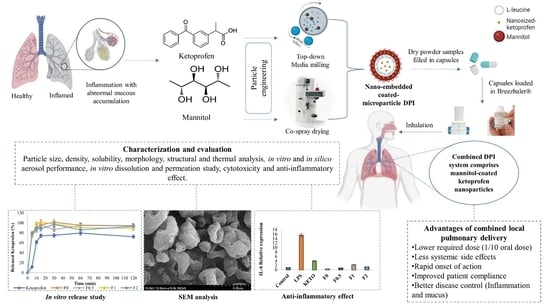
A Novel Combined Dry Powder Inhaler Comprising Nanosized Ketoprofen-Embedded Mannitol-Coated Microparticles for Pulmonary Inflammations: Development, In Vitro–In Silico Characterization, and Cell Line Evaluation
Abstract
:1. Introduction
2. Results
2.1. Holding Time as Short-Term Stability of KN
2.2. Yield and Drug Content of DPI
2.3. Particle Size, Particle Size Distribution, and Zeta Potential
2.4. Solubility
2.5. Morphology
2.6. Contact Angle, Surface Energy, and Cohesion Work
2.7. Thermal Analysis
2.7.1. DSC
2.7.2. TGA
2.8. Structural Analysis
2.9. Density and Flowability
2.10. Aerosol Performance
2.10.1. In Vitro Aerodynamic Characterization
2.10.2. In Silico Characterization
2.11. In Vitro Release Study
2.12. In Vitro Diffusion Study
2.13. The Effect on Mucin Viscosity
2.14. Cytotoxicity Study
2.15. Anti-Inflammatory Effect
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Preparation of Ketoprofen-Containing Nanosuspension (KN)
Holding Time as Short-Term Stability of KN
4.3. Preparation of a Combined Dry Powder Inhaler (DPI)
Yield and Drug Content of DPI
4.4. Characterization and Evaluation
4.4.1. Particle Size, Particle Size Distribution, and Zeta Potential Characterization
4.4.2. Solubility
4.4.3. Morphology
4.4.4. Contact Angle, Surface Energy, and Cohesion Work
4.4.5. Thermal Analysis
DSC
TGA
4.4.6. Structural Analysis
4.4.7. Density and Flowability
4.4.8. Characterization of Aerosol Performance
In Vitro Aerodynamic Characterization
In Silico Characterization
4.4.9. In Vitro Release Study
4.4.10. In Vitro Diffusion Study
4.4.11. Effect on Mucin Viscosity
4.4.12. Cytotoxicity
4.4.13. Anti-Inflammatory Effect
mRNA Extraction and cDNA Synthesis
qPCR Validation of IL-6
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Moldoveanu, B.; Otmishi, P.; Jani, P.; Walker, J.; Sarmiento, X.; Guardiola, J.; Saad, M.; Yu, J. Inflammatory mechanisms in the lung. J. Inflamm. Res. 2008, 2, 1–11. [Google Scholar] [PubMed]
- Fabbri, L.M.; Romagnoli, M.; Corbetta, L.; Casoni, G.; Busljetic, K.; Turato, G.; Ligabue, G.; Ciaccia, A.; Saetta, M.; Papi, A. differences in airway inflammation in patients with fixed airflow obstruction due to asthma or chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2003, 167, 418–424. [Google Scholar] [CrossRef] [PubMed]
- Adler, K.B.; Fischer, B.M.; Wright, D.T.; Cohn, L.A.; Becker, S. Interactions between respiratory epithelial cells and cytokines: Relationships to lung inflammation. Ann. N. Y. Acad. Sci. 1994, 725, 128–145. [Google Scholar] [CrossRef] [PubMed]
- Cesta, M.C.; Zippoli, M.; Marsiglia, C.; Gavioli, E.M.; Mantelli, F.; Allegretti, M.; Balk, R.A. The Role of Interleukin-8 in Lung Inflammation and Injury: Implications for the Management of COVID-19 and Hyperinflammatory Acute Respiratory Distress Syndrome. Front. Pharmacol. 2021, 12, 808797. [Google Scholar] [CrossRef] [PubMed]
- King, P.T. Inflammation in chronic obstructive pulmonary disease and its role in cardiovascular disease and lung cancer. Clin. Transl. Med. 2015, 4, 68. [Google Scholar] [CrossRef] [PubMed]
- Cantin, A.M.; Hartl, D.; Konstan, M.W.; Chmiel, J.F. Inflammation in cystic fibrosis lung disease: Pathogenesis and therapy. J. Cyst. Fibros. 2015, 14, 419–430. [Google Scholar] [CrossRef] [PubMed]
- Widysanto, A.; Mathew, G. Chronic Bronchitis; StatPearls Publishing: Tampa, FL, USA, 2021. [Google Scholar]
- Hauber, H.-P.; Foley, S.C.; Hamid, Q. Mucin overproduction in chronic inflammatory lung disease. Can. Respir. J. 2006, 13, 327–335. [Google Scholar] [CrossRef]
- Perez-Vilar, J.; Sheehan, J.K.; Randell, S.H. Making more MUCS. Am. J. Respir. Cell Mol. Biol. 2003, 28, 267–270. [Google Scholar] [CrossRef]
- Kim, V.; Ramos, F.; Krahnke, J. Clinical issues of mucus accumulation in COPD. Int. J. Chronic Obstr. Pulm. Dis. 2014, 9, 139–150. [Google Scholar] [CrossRef]
- Ye, Q.; He, X.O.; D’Urzo, A. A Review on the Safety and Efficacy of Inhaled Corticosteroids in the Management of Asthma. Pulm. Ther. 2017, 3, 1–18. [Google Scholar] [CrossRef]
- Dahl, R. Systemic side effects of inhaled corticosteroids in patients with asthma. Respir. Med. 2006, 100, 1307–1317. [Google Scholar] [CrossRef] [PubMed]
- Lebedeva, E.S.; Kuzubova, N.N.; Titova, O.N.; Surkova, E.A. Effect of cyclooxygenase-2 inhibition on lung inflammation and hypoxia-inducible factor-1 signalling in COPD model. Eur. Respir. J. 2017, 50 (Suppl. S61), PA3926. Available online: http://erj.ersjournals.com/content/50/suppl_61/PA3926.abstract (accessed on 21 March 2023).
- Ahmed, M.; Azam, F.; Gbaj, A.; Zetrini, A.E.; Abodlal, A.S.; Rghigh, A.; Elmahdi, E.; Hamza, A.; Salama, M.; Bensaber, S.M. Ester Prodrugs of Ketoprofen: Synthesis, In Vitro Stability, In Vivo Biological Evaluation and In Silico Comparative Docking Studies Against COX-1 and COX-2. Curr. Drug. Discov. Technol. 2016, 13, 41–57. [Google Scholar] [CrossRef] [PubMed]
- Rao, P.; Knaus, E.E. Evolution of Nonsteroidal Anti-Inflammatory Drugs (NSAIDs): Cyclooxygenase (COX) Inhibition and Beyond. J. Pharm. Pharm. Sci. 2008, 11, 81s–110s. [Google Scholar] [CrossRef] [PubMed]
- Stigliani, M.; Aquino, R.P.; Del Gaudio, P.; Mencherini, T.; Sansone, F.; Russo, P. Non-steroidal anti-inflammatory drug for pulmonary administration: Design and investigation of ketoprofen lysinate fine dry powders. Int. J. Pharm. 2013, 448, 198–204. [Google Scholar] [CrossRef] [PubMed]
- Rita Patrizia, A.; Mariateresa, S.; Pasquale, D.G.; Teresa, M.; Francesca, S.; Paola, R. Nanospray drying as a novel technique for the manufacturing of inhalable NSAID powders. Sci. World J. 2014, 2014, 838410. [Google Scholar] [CrossRef]
- Teper, A.; Jaques, A.; Charlton, B. Inhaled mannitol in patients with cystic fibrosis: A randomised open-label dose response trial. J. Cyst. Fibros. 2011, 10, 1–8. [Google Scholar] [CrossRef]
- Li, X.; Vogt, F.G.; Hayes, D.; Mansour, H.M. Design, characterization, and aerosol dispersion performance modeling of advanced spray-dried microparticulate/nanoparticulate mannitol powders for targeted pulmonary delivery as dry powder inhalers. J. Aerosol Med. Pulm. Drug Deliv. 2014, 27, 81–93. [Google Scholar] [CrossRef]
- Muralidharan, P.; Malapit, M.; Mallory, E.; Hayes, D.; Mansour, H.M. Inhalable nanoparticulate powders for respiratory delivery. Nanomed. Nanotechnol. Biol. Med. 2015, 11, 1189–1199. [Google Scholar] [CrossRef]
- Scherließ, R.; Bock, S.; Bungert, N.; Neustock, A.; Valentin, L. Particle engineering in dry powders for inhalation. Eur. J. Pharm. Sci. 2022, 172, 106158. [Google Scholar] [CrossRef]
- Tsapis, N.; Bennett, D.; Jackson, B.; Weitz, D.A.; Edwards, D.A. Trojan particles: Large porous carriers of nanoparticles for drug delivery. Proc. Natl. Acad. Sci. USA 2002, 99, 12001–12005. [Google Scholar] [CrossRef] [PubMed]
- Vehring, R. Pharmaceutical Particle Engineering via Spray Drying. Pharm. Res. 2008, 25, 999–1022. [Google Scholar] [CrossRef] [PubMed]
- Muralidharan, P.; Mallory, E.K.; Malapit, M.; Phan, H.; Ledford, J.G.; Hayes, D.; Mansour, H.M. Advanced design and development of nanoparticle/microparticle dual-drug combination lactose carrier-free dry powder inhalation aerosols. RSC Adv. 2020, 10, 41846–41856. [Google Scholar] [CrossRef] [PubMed]
- Raula, J.; Rahikkala, A.; Halkola, T.; Pessi, J.; Peltonen, L.; Hirvonen, J.; Järvinen, K.; Laaksonen, T.; Kauppinen, E.I. Coated particle assemblies for the concomitant pulmonary administration of budesonide and salbutamol sulphate. Int. J. Pharm. 2013, 441, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Deng, Q.; Deng, L.; Miao, Y.; Guo, X.; Li, Y. Particle deposition in the human lung: Health implications of particulate matter from different sources. Environ. Res. 2019, 169, 237–245. [Google Scholar] [CrossRef]
- Darquenne, C. Deposition Mechanisms. J. Aerosol. Med. Pulm. Drug. Deliv. 2020, 33, 181–185. [Google Scholar] [CrossRef]
- Vu, T.V.; Ondracek, J.; Zdímal, V.; Schwarz, J.; Delgado-Saborit, J.M.; Harrison, R.M. Physical properties and lung deposition of particles emitted from five major indoor sources. Air Qual. Atmos. Health 2017, 10, 1–14. [Google Scholar] [CrossRef]
- Heyder, J.; Gebhart, J.; Rudolf, G.; Schiller, C.; Stahlhofen, W. Deposition of particles in the human respiratory tract in the size range 0.005–15 μm. J. Aerosol Sci. 1986, 17, 811–825. [Google Scholar] [CrossRef]
- Silva, A.S.; Sousa, A.M.; Cabral, R.P.; Silva, M.C.; Costa, C.; Miguel, S.P.; Bonifácio, V.D.; Casimiro, T.; Correia, I.J.; Aguiar-Ricardo, A. Aerosolizable gold nano-in-micro dry powder formulations for theragnosis and lung delivery. Int. J. Pharm. 2017, 519, 240–249. [Google Scholar] [CrossRef]
- Kaye, R.S.; Purewal, T.S.; Alpar, H.O. Simultaneously manufactured nano-in-micro (SIMANIM) particles for dry-powder modified-release delivery of antibodies. J. Pharm. Sci. 2009, 98, 4055–4068. [Google Scholar] [CrossRef]
- Singh, S.K.; Srinivasan, K.; Gowthamarajan, K.; Singare, D.S.; Prakash, D.; Gaikwad, N.B. Investigation of preparation parameters of nanosuspension by top-down media milling to improve the dissolution of poorly water-soluble glyburide. Eur. J. Pharm. Biopharm. 2011, 78, 441–446. Available online: https://www.sciencedirect.com/science/article/pii/S0939641111001159 (accessed on 12 January 2023). [CrossRef] [PubMed]
- Canpınar, H.; Gülba, S. A new nanosuspension prepared with wet milling method for oral delivery of highly variable drug Cyclosporine A: Development, optimization and in vivo evaluation. Eur. J. Pharm. Sci. 2022, 171, 106123. [Google Scholar]
- Seville, P.; Learoyd, T.; Li, H.-Y.; Williamson, I.; Birchall, J. Amino acid-modified spray-dried powders with enhanced aerosolisation properties for pulmonary drug delivery. Powder Technol. 2007, 178, 40–50. [Google Scholar] [CrossRef]
- Lewis, D.; Rouse, T.; Singh, D.; Edge, S. Defining the ‘Dose’for Dry Powder Inhalers: The Challenge of Correlating In-Vitro Dose Delivery Results with Clinical Efficacy. Am. Pharm. Rev. 2017, 20, 54–62. [Google Scholar]
- Xia, D.; Shrestha, N.; van de Streek, J.; Mu, H.; Yang, M. Spray drying of fenofibrate loaded nanostructured lipid carriers. Asian J. Pharm. Sci. 2016, 11, 507–515. [Google Scholar] [CrossRef]
- Myat, H.H.; Ritthidej, G.C. Impact of formulation parameters on physical characteristics of spray dried nanoemulsions and their reconstitutions. Asian J. Pharm. Sci. 2016, 11, 197–198. [Google Scholar] [CrossRef]
- Liu, Q.; Guan, J.; Sun, Z.; Shen, X.; Li, L.; Jin, L.; Mao, S. Influence of stabilizer type and concentration on the lung deposition and retention of resveratrol nanosuspension-in-microparticles. Int. J. Pharm. 2019, 569, 118562. Available online: https://www.sciencedirect.com/science/article/pii/S0378517319306064 (accessed on 20 January 2023). [CrossRef]
- Nandiyanto, A.B.D.; Okuyama, K. Progress in developing spray-drying methods for the production of controlled morphology particles: From the nanometer to submicrometer size ranges. Adv. Powder Technol. 2011, 22, 1–19. Available online: https://www.sciencedirect.com/science/article/pii/S0921883110001883 (accessed on 23 February 2023). [CrossRef]
- Han, C.-S.; Kang, J.-H.; Park, E.H.; Lee, H.-J.; Jeong, S.-J.; Kim, D.-W.; Park, C.-W. Corrugated surface microparticles with chitosan and levofloxacin for improved aerodynamic performance. Asian J. Pharm. Sci. 2023, 18, 100815. Available online: https://www.sciencedirect.com/science/article/pii/S1818087623000429 (accessed on 18 March 2023). [CrossRef]
- Focaroli, S.; Mah, P.; Hastedt, J.; Gitlin, I.; Oscarson, S.; Fahy, J.; Healy, A. A Design of Experiment (DoE) approach to optimise spray drying process conditions for the production of trehalose/leucine formulations with application in pulmonary delivery. Int. J. Pharm. 2019, 562, 228–240. Available online: https://www.sciencedirect.com/science/article/pii/S0378517319301796 (accessed on 13 February 2023). [CrossRef]
- Yang, D.-L.; Liu, R.-K.; Wei, Y.; Sun, Q.; Wang, J.-X. Micro-sized nanoaggregates: Spray-drying-assisted fabrication and applications. Particuology 2023, 85, 22–48. [Google Scholar] [CrossRef]
- Lin, L.; Quan, G.; Peng, T.; Huang, Z.; Singh, V.; Lu, M.; Wu, C. Development of fine solid-crystal suspension with enhanced solubility, stability, and aerosolization performance for dry powder inhalation. Int. J. Pharm. 2017, 533, 84–92. [Google Scholar] [CrossRef] [PubMed]
- Pomázi, A.; Ambrus, R.; Sipos, P.; Szabó-Révész, P. Analysis of co-spray-dried meloxicam–mannitol systems containing crystalline microcomposites. J. Pharm. Biomed. Anal. 2011, 56, 183–190. [Google Scholar] [CrossRef] [PubMed]
- Leung, S.S.Y.; Wong, J.; Guerra, H.V.; Samnick, K.; Prud’homme, R.K.; Chan, H.-K. Porous mannitol carrier for pulmonary delivery of cyclosporine A nanoparticles. AAPS J. 2017, 19, 578–586. [Google Scholar] [CrossRef]
- Kang, J.-H.; Kim, Y.-J.; Yang, M.-S.; Shin, D.H.; Kim, D.-W.; Park, I.Y.; Park, C.-W. Co-Spray Dried Nafamostat Mesylate with Lecithin and Mannitol as Respirable Microparticles for Targeted Pulmonary Delivery: Pharmacokinetics and Lung Distribution in Rats. Pharmaceutics 2021, 13, 1519. [Google Scholar] [CrossRef]
- Li, X.; Vogt, F.G.; Hayes, D.; Mansour, H.M. Design, Characterization, and Aerosol Dispersion Performance Modeling of Advanced Co-Spray Dried Antibiotics with Mannitol as Respirable Microparticles/Nanoparticles for Targeted Pulmonary Delivery as Dry Powder Inhalers. J. Pharm. Sci. 2014, 103, 2937–2949. Available online: https://www.sciencedirect.com/science/article/pii/S0022354915304287 (accessed on 22 January 2023). [CrossRef]
- Belotti, S.; Rossi, A.; Colombo, P.; Bettini, R.; Rekkas, D.; Politis, S.; Colombo, G.; Balducci, A.G.; Buttini, F. Spray-dried amikacin sulphate powder for inhalation in cystic fibrosis patients: The role of ethanol in particle formation. Eur. J. Pharm. Biopharm. 2015, 93, 165–172. Available online: https://www.sciencedirect.com/science/article/pii/S0939641115001563 (accessed on 27 March 2023). [CrossRef]
- Zhou, Q.T.; Tang, P.; Leung, S.S.Y.; Chan, J.G.Y.; Chan, H.K. Emerging inhalation aerosol devices and strategies: Where are we headed? Adv. Drug Deliv. Rev. 2014, 75, 3–17. [Google Scholar] [CrossRef]
- Suzuki, Y.; Amaro, M.I.; de Almeida, G.S.; Cabral, L.M.; Healy, A.M.; de Sousa, V.P. Development of a new formulation of roflumilast for pulmonary drug delivery to treat inflammatory lung conditions. Int. J. Pharm. 2018, 550, 89–99. Available online: https://www.sciencedirect.com/science/article/pii/S0378517318306136 (accessed on 20 June 2023). [CrossRef]
- Bosquillon, C.; Lombry, C.; Préat, V.; Vanbever, R. Influence of formulation excipients and physical characteristics of inhalation dry powders on their aerosolization performance. J. Control. Release 2001, 70, 329–339. Available online: https://www.sciencedirect.com/science/article/pii/S016836590000362X (accessed on 1 March 2023). [CrossRef]
- Azari, F.; Ghanbarzadeh, S.; Safdari, R.; Yaqoubi, S.; Adibkia, K.; Hamishehkar, H. Development of a Carrier Free Dry Powder Inhalation Formulation of Ketotifen for Pulmonary Drug Delivery. Drug Res. 2020, 70, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Thorpe, M.; Chapman, K.R.; Fogarty, C.M.; Peckitt, C.; Lassen, C.; Jadayel, D.; Dederichs, J.; Dalvi, M.; Kramer, B. Delivery characteristics and patients’ handling of two single-dose dry-powder inhalers used in COPD. Int. J. COPD 2011, 6, 353–363. [Google Scholar] [CrossRef] [PubMed]
- Longest, P.W.; Holbrook, L.T. In silico models of aerosol delivery to the respiratory tract—Development and applications. Adv. Drug Deliv. Rev. 2012, 64, 296–311. [Google Scholar] [CrossRef] [PubMed]
- Ignjatović, J.; Šušteršič, T.; Bodić, A.; Cvijić, S.; Đuriš, J.; Rossi, A.; Dobričić, V.; Ibrić, S.; Filipović, N. Comparative assessment of in vitro and in silico methods for aerodynamic characterization of powders for inhalation. Pharmaceutics 2021, 13, 1831. [Google Scholar] [CrossRef]
- Haidl, P.; Heindl, S.; Siemon, K.; Bernacka, M.; Cloes, R.M. Inhalation device requirements for patients’ inhalation maneuvers. Respir. Med. 2016, 118, 65–75. [Google Scholar] [CrossRef]
- Roche, N.; Dekhuijzen, P.R.; Mehta, R.; Montembault, M.; Warren, F.; Gupta, A.; Brealey, N.; Moore, A.; Rygg, A. The Evolution of Pressurized Metered-Dose Inhalers from Early to Modern Devices. J. Aerosol Med. Pulm. Drug Deliv. 2016, 29, 311–327. [Google Scholar] [CrossRef]
- Nokhodchi, A.; Chavan, S.; Ghafourian, T. In Vitro Dissolution and Permeability Testing of Inhalation Products: Challenges and Advances. Pharmaceutics 2023, 15, 983. [Google Scholar] [CrossRef]
- Saffari, M.; Ebrahimi, A.; Langrish, T. A novel formulation for solubility and content uniformity enhancement of poorly water-soluble drugs using highly-porous mannitol. Eur. J. Pharm. Sci. 2016, 83, 52–61. Available online: https://www.sciencedirect.com/science/article/pii/S0928098715300907 (accessed on 16 March 2023). [CrossRef]
- Ambrus, R.; Benke, E.; Farkas, Á.; Balásházy, I.; Szabó-Révész, P. Novel dry powder inhaler formulation containing antibiotic using combined technology to improve aerodynamic properties. Eur. J. Pharm. Sci. 2018, 123, 20–27. [Google Scholar] [CrossRef]
- Requena, S.; Ponomarchuk, O.; Castillo, M.; Rebik, J.; Brochiero, E.; Borejdo, J.; Gryczynski, I.; Dzyuba, S.V.; Gryczynski, Z.; Grygorczyk, R.; et al. Imaging viscosity of intragranular mucin matrix in cystic fibrosis cells. Sci. Rep. 2017, 7, 16761. [Google Scholar] [CrossRef]
- Hill, D.B.; Button, B.; Rubinstein, M.; Boucher, R.C. Physiology and Pathophysiology of Human Airwaymucus. Physiol. Rev. 2022, 102, 1757–1836. [Google Scholar] [CrossRef] [PubMed]
- Anderson, W.H.; Coakley, R.D.; Button, B.; Henderson, A.G.; Zeman, K.L.; Alexis, N.E.; Peden, D.B.; Lazarowski, E.R.; Davis, C.W.; Bailey, S.; et al. The relationship of mucus concentration (hydration) to mucus osmotic pressure and transport in chronic bronchitis. Am. J. Respir. Crit. Care Med. 2015, 192, 182–190. [Google Scholar] [CrossRef] [PubMed]
- Odziomek, M.; Sosnowski, T.R.; Gradoń, L. The Influence of Functional Carrier Particles (FCPs) on the Molecular Transport Rate Through the Reconstructed Bronchial Mucus: In Vitro Studies. Transp. Porous Media 2015, 106, 439–454. [Google Scholar] [CrossRef]
- Serisier, D.J.; Carroll, M.P.; Shute, J.K.; A Young, S. Macrorheology of cystic fibrosis, chronic obstructive pulmonary disease normal sputum. Respir. Res. 2009, 10, 63. [Google Scholar] [CrossRef] [PubMed]
- Leal, J.; Smyth, H.D.; Ghosh, D. Physicochemical properties of mucus and their impact on transmucosal drug delivery. Int. J. Pharm. 2017, 532, 555–572. [Google Scholar] [CrossRef] [PubMed]
- Šimková, K.; Thormann, U.; Imanidis, G. Investigation of drug dissolution and uptake from low-density DPI formulations in an impactor–integrated cell culture model. Eur. J. Pharm. Biopharm. 2020, 155, 12–21. Available online: https://www.sciencedirect.com/science/article/pii/S0939641120302265 (accessed on 25 March 2023). [CrossRef] [PubMed]
- Zhang, H.; Dong, S.; Li, Z.; Feng, X.; Xu, W.; Tulinao, C.M.S.; Jiang, Y.; Ding, J. Biointerface engineering nanoplatforms for cancer-targeted drug delivery. Asian J. Pharm. Sci. 2020, 15, 397–415. [Google Scholar] [CrossRef]
- Party, P.; Kókai, D.; Burián, K.; Nagy, A.; Hopp, B.; Ambrus, R. Development of extra-fine particles containing nanosized meloxicam for deep pulmonary delivery: In vitro aerodynamic and cell line measurements. Eur. J. Pharm. Sci. 2022, 176, 106247. Available online: https://www.sciencedirect.com/science/article/pii/S0928098722001324 (accessed on 14 February 2023). [CrossRef]
- Tanaka, T.; Narazaki, M.; Kishimoto, T. IL-6 in inflammation, immunity, and disease. Cold Spring Harb. Perspect. Biol. 2014, 6, a016295. [Google Scholar] [CrossRef]
- Yu, M.; Zheng, X.; Witschi, H.; Pinkerton, K.E. The role of interleukin-6 in pulmonary inflammation and injury induced by exposure to environmental air pollutants. Toxicol. Sci. 2002, 68, 488–497. [Google Scholar] [CrossRef]
- Crestani, B.; Cornillet, P.; Dehoux, M.; Rolland, C.; Guenounou, M.; Aubier, M. Alveolar type II epithelial cells produce interleukin-6 in vitro and in vivo. Regulation by alveolar macrophage secretory products. J. Clin. Investig. 1994, 94, 731–740. [Google Scholar] [CrossRef]
- Shen, Y.; Yang, S.; Shi, Z.; Lin, T.; Zhu, H.; Bi, F.; Yan, S. SeMet Mediates Anti-Inflammation in LPS-Induced U937 Cells Targeting NF-κB Signaling Pathway. Inflammation 2015, 38, 736–744. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, J.A.; Belvisi, M.G.; Akarasereenont, P.; Robbins, R.A.; Kwon, O.J.; Croxtall, J.J.; Vane, J.R. Induction of cyclo-oxygenase-2 by cytokines in human pulmonary epithelial cells: Regulation by dexamethasone. Br. J. Pharmacol. 1994, 113, 1008–1014. [Google Scholar] [CrossRef] [PubMed]
- Grkovich, A.; Johnson, C.A.; Buczynski, M.W.; Dennis, E.A. Lipopolysaccharide-induced cyclooxygenase-2 expression in human u937 macrophages is phosphatidic acid phosphohydrolase-1-dependent. J. Biol. Chem. 2006, 281, 32978–32987. [Google Scholar] [CrossRef] [PubMed]
- Banat, H.; Ambrus, R.; Csóka, I. Drug combinations for inhalation: Current products and future development addressing disease control and patient compliance. Int. J. Pharm. 2023, 643, 123070. [Google Scholar] [CrossRef] [PubMed]
- Celi, S.S.; Fernández-García, R.; Afonso-Urich, A.I.; Ballesteros, M.P.; Healy, A.M.; Serrano, D.R. Co-Delivery of a High Dose of Amphotericin B and Itraconazole by Means of a Dry Powder Inhaler Formulation for the Treatment of Severe Fungal Pulmonary Infections. Pharmaceutics 2023, 15, 2601. [Google Scholar] [CrossRef]
- de Pablo, E.; O’Connell, P.; Fernández-García, R.; Marchand, S.; Chauzy, A.; Tewes, F.; Dea-Ayuela, M.; Kumar, D.; Bolás, F.; Ballesteros, M.; et al. Targeting lung macrophages for fungal and parasitic pulmonary infections with innovative amphotericin B dry powder inhalers. Int. J. Pharm. 2023, 635, 122788. [Google Scholar] [CrossRef]
- Wang, Y.; Zheng, Y.; Zhang, L.; Wang, Q.; Zhang, D. Stability of nanosuspensions in drug delivery. J. Control. Release 2013, 172, 1126–1141. [Google Scholar] [CrossRef]
- Faizi, H.S.; Vora, L.K.; Nasiri, M.I.; Wu, Y.; Mishra, D.; Anjani, Q.K.; Paredes, A.J.; Thakur, R.R.S.; Minhas, M.U.; Donnelly, R.F. Deferasirox Nanosuspension Loaded Dissolving Microneedles for Intradermal Delivery. Pharmaceutics 2022, 14, 2817. [Google Scholar] [CrossRef]
- Chan, S.-Y.; Chung, Y.-Y.; Cheah, X.-Z.; Tan, E.Y.-L.; Quah, J. The characterization and dissolution performances of spray dried solid dispersion of ketoprofen in hydrophilic carriers. Asian J. Pharm. Sci. 2015, 10, 372–385. [Google Scholar] [CrossRef]
- Bilgili, E.; Rahman, M.; Palacios, D.; Arevalo, F. Impact of polymers on the aggregation of wet-milled itraconazole particles and their dissolution from spray-dried nanocomposites. Adv. Powder. Technol. 2018, 29, 2941–2956. [Google Scholar] [CrossRef]
- Peltonen, L.; Hirvonen, J. Pharmaceutical nanocrystals by nanomilling: Critical process parameters, particle fracturing and stabilization methods. J. Pharm. Pharmacol. 2010, 56, 1569–1579. [Google Scholar] [CrossRef] [PubMed]
- Pomázi, A.; Buttini, F.; Ambrus, R.; Colombo, P.; Szabó-Révész, P. Effect of polymers for aerolization properties of mannitol-based microcomposites containing meloxicam. Eur. Polym. J. 2013, 49, 2518–2527. [Google Scholar] [CrossRef]
- Bartos, C.; Szabó-Révész, P.; Bartos, C.; Katona, G.; Jójárt-Laczkovich, O.; Ambrus, R. The Effect of an Optimized Wet Milling Technology on the Crystallinity, Morphology and Dissolution. Molecules 2016, 21, 507. [Google Scholar] [CrossRef] [PubMed]
- Lu, P.; Li, J.; Liu, C.; Yang, J.; Peng, H.; Xue, Z.; Liu, Z. Salvianolic acid B dry powder inhaler for the treatment of idiopathic pulmonary fibrosis. Asian J. Pharm. Sci. 2022, 17, 447–461. [Google Scholar] [CrossRef] [PubMed]
- Laaksonen, T.; Liu, P.; Rahikkala, A.; Peltonen, L.; Kauppinen, E.I.; Hirvonen, J.; Järvinen, K.; Raula, J. Intact Nanoparticulate Indomethacin in Fast-Dissolving Carrier Particles by Combined Wet Milling and Aerosol Flow Reactor Methods. Pharm. Res. 2011, 28, 2403–2411. [Google Scholar] [CrossRef] [PubMed]
- Shetty, N.; Park, H.; Zemlyanov, D.; Mangal, S.; Bhujbal, S.; Zhou, Q. Influence of excipients on physical and aerosolization stability of spray dried high-dose powder formulations for inhalation. Int. J. Pharm. 2018, 544, 222–234. [Google Scholar] [CrossRef]
- Li, L.; Miller, D.; Schmidt, C.P. Production Economics Optimizing inventory’ s contribution to profitability in a regulated utility: The Averch–Johnson effect. Int. J. Prod. Econ. 2016, 175, 132–141. [Google Scholar] [CrossRef]
- Eedara, B.B.; Rangnekar, B.; Doyle, C.; Cavallaro, A.; Das, S.C. The influence of surface active l-leucine and 1,2-dipalmitoyl-sn-glycero-3-phosphatidylcholine (DPPC) in the improvement of aerosolization of pyrazinamide and moxifloxacin co-spray dried powders. Int. J. Pharm. 2018, 542, 72–81. Available online: https://www.sciencedirect.com/science/article/pii/S0378517318301510 (accessed on 15 January 2023). [CrossRef]
- Benetti, A.A.; Bianchera, A.; Buttini, F.; Bertocchi, L.; Bettini, R. Mannitol Polymorphs as Carrier in DPIs Formulations: Isolation Characterization and Performance. Pharmaceutics 2021, 13, 1113. [Google Scholar] [CrossRef]
- Chennakesavulu, S.; Mishra, A.; Sudheer, A.; Sowmya, C.; Reddy, C.S.; Bhargav, E. Pulmonary delivery of liposomal dry powder inhaler formulation for effective treatment of idiopathic pulmonary fibrosis. Asian J. Pharm. Sci. 2018, 13, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Flume, P.A.; Amelina, E.; Daines, C.L.; Charlton, B.; Leadbetter, J.; Guasconi, A.; Aitken, M.L. Efficacy and safety of inhaled dry-powder mannitol in adults with cystic fibrosis: An international, randomized controlled study. J. Cyst. Fibros. 2021, 20, 1003–1009. Available online: https://www.sciencedirect.com/science/article/pii/S1569199321000461 (accessed on 13 March 2023). [CrossRef] [PubMed]
- Ph. Eur. 2.9.34. Bulk density and tapped density of Powders. In European Pharmacopeia 11; EDQM Counsil of Europe: Strasbourg, France, 2023; p. 407.
- USP. <616> Bulk Density and Tapped Density of Powders. In United States Pharmacopeia. 2010, p. 909. Available online: http://ftp.uspbpep.com/v29240/usp29nf24s0_c616.html (accessed on 18 December 2022).
- Ph. Eur. 2.9.18. Preparations for inhalation: Aerodynamic assessment of fine particles. In European Pharmacopeia 11; EDQM Counsil of Europe: Strasbourg, France, 2023; p. 369.
- USP. <601> Aerosols, Nasal Sprays, Metered-Dose Inhalers, and Dry Powder Inhalers. In USP 35; Food and Drug Administration: Rockville, MD, USA, 2012; p. 232. [Google Scholar]
- Koblinger, L.; Hofmann, W. Monte Carlo modeling of aerosol deposition in human lungs. Part I: Simulation of particle transport in a stochastic lung structure. J. Aerosol Sci. 1990, 21, 661–674. Available online: https://www.sciencedirect.com/science/article/pii/002185029090121D (accessed on 25 January 2023). [CrossRef]
- Horváth, A.; Balásházy, I.; Tomisa, G.; Farkas, Á. Significance of breath-hold time in dry powder aerosol drug therapy of COPD patients. Eur. J. Pharm. Sci. 2017, 104, 145–149. [Google Scholar] [CrossRef] [PubMed]
- Chvatal, A.; Farkas, Á.; Balásházy, I.; Szabó-Révész, P.; Ambrus, R. Aerodynamic properties and in silico deposition of meloxicam potassium incorporated in a carrier-free DPI pulmonary system. Int. J. Pharm. 2017, 520, 70–78. [Google Scholar] [CrossRef] [PubMed]
- Farkas, Á.; Lewis, D.; Church, T.; Tweedie, A.; Mason, F.; Haddrell, A.E.; Reid, J.P.; Horváth, A.; Balásházy, I. Experimental and computational study of the effect of breath-actuated mechanism built in the NEXThaler® dry powder inhaler. Int. J. Pharm. 2017, 533, 225–235. Available online: https://www.sciencedirect.com/science/article/pii/S0378517317309237 (accessed on 15 March 2023). [CrossRef] [PubMed]
- Ph. Eur. 2.9.3. Dissolution test for solid dosage forms. In European Pharmacopeia 11; EDQM Counsil of Europe: Strasbourg, France, 2023; p. 348.
- Parlati, C. Respirable Microparticles of Aminoglycoside Antibiotics for Pulmonary Administration. Ph.D. Thesis, University of Parma, Parma, Italy, 2008. [Google Scholar]
- Fröhlich, E.; Mercuri, A.; Wu, S.; Salar-Behzadi, S. Measurements of Deposition, Lung Surface Area and Lung Fluid for Simulation of Inhaled Compounds. Front. Pharmacol. 2016, 7, 181. [Google Scholar] [CrossRef]
- May, S.; Jensen, B.; Weiler, C.; Wolkenhauer, M.; Schneider, M.; Lehr, C.-M. Dissolution Testing of Powders for Inhalation: Influence of Particle Deposition and Modeling of Dissolution Profiles. Pharm. Res. 2014, 31, 3211–3224. [Google Scholar] [CrossRef]
- Gieszinger, P.; Kiss, T.; Szabó-Révész, P.; Ambrus, R. The Development of an In Vitro Horizontal Diffusion Cell to Monitor Nasal Powder Penetration Inline. Pharmaceutics 2021, 13, 809. [Google Scholar] [CrossRef]
- García-Díaz, M.; Birch, D.; Wan, F.; Nielsen, H.M. The role of mucus as an invisible cloak to transepithelial drug delivery by nanoparticles. Adv. Drug Deliv. Rev. 2018, 124, 107–124. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef] [PubMed]











| Sample Name | Sample Description | Yield (%) | Drug Content (%) |
|---|---|---|---|
| F0 | KETO1_LEU1_MAN0 | 52.95 ± 3.54 | 57.47 ± 1.44 |
| F0.5 | KETO1_LEU1_MAN0.5 | 54.86 ± 6.12 | 84.12 ± 2.91 |
| F1 | KETO1_LEU1_MAN1 | 57.29 ± 1.98 | 84.87 ± 7.13 |
| F2 | KETO1_LEU1_MAN2 | 58.68 ± 9.73 | 84.72 ± 3.65 |
| KETO | Ketoprofen_raw | - | - |
| Sample | PS (nm) | PDI | ZP (mV) |
|---|---|---|---|
| F0 | 204.9 ± 3.07 | 0.336 ± 0.003 | −8.88 ± 0.27 |
| F0.5 | 222.5 ± 4.11 | 0.127 ± 0.021 | −12.3 ± 0.43 |
| F1 | 240.7 ± 6.32 | 0.064 ± 0.008 | −7.44 ± 0.18 |
| F2 | 251.4 ± 2.84 | 0.156 ± 0.039 | −11.9 ± 0.33 |
| Sample Name | Solubility * (mg/mL) |
|---|---|
| F0 | 13.93 ± 0.88 |
| F0.5 | 17.77 ± 1.05 |
| F1 | 15.04 ± 0.34 |
| F2 | 17.95 ± 1.71 |
| KETO | 0.42 ± 0.13 |
| Sample Name | F0 | F0.5 | F1 | F2 |
|---|---|---|---|---|
| Bulk Density (g/cm3) | 0.124 ± 0.012 | 0.123 ± 0.003 | 0.120 ± 0.031 | 0.139 ± 0.024 |
| Tapped Density (g/cm3) | 0.180 ± 0.002 | 0.192 ± 0.011 | 0.201 ± 0.009 | 0.228 ± 0.052 |
| Carr’s Index | 31.03 | 35.14 | 40.01 | 39.01 |
| Hausner Ratio | 1.450 | 1.542 | 1.670 | 1.64 |
| MMAD (µm) | 2.40 ± 0.17 | 2.80 ± 0.06 | 4.51 ± 0.41 | 4.90 ± 0.16 |
| FPF (%) | 56.16 ± 2.51 | 71.02 ± 1.19 | 64.32 ± 1.34 | 32.21 ± 3.67 |
| EF (%) | 97.06 ± 3.22 | 96.60 ± 1.65 | 94.82 ± 2.79 | 95.70 ± 2.89 |
| Sample | J * (µg/cm2/h) | RP60 | Kp (cm/h) |
|---|---|---|---|
| KETO | 24.79 ± 5.29 | 1.000 | - |
| F0 | 98.24 ± 11.34 | 3.96 | 0.896 |
| F0.5 | 74.18 ± 18.63 | 2.99 | 0.470 |
| F1 | 121.97 ± 23.12 | 4.92 | 0.877 |
| F2 | 30.04 ± 16.58 | 1.21 | 0.336 |
| Sample | Viscosity (Pa·s) |
|---|---|
| Mucin 10% | 0.035 ± 5.44 |
| F0 | 0.033 ± 1.98 |
| F0.5 | 0.031 ± 2.12 |
| F1 | 0.025 ± 1.37 |
| F2 | 0.030 ± 4.19 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Banat, H.; Csóka, I.; Paróczai, D.; Burian, K.; Farkas, Á.; Ambrus, R. A Novel Combined Dry Powder Inhaler Comprising Nanosized Ketoprofen-Embedded Mannitol-Coated Microparticles for Pulmonary Inflammations: Development, In Vitro–In Silico Characterization, and Cell Line Evaluation. Pharmaceuticals 2024, 17, 75. https://doi.org/10.3390/ph17010075
Banat H, Csóka I, Paróczai D, Burian K, Farkas Á, Ambrus R. A Novel Combined Dry Powder Inhaler Comprising Nanosized Ketoprofen-Embedded Mannitol-Coated Microparticles for Pulmonary Inflammations: Development, In Vitro–In Silico Characterization, and Cell Line Evaluation. Pharmaceuticals. 2024; 17(1):75. https://doi.org/10.3390/ph17010075
Chicago/Turabian StyleBanat, Heba, Ildikó Csóka, Dóra Paróczai, Katalin Burian, Árpád Farkas, and Rita Ambrus. 2024. "A Novel Combined Dry Powder Inhaler Comprising Nanosized Ketoprofen-Embedded Mannitol-Coated Microparticles for Pulmonary Inflammations: Development, In Vitro–In Silico Characterization, and Cell Line Evaluation" Pharmaceuticals 17, no. 1: 75. https://doi.org/10.3390/ph17010075
APA StyleBanat, H., Csóka, I., Paróczai, D., Burian, K., Farkas, Á., & Ambrus, R. (2024). A Novel Combined Dry Powder Inhaler Comprising Nanosized Ketoprofen-Embedded Mannitol-Coated Microparticles for Pulmonary Inflammations: Development, In Vitro–In Silico Characterization, and Cell Line Evaluation. Pharmaceuticals, 17(1), 75. https://doi.org/10.3390/ph17010075