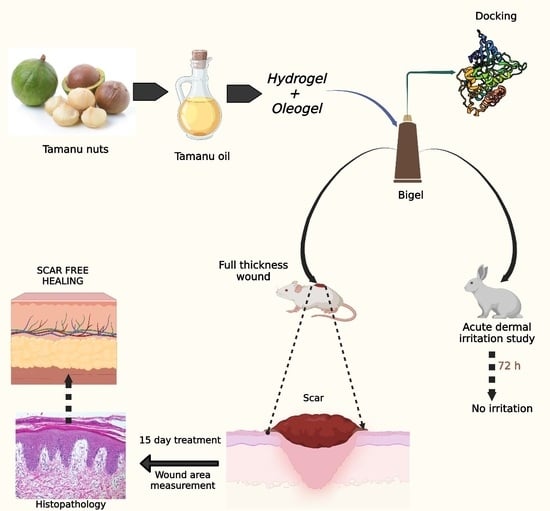
An Integrated Computational and Experimental Approach to Formulate Tamanu Oil Bigels as Anti-Scarring Agent
Abstract
:1. Introduction
2. Results and Discussion
2.1. GC-MS Characterization of Tamanu Oil
2.2. In Silico Studies—Molecular Modeling
2.3. Formulation of Bigels
2.4. Evaluation of Bigel
2.4.1. pH
2.4.2. Viscosity
2.4.3. Spreadability
2.4.4. SEM Analysis
2.5. In Vivo Studies
2.5.1. Acute Dermal Irritation Studies
2.5.2. In Vivo Wound-Healing Studies
2.6. Histopathological Studies
3. Materials and Methods
3.1. Materials
3.2. GC-MS Characterization of Tamanu Oil
3.3. In Silico Studies
3.3.1. Preparation of Ligand and Selection of Protein
3.3.2. Preparation of Protein
3.3.3. Molecular Modeling Studies
3.4. Formulation of Bigels
3.5. Evaluation of Bigels
3.5.1. Organoleptic Evaluation
3.5.2. pH
3.5.3. Spreadability
3.5.4. Viscosity
3.5.5. Scanning Electron Microscopic Analysis
3.6. In Vivo Studies
3.6.1. Acute Dermal Irritation Studies
3.6.2. In Vivo Wound-Healing Studies
3.7. Histopathological Studies
3.8. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Guo, S.; DiPietro, L.A. Factors Affecting Wound Healing. J. Dent. Res. 2010, 89, 219–229. [Google Scholar] [CrossRef] [PubMed]
- Andonova, V.; Peneva, P.; Georgiev, G.V.; Toncheva, V.T.; Apostolova, E.; Peychev, Z.; Dimitrova, S.; Katsarova, M.; Petrova, N.; Kassarova, M. Ketoprofen-Loaded Polymer Carriers in Bigel Formulation: An Approach to Enhancing Drug Photostability in Topical Application Forms. Int. J. Nanomed. 2017, 12, 6221–6238. [Google Scholar] [CrossRef]
- Kamolz, L.-P.; Griffith, M.; Finnerty, C.C.; Kasper, C. Skin Regeneration, Repair, and Reconstruction. BioMed Res. Int. 2015, 2015, 892031. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Lin, C.; Lin, B.; Wang, Z.; Zhang, H.; Wu, F.; Cheng, Y.; Xiang, L.; Guo, D.-J.; Luo, X.; et al. The Anti-Scar Effects of Basic Fibroblast Growth Factor on the Wound Repair In Vitro and In Vivo. PLoS ONE 2013, 8, e59966. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, H.; Patel, S.; Pastar, I. The Role of TGFΒ Signaling in Wound Epithelialization. Adv. Wound Care 2014, 3, 482–491. [Google Scholar] [CrossRef] [PubMed]
- Stoica, A.-I.; Grumezescu, A.M.; Hermenean, A.; Andronescu, E.; Vasile, B.S. Scar-Free Healing: Current Concepts and Future Perspectives. Nanomaterials 2020, 10, 2179. [Google Scholar] [CrossRef] [PubMed]
- Lichtman, M.K.; Otero-Viñas, M.; Falanga, V. Transforming Growth Factor Beta (TGF-β) Isoforms in Wound Healing and Fibrosis. Wound Repair Regen. 2016, 24, 215–222. [Google Scholar] [CrossRef]
- Dweck, A.C.; Meadows, T.T. Tamanu (Calophyllum inophyllum)—The African, Asian, Polynesian and Pacific Panacea. Int. J. Cosmet. Sci. 2002, 24, 341–348. [Google Scholar] [CrossRef]
- Cassien, M.; Mercier, A.; Thétiot-Laurent, S.; Culcasi, M.; Ricquebourg, E.; Asteian, A.; Herbette, G.; Bianchini, J.-P.; Raharivelomanana, P.; Pietri, S. Improving the Antioxidant Properties of Calophyllum inophyllum Seed Oil from French Polynesia: Development and Biological Applications of Resinous Ethanol-Soluble Extracts. Antioxidants 2021, 10, 199. [Google Scholar] [CrossRef]
- Ginigini, J.; Lecellier, G.; Nicolas, M.; Nour, M.; Hnawia, E.; Lebouvier, N.; Herbette, G.; Lockhart, P.J.; Raharivelomanana, P. Chemodiversity of Calophyllum inophyllum L. Oil Bioactive Components Related to Their Specific Geographical Distribution in the South Pacific Region. PeerJ 2019, 7, e6896. [Google Scholar] [CrossRef]
- Prasad, J.S.; Shrivastava, A.; Khanna, A.; Bhatia, G.; Awasthi, S.K.; Narender, T. Antidyslipidemic and Antioxidant Activity of the Constituents Isolated from the Leaves of Calophyllum inophyllum. Phytomedicine 2012, 19, 1245–1249. [Google Scholar] [CrossRef] [PubMed]
- Pribowo, A.; Girish, J.; Gustiananda, M.; Nandhira, R.G.; Hartrianti, P. Potential of Tamanu (Calophyllum inophyllum) Oil for Atopic Dermatitis Treatment. Evid. Based Complement. Altern. Med 2021, 2021, 6332867. [Google Scholar] [CrossRef]
- Saki, E.; Murthy, V.; Khandanlou, R.; Wang, H.; Wapling, J.; Weir, R. Optimisation of Calophyllum inophyllum Seed Oil Nanoemulsion as a Potential Wound Healing Agent. BMC Complement. Med. Ther. 2022, 22, 285. [Google Scholar] [CrossRef]
- Perumal, S.; Ekambaram, S.; Dhanam, T. In Vivo Antiarthritic Activity of the Ethanol Extracts of Stem Bark and Seeds of Calophyllum inophyllum in Freund’s Complete Adjuvant Induced Arthritis. Pharm. Biol. 2017, 55, 1330–1336. [Google Scholar] [CrossRef] [PubMed]
- Erdogan, S.S.; Gur, T.; Terzi, N.K.; Dogan, B. Evaluation of the Cutaneous Wound Healing Potential of Tamanu Oil in Wounds Induced in Rats. J. Wound Care 2021, 30 (Suppl. S9a), Vi–Vx. [Google Scholar] [CrossRef] [PubMed]
- Léguillier, T.; Lecsö-Bornet, M.; Lemus, C.; Rousseau-Ralliard, D.; Lebouvier, N.; Hnawia, E.; Nour, M.; Aalbersberg, W.G.L.; Ghazi, K.; Raharivelomanana, P.; et al. The Wound Healing and Antibacterial Activity of Five Ethnomedical Calophyllum inophyllum Oils: An Alternative Therapeutic Strategy to Treat Infected Wounds. PLoS ONE 2015, 10, e0138602. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.-H.; Liu, Y.-W.; Chen, Z.-F.; Chiou, W.-F.; Tsai, Y.-C.; Chen, C.-C. Calophyllolide Content in Calophyllum inophyllum at Different Stages of Maturity and Its Osteogenic Activity. Molecules 2015, 20, 12314–12327. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, V.-L.; Truong, C.-T.; Nguyen, B.C.Q.; Van Vo, T.-N.; Dao, T.-T.; Nguyen, V.-D.; Trinh, D.-T.T.; Huynh, H.K.; Bui, C.-B. Anti-Inflammatory and Wound Healing Activities of Calophyllolide Isolated from Calophyllum inophyllum Linn. PLoS ONE 2017, 12, e0185674. [Google Scholar] [CrossRef]
- Tsai, S.C.; Yu, L.; Chiang, J.-H.; Liu, F.C.; Lin, W.-H.; Chang, S.-Y.; Lin, W.; Wu, C.H.; Weng, J.R. Anti-Inflammatory Effects of Calophyllum inophyllum L. in RAW264.7 Cells. Oncol. Rep. 2012, 28, 1096–1102. [Google Scholar] [CrossRef]
- Ansel, J.-L.; Lupo, E.; Mijouin, L.; Guillot, S.; Butaud, J.-F.; Ho, R.; Lecellier, G.; Raharivelomanana, P.; Pichon, C. Biological Activity of Polynesian Calophyllum inophyllum Oil Extract on Human Skin Cells. Planta Medica 2016, 82, 961–966. [Google Scholar] [CrossRef]
- Shakeel, A.; Farooq, U.; Gabriele, D.; Marangoni, A.G.; Lupi, F.R. Bigels and Multi-Component Organogels: An Overview from Rheological Perspective. Food Hydrocoll. 2021, 111, 106190. [Google Scholar] [CrossRef]
- Baltuonytė, G.; Eisinaitė, V.; Kazernavičiūtė, R.; Vinauskienė, R.; Jasutienė, I.; Leskauskaitė, D. Novel Formulation of Bigel-Based Vegetable Oil Spreads Enriched with Lingonberry Pomace. Foods 2022, 11, 2213. [Google Scholar] [CrossRef] [PubMed]
- Martín-Illana, A.; Notario-Pérez, F.; Cazorla-Luna, R.; Ruiz-Caro, R.; Bonferoni, M.C.; Tamayo, A.; Veiga, M.D. Bigels as Drug Delivery Systems: From Their Components to Their Applications. Drug Discov. Today 2022, 27, 1008–1026. [Google Scholar] [CrossRef]
- Keerthy, P.; Patil, S.B. A Clinical Study to Evaluate the Efficacy of Murivenna Application on Episiotomy Wound. J. Ayurveda Integr. Med. Sci. 2020, 5, 65–71. [Google Scholar] [CrossRef]
- Hepsibah, P.T.; Rosamma, M.P.; Prasad, N.B.; Kumar, P.S. Standardisation of murivenna and hemajeevanti taila. Anc. Sci. Life 1993, 12, 428–434. [Google Scholar] [PubMed]
- Manikandan, A.; Mani, M.P.; Jaganathan, S.K.; Rajasekar, R.; Mani, M.P. Formation of Functional Nanofibrous Electrospun Polyurethane and Murivenna Oil with Improved Haemocompatibility for Wound Healing. Polym. Test. 2017, 61, 106–113. [Google Scholar] [CrossRef]
- Itoigawa, M.; Ito, C.; Tan, H.T.W.; Kuchide, M.; Tokuda, H.; Nishino, H.; Furukawa, H. Cancer Chemopreventive Agents, 4-Phenylcoumarins from Calophyllum inophyllum. Cancer Lett. 2001, 169, 15–19. [Google Scholar] [CrossRef]
- Saravanan, R.; Dhachinamoorthi, D.; Senthilkumar, K.; Thamizhvanan, K. Antimicrobial activity of various extracts from various parts of Calophyllum inophyllum L. J. Appl. Pharm. Sci. 2011, 1, 12. [Google Scholar]
- Creagh, T.; Ruckle, J.; Tolbert, D.T.; Giltner, J.; Eiznhamer, D.A.; Dutta, B.; Flavin, M.T.; Xu, Z. Safety and Pharmacokinetics of Single Doses of (+)-Calanolide A, a Novel, Naturally Occurring Nonnucleoside Reverse Transcriptase Inhibitor, in Healthy, Human Immunodeficiency Virus-Negative Human Subjects. Antimicrob. Agents Chemother. 2001, 45, 1379–1386. [Google Scholar] [CrossRef]
- Saechan, C.; Kaewsrichan, J.; Leelakanok, N.; Petchsomrit, A. Antioxidant in Cosmeceutical Products Containing Calophyllum inophyllum Oil. OCL 2021, 28, 28. [Google Scholar] [CrossRef]
- White, L.A.; Mitchell, T.; Brinckerhoff, C.E. Transforming Growth Factor β Inhibitory Element in the Rabbit Matrix Metalloproteinase-1 (Collagenase-1) Gene Functions as a Repressor of Constitutive Transcription. Biochim. Et Biophys. Acta 2000, 1490, 259–268. [Google Scholar] [CrossRef] [PubMed]
- Pakyari, M.; Farrokhi, A.; Maharlooei, M.K.; Ghahary, A. Critical Role of Transforming Growth Factor Beta in Different Phases of Wound Healing. Adv. Wound Care 2013, 2, 215–224. [Google Scholar] [CrossRef] [PubMed]
- Tebben, A.J.; Ruzanov, M.; Gao, M.; Xie, D.; Kiefer, S.E.; Yan, C.; Newitt, J.A.; Zhang, L.; Kim, K.; Lu, H.; et al. Crystal structures of apo and inhibitor-bound TGFβR2 kinase domain: Insights into TGFβR isoform selectivity. Acta Cryst. 2016, D72, 658–674. [Google Scholar] [CrossRef]
- Kalimuthu, A.K.; Pavadai, P.; Panneerselvam, T.; Babkiewicz, E.; Pijanowska, J.; Mrówka, P.; Rajagopal, G.; Deepak, V.; Sundar, K.; Maszczyk, P.; et al. Cytotoxic Potential of Bioactive Compounds from Aspergillus flavus, an Endophytic Fungus Isolated from Cynodon dactylon, against Breast Cancer: Experimental and Computational Approach. Molecules 2022, 27, 8814. [Google Scholar] [CrossRef] [PubMed]
- Kalimuthu, A.K.; Panneerselvam, T.; Pavadai, P.; Pandian SR, K.; Sundar, K.; Murugesan, S.; Ammunje, D.N.; Kumar, S.; Arunachalam, S.; Kunjiappan, S. Pharmacoinformatics-based investigation of bioactive compounds of Rasam (South Indian recipe) against human cancer. Sci. Rep. 2021, 11, 21488. [Google Scholar] [CrossRef] [PubMed]
- Gade, A.C.; Murahari, M.; Pavadai, P.; Kumar, M.S. Virtual Screening of a Marine Natural Product Database for In Silico Identification of a Potential Acetylcholinesterase Inhibitor. Life 2023, 13, 1298. [Google Scholar] [CrossRef]
- Hua, L.; Anjum, F.; Shafie, A.; Ashour, A.A.; Almalki, A.A.; Alqarni, A.A.; Banjer, H.J.; Almaghrabi, S.A.; He, S.; Xu, N. Identifying Promising GSK3β Inhibitors for Cancer Management: A Computational Pipeline Combining Virtual Screening and Molecular Dynamics Simulations. Front. Chem. 2023, 11, 1200490. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Zhang, J.; Hu, C.Q.; Zhang, X.; Ma, B.; Zhang, P. In Silico ADME and Toxicity Prediction of Ceftazidime and Its Impurities. Front. Pharmacol. 2019, 10, 434. [Google Scholar] [CrossRef]
- Sharmeen, J.B.; Mahomoodally, F.; Zengin, G.; Maggi, F. Essential Oils as Natural Sources of Fragrance Compounds for Cosmetics and Cosmeceuticals. Molecules 2021, 26, 666. [Google Scholar] [CrossRef]
- Lukić, M.; Pantelić, I.; Savić, S. Towards Optimal PH of the Skin and Topical Formulations: From the Current State of the Art to Tailored Products. Cosmetics 2021, 8, 69. [Google Scholar] [CrossRef]
- Sandeep, D.S. Development, Characterization, and In vitro Evaluation of Aceclofenac Emulgel. Asian J. Pharm. 2020, 14, 1–7. [Google Scholar]
- Szulc-Musioł, B.; Dolińska, B.; Kołodziejska, J.; Ryszka, F. Influence of Plasma on the Physical Properties of Ointments with Quercetin. Acta Pharm. 2017, 67, 569–578. [Google Scholar] [CrossRef] [PubMed]
- Ilomuanya, M.O.; Hameedat, A.T.; Akang, E.E.U.; Ekama, S.O.; Silva, B.O.; Akanmu, A.S. Development and Evaluation of Mucoadhesive Bigel Containing Tenofovir and Maraviroc for HIV Prophylaxis. Future J. Pharm. Sci. 2020, 6, 81. [Google Scholar] [CrossRef] [PubMed]
- OECD. Test No. 404: Acute Dermal Irritation/Corrosion; OECD: Paris, France, 2015. [Google Scholar]
- Nagar, H.; Srivastava, A.; Srivastava, R.; Kurmi, M.L.; Chandel, H.S.; Ranawat, M.S. Pharmacological Investigation of the Wound Healing Activity of Cestrum nocturnum (L.) Ointment in Wistar Albino Rats. J. Pharm. 2016, 2016, 9249040. [Google Scholar] [CrossRef] [PubMed]
- Balakrishnan, B.; Mohanty, M.; Fernandez, A.C.; Mohanan, P.V.; Jayakrishnan, A. Evaluation of the Effect of Incorporation of Dibutyryl Cyclic Adenosine Monophosphate in an in Situ-Forming Hydrogel Wound Dressing Based on Oxidized Alginate and Gelatin. Biomaterials 2006, 27, 1355–1361. [Google Scholar] [CrossRef]
- Artanti, N.; Dewijanti, I.D.; Muzdalifah, D.; Windarsih, A.; Suratno, S.; Handayani, S. Alpha glucosidase inhibitory activity of combination of Caesalpinia sappan L. and Garcinia mangostana extract. J. Appl. Pharm. Sci. 2023, 13, 189–198. [Google Scholar] [CrossRef]
- Vellur, S.; Pavadai, P.; Babkiewicz, E.; Ram Kumar Pandian, S.; Maszczyk, P.; Kunjiappan, S. An In Silico Molecular Modelling-Based Prediction of Potential Keap1 Inhibitors from Hemidesmus indicus (L.) R.Br. against Oxidative-Stress-Induced Diseases. Molecules 2023, 28, 4541. [Google Scholar] [CrossRef]
- Sabale, V.; Kunjwani, H.K.; Sabale, P.M. Formulation and in Vitro Evaluation of the Topical Antiageing Preparation of the Fruit of Benincasa Hispida. J. Ayurveda Integr. Med. 2011, 2, 124–128. [Google Scholar] [CrossRef]





| Sl.No. | Compounds | Component RT (Min) | SMILES | Percentage |
|---|---|---|---|---|
| 1 | Calanolide A | 31.03 | CCCC1=CC(=O)OC2=C1C3=C(C=CC(O3)(C)C)C4=C2[C@H]([C@@H]([C@H](O4)C)C)O | 2.02 |
| 2 | Calophyllolide | 33.52 | CC=C(C)C(=O)C1=C(C2=C(C3=C1OC(=O)C=C3C4=CC=CC=C4)OC(C=C2)(C)C)OC | 1.92 |
| 3 | Inophyllum C | 24.91 | C[C@@H]1[C@H](OC2=C(C1=O)C3=C(C(=CC(=O)O3)C4=CC=CC=C4)C5=C2C=CC(O5)(C)C)C | 3.65 |
| 4 | Oleic acid | 26.16 | CCCCCCCC/C=C\CCCCCCCC(=O)O | 0.85 |
| 5 | Linoleic acid | 26.16 | CCCCC/C=C\C/C=C\CCCCCCCC(=O)O | 0.64 |
| 6 | Palmitic acid | 23.27 | CCCCCCCCCCCCCCCC(=O)O | 0.23 |
| 7 | Stearic acid | 26.38 | CCCCCCCCCCCCCCCCCC(=O)O | 0.98 |
| 8 | 4-Norlanosta-17(20),24-diene-11,16-diol-21-oic acid, 3-oxo-16,21-lactone | 29.66 | CC1C2CCC3(C(C2(CCC1=O)C)C(CC4C3(CC5C4=C(C(=O)O5)CCC=C(C)C)C)O)C | 2.3 |
| 9 | Pentacosanoic acid | 25.28 | CCCCCCCCCCCCCCCCCCCCCCCCC(=O)O | 0.66 |
| Compounds | Docking Score kcal/mol | H-Bond Interactions | Hydrophobic Interactions | External Bond Interactions | |
|---|---|---|---|---|---|
| Residue | Distance (Å) | ||||
| Calophyllolide | −8.6 | Ser287 | 2.3 | Val219, Lys337 | - |
| Inophyllum C | −11.3 | His283 | 2.47 | Val219, Ala230, Lys232, Leu260, Leu340, Ala350, Asp351 | - |
| Calanolide A | −9.8 | Ser280, His283 | 2.88 2.31 | Ile211, Val219, Lys232, Leu260, Leu340 | - |
| Oleic acid | −5.3 | Ser280 | 2.56 | Ile211, Val219, Leu260, Leu340, Ala350, Asp351 | - |
| Linoleic acid | −6.4 | Ser280, His283 | 3.25 1.87 | Ile211, Val219, Lys232, Tyr249, Leu260, Phe262, Asp351 | - |
| Palmitic acid | −5.9 | His283 | 2.9 | Lys232, Tyr249, Phe262, Leu278, Leu340, Lys232 | - |
| 4-Norlanosta-17(20),24-diene-11,16-diol-21-oic acid, 3-oxo-16,21-lactone | −11.1 | Ser287 | 3.08 | Ile211, Val219, Ala230, Leu260, Tyr282, Lys337, Leu340, Ala350, Asp351 | Lys232 (salt bridge) |
| Hyenic acid | −5 | Val231 | 3.29 | Ile211, Val219, Lys232, Leu260, Lys337, Leu340, Ala350, Asp351 | |
| Staurosporine | −8.6 | Ser280, Asp281, His 283 | 2.57 2.76 1.94 | Ile 211, Gly212, Lys337 | Val219, Ala230, Lys232, Leu340, Ala350 π stacking |
| Parameters | BG1 | BG2 | BG3 | BG4 | BG5 | BG6 | BG7 | BG8 |
|---|---|---|---|---|---|---|---|---|
| pH | 5.82 ± 0.05 | 5.96 ± 0.06 | 5.92 ± 0.12 | 6.04 ± 0.07 | 5.96 ± 0.17 | 5.58 ± 0.03 | 5.62 ± 0.13 | 5.76 ± 0.10 |
| Spreadability (cm) | 6.50 ± 0.36 | 6.10 ± 0.26 | 5.93 ± 0.25 | 5.63 ± 0.30 | 5.46 ± 0.40 | 5.36 ± 0.05 | 5.30 ± 0.10 | 5.26 ± 0.15 |
| Viscosity (cps) | 220.4 ± 0.96 | 238.9 ± 0.85 | 252.9 ± 0.65 | 273.8 ± 0.05 | 313.0 ± 0.15 | 337.4 ± 0.25 | 378.4 ± 0.05 | 391.5 ± 0.28 |
| Group | Control | Tamanu Oil | Murivenna | BG1 | BG4 | BG8 |
|---|---|---|---|---|---|---|
| Day 3 | 0.573 ± 0.0049 | 0.538 ± 0.0047 ** | 0.555 ± 0.0042 ns | 0.561 ± 0.0094 ns | 0.511 ± 0.0060 ** | 0.505 ± 0.0042 ** |
| Day 6 | 0.470 ± 0.0057 | 0.460 ± 0.0051 ns | 0.451 ± 0.0047 * | 0.463 ± 0.0042 ns | 0.415 ± 0.00428 ** | 0.405 ± 0.0042 ** |
| Day 9 | 0.418 ± 0.0094 | 0.350 ± 0.0036 ** | 0.358 ± 0.0095 ** | 0.380 ± 0.0057 ** | 0.273 ± 0.0066 ** | 0.248 ± 0.0095 ** |
| Day 12 | 0.251 ± 0.0104 | 0.112 ± 0.0107 ** | 0.098 ± 0.0047 ** | 0.1283 ± 0.0060 ** | 0.0833 ± 0.0066 ** | 0.00 ± 0.00 ** |
| Day 15 | 0.083 ± 0.0025 | 0.00 ± 0.00 ** | 0.00 ± 0.00 ** | 0.00 ± 0.00 ** | 0.00 ± 0.00 ** | 0.00 ± 0.00 ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krishnappa, M.; Abraham, S.; Furtado, S.C.; Krishnamurthy, S.; Rifaya, A.; Asiri, Y.I.; Chidambaram, K.; Pavadai, P. An Integrated Computational and Experimental Approach to Formulate Tamanu Oil Bigels as Anti-Scarring Agent. Pharmaceuticals 2024, 17, 102. https://doi.org/10.3390/ph17010102
Krishnappa M, Abraham S, Furtado SC, Krishnamurthy S, Rifaya A, Asiri YI, Chidambaram K, Pavadai P. An Integrated Computational and Experimental Approach to Formulate Tamanu Oil Bigels as Anti-Scarring Agent. Pharmaceuticals. 2024; 17(1):102. https://doi.org/10.3390/ph17010102
Chicago/Turabian StyleKrishnappa, Megha, Sindhu Abraham, Sharon Caroline Furtado, Shwetha Krishnamurthy, Aynul Rifaya, Yahya I. Asiri, Kumarappan Chidambaram, and Parasuraman Pavadai. 2024. "An Integrated Computational and Experimental Approach to Formulate Tamanu Oil Bigels as Anti-Scarring Agent" Pharmaceuticals 17, no. 1: 102. https://doi.org/10.3390/ph17010102
APA StyleKrishnappa, M., Abraham, S., Furtado, S. C., Krishnamurthy, S., Rifaya, A., Asiri, Y. I., Chidambaram, K., & Pavadai, P. (2024). An Integrated Computational and Experimental Approach to Formulate Tamanu Oil Bigels as Anti-Scarring Agent. Pharmaceuticals, 17(1), 102. https://doi.org/10.3390/ph17010102