Infect. Dis. 1989, 11, 954–963. [Google Scholar] [CrossRef]
- Dostal, G.H.; Gamelli, R.L. The differential effect of corticosteroids on wound disruption strength in mice. Arch. Surg. 1990, 125, 636–640. [Google Scholar] [CrossRef]
- Howes, E.L.; Plotz, C.M.; Blunt, J.W.; Ragan, C. Retardation of wound healing by cortisone. Surgery 1950, 28, 177–181. [Google Scholar]
- Meadows, E.C.; Prudden, J.F. A study of the influence of adrenal steroids on the strength of healing wounds; preliminary report. Surgery 1953, 33, 841–848. [Google Scholar] [PubMed]
- Pearce, C.W.; Foot, N.C.; Jordan, G.L., Jr.; Law, S.W.; Wantz, G.E., Jr. The effect and interrelation of testosterone, cortisone, and protein nutrition on wound healing. Surg. Gynecol. Obstet. 1960, 111, 274–284. [Google Scholar] [PubMed]
- Lindstedt, E.; Sandblom, P. Wound healing in man: Tensile strength of healing wounds in some patient groups. Ann. Surg. 1975, 181, 842–846. [Google Scholar] [CrossRef] [PubMed]
- Sandblom, P.; Petersen, P.; Muren, A. Determination of the tensile strength of the healing wound as a clinical test. Acta Chir. Scand. 1953, 105, 252–257. [Google Scholar] [PubMed]
- Johannesdottir, S.A.; Horváth-Puhó, E.; Dekkers, O.M.; Cannegieter, S.C.; Jørgensen, J.O.L.; Ehrenstein, V.; Vandenbroucke, J.P.; Pedersen, L.; Sørensen, H.T. Use of glucocorticoids and risk of venous thromboembolism: A nationwide population-based case-control study. JAMA Intern. Med. 2013, 173, 743–752. [Google Scholar] [CrossRef]
- Orsi, F.A.; Lijfering, W.M.; Geersing, G.J.; Rosendaal, F.R.; Dekkers, O.M.; le Cessie, S.; Cannegieter, S.C. Glucocorticoid use and risk of first and recurrent venous thromboembolism: Self-controlled case-series and cohort study. Br. J. Haematol. 2021, 193, 1194–1202. [Google Scholar] [CrossRef]
- Carlson, B.C.; Robinson, W.A.; Wanderman, N.R.; Sebastian, A.S.; Nassr, A.; Freedman, B.A.; Anderson, P.A. A review and clinical perspective of the impact of osteoporosis on the spine. Geriatr. Orthop. Surg. Rehabil. 2019, 10, 2151459319861591. [Google Scholar] [CrossRef]


| Author (Year) | Database | Study Period | Expose/Control | Surgical Procedure | CPT or ICD Codes * | Outcome of Interest | Adjustment Method |
|---|---|---|---|---|---|---|---|
| Aziz KT et al. (2021) [13] | ACS-NSQIP | 2011–2018 | Steroids: 1662 Non-steroids: 98,308 | Shoulder surgery (Exclude arthroplasty) | 29827, 23410, 23412, 29828, 29430, 24342, 23020, 23130, 23415, 23420, 29826, 23420, 29824, 29805, 29806, 29807, 29825, 29822, 29823, 29819, 29820, 29821, 23455, 23460, 23465, 23662, 23466 | Overall complications, infectious complications | Multivariable logistic regression |
| Boddapati V et al. (2018) [19] | ACS-NSQIP | 2005–2015 | Steroids: 3714 Non-steroids: 97,818 | Total hip arthroplasty | 27130 | Mortality, overall complications, wound dehiscence, infectious complications, thromboembolism, re-admission, re-operation | Propensity score matching |
| Boylan MR et al. (2016) [20] | SPARCS | 2003–2010 | Steroids: 402 Non-steroids: 104,720 | Primary total hip arthroplasty | Inclusion: ICD-9: 81.51, 00.74, 00.75, 00.76, 00.77 Exclusion: ICD-9: 899, 88 (exclude) | Thromboembolism, re-admission, re-operation | Propensity score matching |
| Cloney MB et al. (2018) [21] | ACS-NSQIP | 2006–2013 | Steroids: 353 Non-steroids: 8139 | Posterior lumbar fusion | Inclusion: 22612 Exclusion: 22849, 22850, 22852, 22855; 0090T, 0092T, 0093T, 0095T, 0096T, 0098T, 0163T, 0164T, 0165T, 0163T, 0164T, 0165T, 0195T, 0196T, 0202T | Overall complications, wound dehiscence, infectious complications, thromboembolism, re-admission, re-operation | Multivariable logistic regression |
| Fassihi SC et al. (2020) [15] | ACS-NSQIP | 2007–2016 | Steroids: 474 Non-steroids: 10,499 | Revision total knee arthroplasty † | 27486, 27487, 27488 | Mortality, wound dehiscence, infectious complications, re-operation | Multivariable logistic regression |
| Ifarraguerri AM et al. (2023) [22] | ACS-NSQIP | 2006–2018 | Steroids: 178 Non-steroids: 10,153 | ORIF for ankle fractures | CPT: 27766, 27792, 27814, 27822, 27823 ICD-9: 824.0–824.9 ICD-10: S82.5, S82.6, S82.84, S82.85, S82.87, S82.89 | Mortality, wound dehiscence, infectious complications, thromboembolism, re-admission, re-operation | Multivariable logistic regression |
| Kantar RS et al. (2015) [23] | ACS-NSQIP | 2008–2012 | Steroids: 6575 Non-steroids: 223,029 | All major surgical procedures stratified by subspecialty (orthopedic surgery) | Not specified | Thromboembolism | Multivariable logistic regression |
| Kebaish KJ et al. (2021) [12] | ACS-NSQIP | 2005–2016 | Steroids: 5243 Non-steroids: 135,276 | Elective posterior lumbar spine surgery ‡ | Inclusion: 63005, 63012, 63017, 63030, 63035, 63042, 63044, 63047, 63048; 22612, 22614; 22630, 22632, 22633, 22634 Exclusion: 22558, 22585, 22845, 22846, 22847, 22853 | Mortality, overall complications, wound dehiscence, infectious complications, thromboembolism, re-admission, re-operation | Propensity score matching and Multivariable logistic regression |
| Kittle H et al. (2020) [24] | ACS-NSQIP | 2010–2017 | Steroids: 14,774 Non-steroids: 388,792 | Total joint arthroplasty § | 27447, 27130, 27134, 27137, 27138, 27486, 27487 | Mortality, wound dehiscence, infectious complications, thromboembolism, re-admission | NA |
| Lee R et al. (2020) [25] | ACS-NSQIP | 2005–2016 | Steroids: 198 Non-steroids: 5179 | Anterior cervical discectomy and fusion | 22551, 22554 | Mortality, wound dehiscence, infectious complications, thromboembolism, re-admission, re-operation | Propensity score matching |
| Newton WN et al. (2023) [26] | ACS-NSQIP | 2005–2020 | Steroids: 93 Non-steroids: 1205 | Salvage operations for wrist arthritis ‖ | 25215, 25820, 25825, 25800, 25805, 25810, 25446 | Overall complications, wound dehiscence, infectious complications, thromboembolism, re-admission, re-operation | Multivariable logistic regression |
| Quan et al. (2022) [27] | ACS-NSQIP | 2007–2018 | Steroids: 360 Non-steroids: 16,145 | ORIF for DRFs | 25607, 25608, 25609 | Mortality, overall complications, infectious complications, thromboembolism, re-operation | Multivariable logistic regression |
| Roberts S et al. (2021) [28] | PearlDiver | 2007–2017 | Steroids (>6 m) ¶: 2611 Steroids (<6 m): 2800 Non-steroids: 3704 | Posterior/transforaminal lumbar interbody fusion | 22630, 22632, 22633, 22634 | Wound dehiscence, infectious complications, re-admission, re-operation | Matched unexposed cohort |
| Singla A et al. (2019) [29] | PearlDiver | 2005–2012 | Steroids: 11,687 Non-steroids: 348,318 | Elective posterior lumbar fusion | ICD-9- P-8106, ICD-9-P-8107, ICD-9-P-8108 | Mortality, infectious complications | Multivariable logistic regression |
| Tihista M et al. (2020) [14] | ACS-NSQIP | 2005–2016 | Steroids: 1044 Non-steroids: 25,690 | Lumbar decompression procedures | 63005, 63017, 63030, 63042, 63047 | Mortality, overall complications, wound dehiscence, infectious complications, thromboembolism, re-admission, re-operation | Multivariable logistic regression |
| White SJW et al. (2019a) [30] | ACS-NSQIP | 2008–2015 | Steroids: 289 Non-steroids: 9194 | Elective anterior lumbar fusion | 22558 | Mortality, wound dehiscence, infectious complications, thromboembolism | Multivariable logistic regression |
| White SJW et al. (2019b) [31] | ACS-NSQIP | 2008–2015 | Steroids: 418 Non-steroids: 7518 | Elective adult spinal deformity surgery | 22595, 22600, 22612, 22630, 22633, 22551, 22554, 22558 | Mortality, wound dehiscence, infectious complications, thromboembolism | Multivariable logistic regression |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hung, Y.-T.; Hung, W.-K.; Chi, C.-C. Effects of Preoperative Chronic Steroid Use on Postoperative Outcomes in Orthopedic Surgery: A Systematic Review and Meta-Analysis. Pharmaceuticals 2023, 16, 1328. https://doi.org/10.3390/ph16091328
Hung Y-T, Hung W-K, Chi C-C. Effects of Preoperative Chronic Steroid Use on Postoperative Outcomes in Orthopedic Surgery: A Systematic Review and Meta-Analysis. Pharmaceuticals. 2023; 16(9):1328. https://doi.org/10.3390/ph16091328
Chicago/Turabian StyleHung, Yu-Ting, Wei-Kai Hung, and Ching-Chi Chi. 2023. "Effects of Preoperative Chronic Steroid Use on Postoperative Outcomes in Orthopedic Surgery: A Systematic Review and Meta-Analysis" Pharmaceuticals 16, no. 9: 1328. https://doi.org/10.3390/ph16091328
APA StyleHung, Y. -T., Hung, W. -K., & Chi, C. -C. (2023). Effects of Preoperative Chronic Steroid Use on Postoperative Outcomes in Orthopedic Surgery: A Systematic Review and Meta-Analysis. Pharmaceuticals, 16(9), 1328. https://doi.org/10.3390/ph16091328