Recent Advances in the Management of Rosacea through Natural Compounds
Abstract
:1. Introduction
2. Methods
3. Pathophysiology and Mechanisms in Rosacea
4. Standard Management and Treatment Options for Rosacea
5. Natural Compounds for the Management of Rosacea
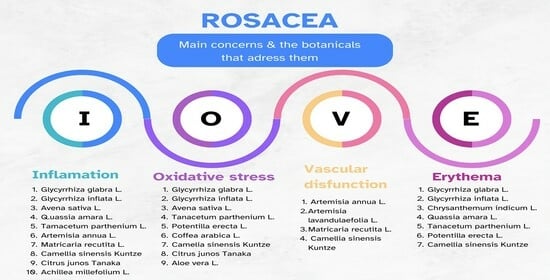
5.1. Glycyrrhiza glabra L. and Glycyrrhiza inflata L. (Licorice)
5.2. Chrysanthemum indicum L. (Indian Chrysanthemum)
5.3. Avena sativa L. (Colloidal Oat)
5.4. Quassia amara L. (Bitter Wood)
5.5. Tenacetum parthenium L. (Feverfew)
5.6. Artemisia annua L. (Wormwood)
5.7. Matricaria recutita L. (Chamomile)
5.8. Potentilla erecta L. (Tormentil)
5.9. Camellia sinensis Kuntze (Green Tea)
5.10. Artemisia lavandulaefolia L.
5.11. Citrus junos Tanaka (Yuzu Citrus Fruit)
5.12. Achillea millefolium L. (Yarrow)
5.13. Coffea arabica L. (Coffeeberry)
5.14. Aloe vera L.
6. Limitations
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Korting, H.C.; Schöllmann, C. Current Topical and Systemic Approaches to Treatment of Rosacea. J. Eur. Acad. Dermatol. Venereol. 2009, 23, 876–882. [Google Scholar] [CrossRef] [PubMed]
- Gether, L.; Overgaard, L.K.; Egeberg, A.; Thyssen, J.P. Incidence and Prevalence of Rosacea: A Systematic Review and Meta-Analysis. Br. J. Dermatol. 2018, 179, 282–289. [Google Scholar] [CrossRef] [PubMed]
- Scheinfeld, N.; Berk, T. A Review of the Diagnosis and Treatment of Rosacea. Postgrad. Med. 2010, 122, 139–143. [Google Scholar] [CrossRef] [PubMed]
- Wollina, U.; Verma, S.B. Rosacea and Rhinophyma: Not Curse of the Celts but Indo Eurasians. J. Cosmet. Dermatol. 2009, 8, 234–235. [Google Scholar] [CrossRef] [PubMed]
- Wilkin, J.; Dahl, M.; Detmar, M.; Drake, L.; Feinstein, A.; Odom, R.; Powell, F. Standard Classification of Rosacea: Report of the National Rosacea Society Expert Committee on the Classification and Staging of Rosacea. J. Am. Acad. Dermatol. 2002, 46, 584–587. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.; Almeida, L.M.C.; Bewley, A.; Cribier, B.; Dlova, N.C.; Gallo, R.; Kautz, G.; Mannis, M.; Oon, H.H.; Rajagopalan, M.; et al. Updating the Diagnosis, Classification and Assessment of Rosacea: Recommendations from the Global ROSacea COnsensus (ROSCO) Panel. Br. J. Dermatol. 2017, 176, 431–438. [Google Scholar] [CrossRef] [PubMed]
- van Zuuren, E.J.; Arents, B.W.M.; van der Linden, M.M.D.; Vermeulen, S.; Fedorowicz, Z.; Tan, J. Rosacea: New Concepts in Classification and Treatment. Am. J. Clin. Dermatol. 2021, 22, 457–465. [Google Scholar] [CrossRef] [PubMed]
- Gallo, R.L.; Granstein, R.D.; Kang, S.; Mannis, M.; Steinhoff, M.; Tan, J.; Thiboutot, D. Standard Classification and Pathophysiology of Rosacea: The 2017 Update by the National Rosacea Society Expert Committee. J. Am. Acad. Dermatol. 2018, 78, 148–155. [Google Scholar] [CrossRef]
- Grading System for Rosacea|Rosacea.org. Available online: https://www.rosacea.org/physicians/grading-system-for-rosacea/view-online#toc15 (accessed on 23 September 2023).
- Sharma, A.; Kroumpouzos, G.; Kassir, M.; Galadari, H.; Goren, A.; Grabbe, S.; Goldust, M. Rosacea Management: A Comprehensive Review. J. Cosmet. Dermatol. 2022, 21, 1895–1904. [Google Scholar] [CrossRef]
- Two, A.M.; Wu, W.; Gallo, R.L.; Hata, T.R. Rosacea: Part I. Introduction, Categorization, Histology, Pathogenesis, and Risk Factors. J. Am. Acad. Dermatol. 2015, 72, 749–758. [Google Scholar] [CrossRef]
- Chauhan, N.; Ellis, D.A.F. Rosacea: Pathophysiology and Management Principles. Facial Plast. Surg. Clin. North Am. 2013, 21, 127–136. [Google Scholar] [CrossRef] [PubMed]
- Barakji, Y.A.; Rønnstad, A.T.M.; Christensen, M.O.; Zachariae, C.; Wienholtz, N.K.F.; Halling, A.S.; Maul, J.T.; Thomsen, S.F.; Egeberg, A.; Thyssen, J.P. Assessment of Frequency of Rosacea Subtypes in Patients with Rosacea: A Systematic Review and Meta-Analysis. JAMA Dermatol. 2022, 158, 617–625. [Google Scholar] [CrossRef] [PubMed]
- Thiboutot, D.; Anderson, R.; Cook-Bolden, F.; Draelos, Z.; Gallo, R.L.; Granstein, R.D.; Kang, S.; Macsai, M.; Gold, L.S.; Tan, J. Standard Management Options for Rosacea: The 2019 Update by the National Rosacea Society Expert Committee. J. Am. Acad. Dermatol. 2020, 82, 1501–1510. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Matsuo, T.; Matsuo, C.; Abe, T. Traditional Chinese Medicines and Prescriptions Brought from China to Japan by a Monk (Jianzhen, Japanese: Ganjin): A Historical Review. Compounds 2022, 2, 267–284. [Google Scholar] [CrossRef]
- Mukherjee, P.K.; Wahile, A. Integrated Approaches towards Drug Development from Ayurveda and Other Indian System of Medicines. J. Ethnopharmacol. 2006, 103, 25–35. [Google Scholar] [CrossRef]
- WHO Global Centre for Traditional Medicine. Available online: https://www.who.int/initiatives/who-global-centre-for-traditional-medicine (accessed on 5 January 2024).
- Kaur, R.; Kaur, H.; Dhindsa, A.S. Glycyrrhiza Glabra: A Phytopharmacological Review. Int. J. Pharm. Sci. Res. 2013, 4, 2470. [Google Scholar] [CrossRef]
- Schwab, V.D.; Sulk, M.; Seeliger, S.; Nowak, P.; Aubert, J.; Mess, C.; Rivier, M.; Carlavan, I.; Rossio, P.; Metze, D.; et al. Neurovascular and Neuroimmune Aspects in the Pathophysiology of Rosacea. J. Investig. Dermatol. Symp. Proc. 2011, 15, 53–62. [Google Scholar] [CrossRef]
- Laquer, V.; Hoang, V.; Nguyen, A.; Kelly, K.M. Angiogenesis in Cutaneous Disease: Part II. J. Am. Acad. Dermatol. 2009, 61, 945–958. [Google Scholar] [CrossRef]
- Holmes, A.D.; Steinhoff, M. Integrative Concepts of Rosacea Pathophysiology, Clinical Presentation and New Therapeutics. Exp. Dermatol. 2017, 26, 659–667. [Google Scholar] [CrossRef]
- Abram, K.; Silm, H.; Maaroos, H.I.; Oona, M. Risk Factors Associated with Rosacea. J. Eur. Acad. Dermatol. Venereol. 2010, 24, 565–571. [Google Scholar] [CrossRef]
- Buddenkotte, J.; Steinhoff, M. Recent Advances in Understanding and Managing Rosacea. F1000Research 2018, 7, 1885. [Google Scholar] [CrossRef]
- Woo, Y.R.; Lim, J.H.; Cho, D.H.; Park, H.J. Rosacea: Molecular Mechanisms and Management of a Chronic Cutaneous Inflammatory Condition. Int. J. Mol. Sci. 2016, 17, 1562. [Google Scholar] [CrossRef]
- Yuan, X.; Li, J.; Li, Y.; Deng, Z.; Zhou, L.; Long, J.; Tang, Y.; Zuo, Z.; Zhang, Y.; Xie, H. Artemisinin, a Potential Option to Inhibit Inflammation and Angiogenesis in Rosacea. Biomed. Pharmacother. 2019, 117, 109181. [Google Scholar] [CrossRef]
- Dall’Oglio, F.; Nasca, M.R.; Micali, G. Emerging Topical Drugs for the Treatment of Rosacea. Expert. Opin. Emerg. Drugs 2021, 26, 27–38. [Google Scholar] [CrossRef] [PubMed]
- Roh, K.B.; Jang, Y.; Cho, E.; Park, D.; Kweon, D.H.; Jung, E. Chlorogenic Acid Isomers Isolated from Artemisia Lavandulaefolia Exhibit Anti-Rosacea Effects In Vitro. Biomedicines 2022, 10, 463. [Google Scholar] [CrossRef]
- Lazaridou, E.; Korfitis, C.; Kemanetzi, C.; Sotiriou, E.; Apalla, Z.; Vakirlis, E.; Fotiadou, C.; Lallas, A.; Ioannides, D. Rosacea and Helicobacter Pylori: Links and Risks. Clin. Cosmet. Investig. Dermatol. 2017, 10, 305. [Google Scholar] [CrossRef] [PubMed]
- Wollina, U. Is Rosacea a Systemic Disease? Clin. Dermatol. 2019, 37, 629–635. [Google Scholar] [CrossRef]
- Gupta, A.K.; Chaudhry, M.M. Rosacea and Its Management: An Overview. J. Eur. Acad. Dermatol. Venereol. 2005, 19, 273–285. [Google Scholar] [CrossRef]
- Fisk, W.A.; Lev-Tov, H.A.; Clark, A.K.; Sivamani, R.K. Phytochemical and Botanical Therapies for Rosacea: A Systematic Review. Phytother. Res. 2015, 29, 1439–1451. [Google Scholar] [CrossRef]
- Coping with Rosacea|Rosacea.org. Available online: https://www.rosacea.org/patients/materials/coping-with-rosacea/identifying-rosacea-triggers (accessed on 11 January 2024).
- Abokwidir, M.; Feldman, S.R. Rosacea Management. Ski. Appendage Disord. 2016, 2, 26–34. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, J.; Gendrisch, F.; Schempp, C.M.; Wölfle, U. New Herbal Biomedicines for the Topical Treatment of Dermatological Disorders. Biomedicines 2020, 8, 27. [Google Scholar] [CrossRef] [PubMed]
- Flieger, J.; Flieger, W.; Baj, J.; Maciejewski, R. Antioxidants: Classification, Natural Sources, Activity/Capacity Measurements, and Usefulness for the Synthesis of Nanoparticles. Materials 2021, 14, 4135. [Google Scholar] [CrossRef] [PubMed]
- Schaller, M.; Almeida, L.M.C.; Bewley, A.; Cribier, B.; Del Rosso, J.; Dlova, N.C.; Gallo, R.L.; Granstein, R.D.; Kautz, G.; Mannis, M.J.; et al. Recommendations for Rosacea Diagnosis, Classification and Management: Update from the Global ROSacea COnsensus 2019 Panel. Br. J. Dermatol. 2020, 182, 1269–1276. [Google Scholar] [CrossRef] [PubMed]
- Rosacea Triggers Survey|Rosacea.org. Available online: https://www.rosacea.org/patients/rosacea-triggers/rosacea-triggers-survey (accessed on 8 January 2024).
- Thornfeldt, C. Cosmeceuticals Containing Herbs: Fact, Fiction, and Future. Dermatol. Surg. 2005, 31 Pt 2, 873–881. [Google Scholar] [CrossRef] [PubMed]
- Sharma, V.; Katiyar, A.; Agrawal, R.C.; Sharma, V.; Katiyar, A. Glycyrrhiza Glabra: Chemistry and Pharmacological Activity 4. In Sweeteners; Springer: Cham, The Netherland, 2018. [Google Scholar] [CrossRef]
- Cerulli, A.; Masullo, M.; Montoro, P.; Piacente, S. Licorice (Glycyrrhiza Glabra, G. Uralensis, and G. Inflata) and Their Constituents as Active Cosmeceutical Ingredients. Cosmetics 2022, 9, 7. [Google Scholar] [CrossRef]
- Ye, J.-H.; Lu, J.; Liang, Y.-R. Analysis of Chemical Composition of Chrysanthemum Indicum Flowers by GC/MS and HPLC Fluorine Metabolism in Tea Plant View Project. J. Med. Plants Res. 2010, 89, 421–426. [Google Scholar]
- Sharma, N.; Radha; Kumar, M.; Kumari, N.; Puri, S.; Rais, N.; Natta, S.; Dhumal, S.; Navamaniraj, N.; Chandran, D.; et al. Phytochemicals, Therapeutic Benefits and Applications of Chrysanthemum Flower: A Review. Heliyon 2023, 9, e20232. [Google Scholar] [CrossRef]
- Kim, I.S.; Hwang, C.W.; Yang, W.S.; Kim, C.H. Multiple Antioxidative and Bioactive Molecules of Oats (Avena sativa L.) in Human Health. Antioxidants 2021, 10, 1454. [Google Scholar] [CrossRef]
- Balkrishna, A.; Singh, S.; Srivastava, D.; Mishra, S.; Rajput, S.K.; Arya, V. Quassia amara L.: A Comprehensive Review of Its Ethnomedicinal Uses, Phytochemistry, Pharmacology and Toxicity. J. Phytopharm. 2022, 11, 194–199. [Google Scholar] [CrossRef]
- Lechkova, B.; Karcheva-Bahchevanska, D.; Ivanov, K.; Todorova, V.; Benbassat, N.; Penkova, N.; Atanassova, P.; Peychev, L.; Hrischev, P.; Peychev, Z.; et al. A Study of the Chemical Composition, Acute and Subacute Toxicity of Bulgarian Tanacetum parthenium Essential Oil. Molecules 2023, 28, 4906. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Chen, F.; Wang, X.; Kim, H.J.; He, G.Q.; Haley-Zitlin, V.; Huang, G. Antioxidant Constituents in Feverfew (Tanacetum parthenium) Extract and Their Chromatographic Quantification. Food Chem. 2006, 96, 220–227. [Google Scholar] [CrossRef]
- Pareek, A.; Suthar, M.; Rathore, G.S.; Bansal, V.; Pareek, M.A. Feverfew (Tanacetum parthenium L.): A Systematic Review. Pharmacogn. Rev. 2011, 5, 103–110. [Google Scholar] [CrossRef]
- Ćavar, S.; Maksimović, M.; Vidic, D.; Parić, A. Chemical Composition and Antioxidant and Antimicrobial Activity of Essential Oil of Artemisia annua L. from Bosnia. Ind. Crops Prod. 2012, 37, 479–485. [Google Scholar] [CrossRef]
- Iqbal, S.; Younas, U.; Chan, K.W.; Zia-Ul-Haq, M.; Ismail, M. Chemical Composition of Artemisia annua L. Leaves and Antioxidant Potential of Extracts as a Function of Extraction Solvents. Molecules 2012, 17, 6020–6032. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, J.F.S.; Luthria, D.L.; Sasaki, T.; Heyerick, A. Flavonoids from Artemisia annua L. as Antioxidants and Their Potential Synergism with Artemisinin against Malaria and Cancer. Molecules 2010, 15, 3135–3170. [Google Scholar] [CrossRef] [PubMed]
- Singh, O.; Khanam, Z.; Misra, N.; Srivastava, K.; Singh, M.O. Chamomile (Matricaria chamomilla L.): An Overview. Pharmacogn. Rev. 2011, 5, 82–95. [Google Scholar] [CrossRef] [PubMed]
- Tomczyk, M.; Latté, K.P. Potentilla-A Review of Its Phytochemical and Pharmacological Profile. J. Ethnopharmacol. 2009, 122, 184–204. [Google Scholar] [CrossRef] [PubMed]
- Zhao, T.; Li, C.; Wang, S.; Song, X. Green Tea (Camellia sinensis): A Review of Its Phytochemistry, Pharmacology, and Toxicology. Molecules 2022, 27, 3909. [Google Scholar] [CrossRef] [PubMed]
- Cha, J.D.; Jeong, M.R.; Choi, H.J.; Jeong, S.I.; Moon, S.E.; Yun, S.I.; Kim, Y.H.; Kil, B.S.; Song, Y.H. Chemical Composition and Antimicrobial Activity of the Essential Oil of Artemisia lavandulaefolia. Planta Med. 2005, 71, 575–577. [Google Scholar] [CrossRef]
- Shim, J.-H.; Chae, J.-I.; Cho, S.-S. Identification and Extraction Optimization of Active Constituents in Citrus Junos Seib Ex TANAKA Peel and Its Biological Evaluation. Molecules 2019, 24, 680. [Google Scholar] [CrossRef]
- El-Toumy, S.A.; Hussein, A.A. Cold Pressed Yuzu (Citrus Junos Sieb. Ex Tanaka) Oil. In Cold Pressed Oils; Green Technology, Bioactive Compounds, Functionality, and Applications; Academic Press: Cambridge, MA, USA, 2020; pp. 711–718. [Google Scholar] [CrossRef]
- Verma, R.S.; Joshi, N.; Padalia, R.C.; Goswami, P.; Singh, V.R.; Chauhan, A.; Verma, S.K.; Iqbal, H.; Verma, R.K.; Chanda, D.; et al. Chemical Composition and Allelopathic, Antibacterial, Antifungal and in Vitro Acetylcholinesterase Inhibitory Activities of Yarrow (Achillea millefolium L.) Native to India. Ind. Crops Prod. 2017, 104, 144–155. [Google Scholar] [CrossRef]
- Heimbach, J.T.; Marone, P.A.; Hunter, J.M.; Nemzer, B.V.; Stanley, S.M.; Kennepohl, E. Safety Studies on Products from Whole Coffee Fruit. Food Chem. Toxicol. 2010, 48, 2517–2525. [Google Scholar] [CrossRef] [PubMed]
- Sofía Torres-Valenzuela, L.; Andrea Serna-Jiménez, J.; Martínez, K. Coffee By-Products: Nowadays and Perspectives. In Coffee-Production and Research; IntechOpen: London, UK, 2020. [Google Scholar] [CrossRef]
- Hamman, J.H. Composition and Applications of Aloe Vera Leaf Gel. Molecules 2008, 13, 1599. [Google Scholar] [CrossRef] [PubMed]
- Tin, N.H.; Tien, N.M.; Tien, L.T.D.; Trinh, B.T.N.; Van Thanh, V.; Khang, D.T.; Hang, P.T.; Yen, T.H.; Hai, T.Q.; Tam, T.T.T. Phytochemical and Pharmacological Review of Licorice (Glycyrrhiza sp.)—A Traditional Local Herb for the Future of Medicine. Tạp chí Y Dược học Cần Thơ 2022, 4, 71–80. [Google Scholar] [CrossRef]
- Frattaruolo, L.; Carullo, G.; Brindisi, M.; Mazzotta, S.; Bellissimo, L.; Rago, V.; Curcio, R.; Dolce, V.; Aiello, F.; Cappello, A.R. Antioxidant and Anti-Inflammatory Activities of Flavanones from Glycyrrhiza glabra L. (Licorice) Leaf Phytocomplexes: Identification of Licoflavanone as a Modulator of NF-KB/MAPK Pathway. Antioxidants 2019, 8, 186. [Google Scholar] [CrossRef] [PubMed]
- Quintana, S.E.; Cueva, C.; Villanueva-Bermejo, D.; Moreno-Arribas, M.V.; Fornari, T.; García-Risco, M.R. Antioxidant and Antimicrobial Assessment of Licorice Supercritical Extracts. Ind. Crops Prod. 2019, 139, 111496. [Google Scholar] [CrossRef]
- Kolbe, L.; Immeyer, J.; Batzer, J.; Wensorra, U.; Dieck, K.T.; Mundt, C.; Wolber, R.; Stäb, F.; Schönrock, U.; Ceilley, R.I.; et al. Anti-Inflammatory Efficacy of Licochalcone A: Correlation of Clinical Potency and in Vitro Effects. Arch. Dermatol. Res. 2006, 298, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Saeedi, M.; Morteza-Semnani, K.; Ghoreishi, M.R. The Treatment of Atopic Dermatitis with Licorice Gel. J. Dermatol. Treat. 2003, 14, 153–157. [Google Scholar] [CrossRef]
- Yokota, T.; Nishio, H.; Kubota, Y.; Mizoguchi, M. The Inhibitory Effect of Glabridin from Licorice Extracts on Melanogenesis and Inflammation. Pigment. Cell Res. 1998, 11, 355–361. [Google Scholar] [CrossRef]
- Weber, T.M.; Ceilley, R.I.; Buerger, A.; Kolbe, L.; Trookman, N.S.; Rizer, R.L.; Schoelermann, A. Skin Tolerance, Efficacy, and Quality of Life of Patients with Red Facial Skin Using a Skin Care Regimen Containing Licochalcone A. J. Cosmet. Dermatol. 2006, 5, 227–232. [Google Scholar] [CrossRef]
- Rigopoulos, D.; Kalogeromitros, D.; Gregoriou, S.; Pacouret, J.M.; Koch, C.; Fisher, N.; Bachmann, K.; Brown, M.; Schwarz, E.; Camel, E.; et al. Randomized Placebo-Controlled Trial of a Flavonoid-Rich Plant Extract-Based Cream in the Treatment of Rosacea. J. Eur. Acad. Dermatol. Venereol. 2005, 19, 564–568. [Google Scholar] [CrossRef]
- Colloidal Oatmeal: History, Chemistry and Clinical Properties—PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/17373175/ (accessed on 6 May 2023).
- Cerio, R.; Dohil, M.; Downie, J.; Magina, S.; Mahé, E.; Stratigos, A.J. Mechanism of Action and Clinical Benefits of Colloidal Oatmeal for Dermatologic Practice. J. Drugs Dermatol. 2010, 9, 1116–1120. [Google Scholar]
- Sur, R.; Nigam, A.; Grote, D.; Liebel, F.; Southall, M.D. Avenanthramides, Polyphenols from Oats, Exhibit Anti-Inflammatory and Anti-Itch Activity. Arch. Dermatol. Res. 2008, 300, 569–574. [Google Scholar] [CrossRef]
- Nebus, J.; Nollent, V.; Wallo, W. New Learnings on the Clinical Benefits of Colloidal Oatmeal in Atopic Dermatitis. Dermatologist 2012. Available online: https://www.hmpgloballearningnetwork.com/site/thederm/site/cathlab/event/new-learnings-clinical-benefits-colloidal-oatmeal-atopic-dermatitis-1 (accessed on 30 January 2024).
- “Natural” Ingredients in Cosmetic Dermatology—PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/19562883/ (accessed on 6 May 2023).
- Southall, M.; Johnson, J.; Ilnytska, O.; Kaur, S.; Chon, S.; Reynertson, K.A.; Nebus, J.; Garay, M.; Mahmood, K.; Southall, M.D. Colloidal Oatmeal (Avena sativa) Improves Skin. Barrier Through Multi-Therapy Activity. J. Drugs Dermatol. 2016, 15, 684–690. [Google Scholar]
- Toma, W.; Gracioso, J.S.; Hiruma-Lima, C.A.; Andrade, F.D.P.; Vilegas, W.; Souza Brito, A.R.M. Evaluation of the Analgesic and Antiedematogenic Activities of Quassia amara Bark Extract. J. Ethnopharmacol. 2003, 85, 19–23. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, A.; Diehl, C. Evaluation of the Efficacy and Tolerance of a Topical Gel with 4% Quassia Extract in the Treatment of Rosacea. J. Clin. Pharmacol. 2012, 52, 84–88. [Google Scholar] [CrossRef] [PubMed]
- Pittler, M.H.; Ernst, E. Feverfew for Preventing Migraine. Cochrane Database Syst. Rev. 2004, CD002286. [Google Scholar] [CrossRef]
- Groenewegen, W.A.; Heptinstall, S. A Comparison of the Effects of an Extract of Feverfew and Parthenolide, a Component of Feverfew, on Human Platelet Activity In-Vitro. J. Pharm. Pharmacol. 1990, 42, 553–557. [Google Scholar] [CrossRef] [PubMed]
- Baumann, L.S. Less-Known Botanical Cosmeceuticals. Dermatol. Ther. 2007, 20, 330–342. [Google Scholar] [CrossRef] [PubMed]
- Martin, K.; Sur, R.; Liebel, F.; Tierney, N.; Lyte, P.; Garay, M.; Oddos, T.; Anthonavage, M.; Shapiro, S.; Southall, M. Parthenolide-Depleted Feverfew (Tanacetum parthenium) Protects Skin from UV Irradiation and External Aggression. Arch. Dermatol. Res. 2008, 300, 69–80. [Google Scholar] [CrossRef]
- Sur, R.; Martin, K.; Liebel, F.; Lyte, P.; Shapiro, S.; Southall, M. Anti-Inflammatory Activity of Parthenolide-Depleted Feverfew (Tanacetum parthenium). Inflammopharmacology 2009, 17, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Anti-Inflammatory Ingredients—PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/18681154/ (accessed on 9 May 2023).
- Ribet, V.; Nebus, J.; Wallo, W.; Smith, G.; Kurtz, E.S.; Black, D.; Jean Louis Alibert, C.; Coutanceau, C.; Theunis, J. Evaluating Topical Preparations in Individuals with Sensitive Skin. J. Am. Acad. Dermatol. 2005, 52, P90. [Google Scholar] [CrossRef]
- Wang, G.J.; Gao, X.Y.; Wu, Y.; He, H.Q.; Yu, Y.; Qin, H.H.; Shen, W.T. Evaluation of the Efficacy and Tolerance of Artemether Emulsion for the Treatment of Papulopustular Rosacea: A Randomized Pilot Study. J. Dermatol. Treat. 2019, 30, 809–812. [Google Scholar] [CrossRef] [PubMed]
- Nagula, R.L.; Wairkar, S. Recent Advances in Topical Delivery of Flavonoids: A Review. J. Control. Release 2019, 296, 190–201. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Zeng, Q.; Chen, X.; Wang, G.; Zhang, H.; Yu, A.; Wang, H.; Hu, Y. The Therapeutic Effect of Artesunate on Rosacea through the Inhibition of the JAK/STAT Signaling Pathway. Mol. Med. Rep. 2018, 17, 8385–8390. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Heo, Y.; Kim, Y.C. Effect of German Chamomile Oil Application on Alleviating Atopic Dermatitis-like Immune Alterations in Mice. J. Vet. Sci. 2010, 11, 35. [Google Scholar] [CrossRef]
- Emer, J.; Waldorf, H.; Berson, D. Botanicals and Anti-Inflammatories: Natural Ingredients for Rosacea. In Seminars in Cutaneous Medicine and Surgery; W.B. Saunders Ltd.: St. Louis, MO, USA, 2011; pp. 148–155. [Google Scholar] [CrossRef]
- Guarrera, M.; Turbino, L.; Rebora, A. The Anti-Inflammatory Activity of Azulene. J. Eur. Acad. Dermatol. Venereol. 2001, 15, 486–487. [Google Scholar] [CrossRef] [PubMed]
- Kaltalioglu, K.; Balabanli, B.; Coskun-Cevher, S. Phenolic, Antioxidant, Antimicrobial, and In-Vivo Wound Healing Properties of Potentilla Erecta L. Root Extract in Diabetic Rats. Iran. J. Pharm. Res. 2020, 19, 264. [Google Scholar] [CrossRef]
- Chiu, A.E.; Chan, J.L.; Kern, D.G.; Kohler, S.; Rehmus, W.E.; Kimball, A.B. Double-Blinded, Placebo-Controlled Trial of Green Tea Extracts in the Clinical and Histologic Appearance of Photoaging Skin. Dermatol. Surg. 2005, 31 Pt 2, 855–860. [Google Scholar] [CrossRef]
- Yoon, J.Y.; Kwon, H.H.; Min, S.U.; Thiboutot, D.M.; Suh, D.H. Epigallocatechin-3-Gallate Improves Acne in Humans by Modulating Intracellular Molecular Targets and Inhibiting P. Acnes. J. Investig. Dermatol. 2013, 133, 429–440. [Google Scholar] [CrossRef]
- Belo, S.E.D.; Gaspar, L.R.; Campos, P.M.B.G.M. Photoprotective Effects of Topical Formulations Containing a Combination of Ginkgo Biloba and Green Tea Extracts. Phytother. Res. 2011, 25, 1854–1860. [Google Scholar] [CrossRef]
- Morley, N.; Clifford, T.; Salter, L.; Campbell, S.; Gould, D.; Curnow, A. The Green Tea Polyphenol (-)-Epigallocatechin Gallate and Green Tea Can Protect Human Cellular DNA from Ultraviolet and Visible Radiation-Induced Damage. Photodermatol. Photoimmunol. Photomed. 2005, 21, 15–22. [Google Scholar] [CrossRef]
- Yusuf, N.; Irby, C.; Katiyar, S.K.; Elmets, C.A. Photoprotective Effects of Green Tea Polyphenols. Photodermatol. Photoimmunol. Photomed. 2007, 23, 48–56. [Google Scholar] [CrossRef]
- Marijan, M.; Tomi’c, D.T.; Strawa, J.W.; Jakupovi´c, L.J.; Ini´c, S.I.; Jug, M.; Tomczyk, M.; Zovko, M.; Konči´c, K. Optimization of Cyclodextrin-Assisted Extraction of Phenolics from Helichrysum Italicum for Preparation of Extracts with Anti-Elastase and Anti-Collagenase Properties. Metabolites 2023, 13, 257. [Google Scholar] [CrossRef]
- Zou, Z.; Xi, W.; Hu, Y.; Nie, C.; Zhou, Z. Antioxidant Activity of Citrus Fruits. Food Chem. 2016, 196, 885–896. [Google Scholar] [CrossRef]
- Hirota, R.; Roger, N.N.; Nakamura, H.; Song, H.S.; Sawamura, M.; Suganuma, N. Anti-Inflammatory Effects of Limonene from Yuzu (Citrus Junos Tanaka) Essential Oil on Eosinophils. J. Food Sci. 2010, 75, H87–H92. [Google Scholar] [CrossRef]
- Jeon, H.W.; Na, E.Y.; Yun, S.J.; Lee, S.C.; Lee, J.B. Citron Essential Oils Alleviate the Mediators Related to Rosacea Pathophysiology in Epidermal Keratinocytes. Ann. Dermatol. 2018, 30, 653–661. [Google Scholar] [CrossRef]
- Becker, L.C.; Bergfeld, W.F.; Belsito, D.V.; Hill, R.A.; Klaassen, C.D.; Liebler, D.C.; Marks, J.G.; Shank, R.C.; Slaga, T.J.; Snyder, P.W.; et al. Safety Assessment of Achillea Millefolium as Used in Cosmetics. Int. J. Toxicol. 2016, 35 (Suppl. S3), S5–S15. [Google Scholar] [CrossRef]
- Strzępek-Gomółka, M.; Gaweł-Bęben, K.; Kukula-Koch, W. Achillea Species as Sources of Active Phytochemicals for Dermatological and Cosmetic Applications. In Oxidative Medicine and Cellular Longevity; Hindawi Limited: London, UK, 2021. [Google Scholar] [CrossRef]
- Farris, P. Idebenone, Green Tea, and Coffeeberry® Extract: New and Innovative Antioxidants. Dermatol. Ther. 2007, 20, 322–329. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.F.; Ouyang, S.H.; Tu, L.F.; Wang, X.; Yuan, W.L.; Wang, G.E.; Wu, Y.P.; Duan, W.J.; Yu, H.M.; Fang, Z.Z.; et al. Caffeine Protects Skin from Oxidative Stress-Induced Senescence through the Activation of Autophagy. Theranostics 2018, 8, 5713–5730. [Google Scholar] [CrossRef] [PubMed]
- Saewan, N. Effect of Coffee Berry Extract on Anti-Aging for Skin and Hair—In Vitro Approach. Cosmetics 2022, 9, 66. [Google Scholar] [CrossRef]
- Hekmatpou, D.; Mehrabi, F.; Rahzani, K.; Aminiyan, A. The Effect of Aloe Vera Clinical Trials on Prevention and Healing of Skin Wound: A Systematic Review. Iran. J. Med. Sci. 2019, 44, 1. [Google Scholar]
- Aloe Vera in Dermatology: A Brief Review—PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/19218914/ (accessed on 9 May 2023).
- Bedi, M.K.; Shenefelt, P.D. Herbal Therapy in Dermatology. Arch. Dermatol. 2002, 138, 232–242. [Google Scholar] [CrossRef]
- Radha, M.H.; Laxmipriya, N.P. Evaluation of Biological Properties and Clinical Effectiveness of Aloe Vera: A Systematic Review. J. Tradit. Complement. Med. 2015, 5, 21–26. [Google Scholar] [CrossRef]
- Saini, D.K.; Saini, M.R. Evaluation of Radioprotective Efficacy and Possible Mechanism of Action of Aloe Gel. Environ. Toxicol. Pharmacol. 2011, 31, 427–435. [Google Scholar] [CrossRef]
- Syed, T.A.; Ahmad, S.A.; Holt, A.H.; Ahmad, S.A.; Ahmad, S.H.; Afzal, M. Management of Psoriasis with Aloe Vera Extract in a Hydrophilic Cream: A Placebo-Controlled, Double-Blind Study. Trop. Med. Int. Health 1996, 1, 505–509. [Google Scholar] [CrossRef]
- Choonhakarn, C.; Busaracome, P.; Sripanidkulchai, B.; Sarakarn, P. A Prospective, Randomized Clinical Trial Comparing Topical Aloe Vera with 0.1% Triamcinolone Acetonide in Mild to Moderate Plaque Psoriasis. J. Eur. Acad. Dermatol. Venereol. 2010, 24, 168–172. [Google Scholar] [CrossRef]
- Somboonwong, J.; Thanamittramanee, S.; Jariyapongskul, A.; Patumraj, S. Therapeutic effects of Aloe vera on cutaneous microcirculation and wound healing in second degree burn model in rats. J. Med. Assoc. Thai. 2000, 83, 417–425. [Google Scholar]


| Primary (major) features | Flushing (erythema) | Redness that is visible and usually prolonged over the central face. It occurs rapidly after being exposed to triggers. |
| Papules and pustules | Papules with or without pustules; occasional nodules. | |
| Telangiectasia | The presence of red blood vessels on the surface of the cheeks. This feature is rarely visible in darker phototypes. | |
| Ocular manifestations | It may be a feature or a separate form of rosacea altogether. Characterized by lid inflammation or conjunctival redness. In time, it may result in corneal damage. | |
| Secondary features | Burning or stinging | Acute sensation of burning or stinging, especially in the central area. |
| Dry appearance | Rough and scaly skin, especially around the nose and cheeks area. | |
| Edema | It is usually present together with erythema but not conditioned by it. It appears when exposed to triggers and may last for a few days. | |
| Phymatous changes | Thickening or fibrosis of the skin with a bulbous character. The most common form is rhinophyma. |
| Clinical Subtypes | Description |
|---|---|
| Erythemato-telangiectatic (ETR) (subtype I) | Nontransient episodes of flushing; often with stinging and itching; mostly persistent central facial erythema; and telangiectasia is common. |
| Papulopustular (subtype II) | Persistent facial erythema; papules or pustules or both, with a central facial localization. |
| Phymatous (subtype III) | Thickened skin, irregular surface nodularities; enlargement of skin tissue. It occurs mostly in the nose area (rhinophyma), but it also affects the chin, forehead, cheeks, or ears. Telangiectasia is also present. |
| Ocular (subtype IV) | Includes inflammation of the lids and conjunctival telangiectasias. Foreign body sensations in the eye are also common. Patients also complain about dryness, itching, ocular photosensitivity, or even periorbital edema. |
| Product | Source | Active Components |
|---|---|---|
| Licorice root | Glycyrrhiza glabra L., Glycyrrhiza inflata L. | Saponins (glycyrrhizin), flavanones (liquiritin, liquiritigenin), chalcones (isoliquiritigenin, licochalcone), and isoflavones (glabridin, 4t-O-methylglabridin) [39,40]. |
| Indian chrysanthemum flower | Chrysanthemum indicum L. | Flavonols (rutin, quercetin), flavones (luteolin), chlorogenic acids, and volatile oils (Germacrene D, α-neoclovene, eucalyptol, and α-pinene) [41,42]. |
| Colloidal oat powder | Avena sativa L. | Phenolic acids (caffeic acid, coumaric acid), alkaloids (avenanthramides), unsaturated fatty acids (palmitic, oleic, and linoleic acids), and β-glucan [43]. |
| Amargo wood | Quassia amara L. | Steroids (β-sitosterol, stigmasterol), triterpenes, and bitter principles (quassia, neoquassin) [44]. |
| Feverfew leaves | Tanacetum parthenium L. | Volatile oils (camphor, trans-chrisantenyl acetate) [45], flavones (luteolin, apigenin) [46], and sesquiterpene (parthenolides) [47]. |
| Wormwood plant | Artemisia annua L. | Volatile oils (camphor, 1,8-cineole, α-pinene) [48], terpenes (artemisinin), and flavones (apigenin, luteolin, and chrysin) [49,50]. |
| Chamomile flowers | Matricaria recutita L. | Chlorogenic acids, phenolic acids (caffeic acid), flavones (apigenin, luteolin), and volatile oils (β-farnesene, farnesol, chamazulene, and α-bisabolol) [51]. |
| Tormentil rhizome/flower | Potentilla erecta L. | Flavonols (rutin), flavanones (hesperidin), tannins, triterpenoids (ursolic acid, tormentic acid), and b-sitosterol [52]. |
| Green Tea leaves | Camellia sinensis L. | Flavonols (quercetin, kaempferol, and myricitin), catechins (catechin, epigallocatechin, and epigallocatechin gallate), and polysaccharides [53]. |
| Artemisia lavandulaefolia essential oil | Artemisia lavandulaefolia L. | Essential oils (β-caryophyllene, cis-chrysanthenol, and camphor) [54] and phenolic acids (3,5-dicaffeoylquinic acid–isochlorogenic acid A, and 4,5-dicaffeoylquinic acid–isochlorogenic acid C) [27]. |
| Yuzu citrus fruit | Citrus junos Tanaka | Flavanoles (hesperidin) [55] and essential oils (limonene, a-pinene, b-pinene, and terpinene) [56]. |
| Achillea spp. flower | Achillea millefolium L. | Flavones (apeginenin, lutelin), flavanols (rutin, kampherol), and essential oils (chamazulene, b-pinene, camphor, and bisabolol) [57]. |
| Coffeeberry beans | Coffea arabica L. | Alkaloids (caffeine, trigonelline), chlorogenic acids, and lipids (triglycerides, linoleic acid, and palmitic acid) [58,59]. |
| Aloe vera plant | Aloe vera L. | Polysaccharides (acetylated glucomannan, arabinan), proteins (lecitin), vitamins (B1, B2, B6, beta-carotene, and folic acid), and amino acids [60]. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Semenescu, I.; Similie, D.; Diaconeasa, Z.; Danciu, C. Recent Advances in the Management of Rosacea through Natural Compounds. Pharmaceuticals 2024, 17, 212. https://doi.org/10.3390/ph17020212
Semenescu I, Similie D, Diaconeasa Z, Danciu C. Recent Advances in the Management of Rosacea through Natural Compounds. Pharmaceuticals. 2024; 17(2):212. https://doi.org/10.3390/ph17020212
Chicago/Turabian StyleSemenescu, Iulia, Diana Similie, Zorita Diaconeasa, and Corina Danciu. 2024. "Recent Advances in the Management of Rosacea through Natural Compounds" Pharmaceuticals 17, no. 2: 212. https://doi.org/10.3390/ph17020212
APA StyleSemenescu, I., Similie, D., Diaconeasa, Z., & Danciu, C. (2024). Recent Advances in the Management of Rosacea through Natural Compounds. Pharmaceuticals, 17(2), 212. https://doi.org/10.3390/ph17020212