Exploring Immersion Coating as a Cost-Effective Method for Small-Scale Production of Enteric-Coated Gelatin Capsules
Abstract
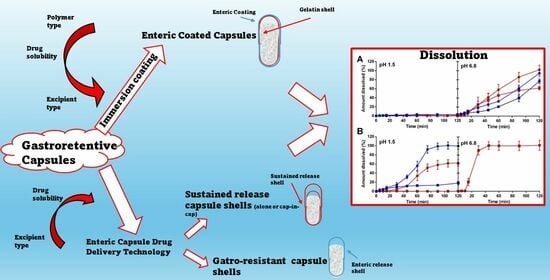
:1. Introduction
2. Results and Discussion
2.1. Coating Solution Rheology
2.2. Dissolution Studies
2.2.1. Capsules Coated with Eudragit Polymers
2.2.2. Capsules Coated with Hypromellose Acetate Succinate Polymers
2.2.3. Commercial Capsules for Enteric/Delayed Release
3. Materials and Methods
3.1. Materials
3.2. Blend Preparation and Capsule Filling
3.3. Coating Solution Formulation
3.4. Film Solution Rheology
3.5. Capsule Coating
3.6. Dissolution Studies
- The dosage forms were positioned in the basket within the vessel, where a liquid simulating stomach conditions (pH 1.5) was previously introduced and heated to 37 °C. In this medium, the capsules were expected to remain stable without releasing the API; the maximum allowable drug release was set at 10% or less [26] over a 2 h period.
- Immediately following the first step, the basket containing the capsules was transferred to a vessel filled with a liquid simulating intestinal fluids (pH 6.8) and left for an additional two hours.
- Gastric-like medium (minutes): 5-10-15-30-45-60-75-90-105-120;
- Intestinal-like medium (minutes): 5-10-15-30-45-60-90-120.
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Salawi, A. Pharmaceutical Coating and Its Different Approaches, a Review. Polymers 2022, 14, 3318. [Google Scholar] [CrossRef] [PubMed]
- Seo, K.-S.; Bajracharya, R.; Lee, S.H.; Han, H.-K. Pharmaceutical Application of Tablet Film Coating. Pharmaceutics 2020, 12, 853. [Google Scholar] [CrossRef] [PubMed]
- Faisal, W.; Farag, F.; Abdellatif, A.A.H.; Abbas, A. Taste Masking Approaches for Medicines. Curr. Drug Deliv. 2018, 15, 167–185. [Google Scholar] [CrossRef] [PubMed]
- Joshi, S.; Petereit, H.-U. Film coatings for taste masking and moisture protection. Int. J. Pharm. 2013, 457, 395–406. [Google Scholar] [CrossRef] [PubMed]
- Odani, N.; Mohan, S.; Kato, E.; Feng, H.; Li, Y.; Hossain, M.N.; Drennen, J.K.; Anderson, C.A. Determining the effect of photodegradation on film coated nifedipine tablets with terahertz based coating thickness measurements. Eur. J. Pharm. Biopharm. 2019, 145, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Felton, L.A.; Porter, S.C. An update on pharmaceutical film coating for drug delivery. Expert Opin. Drug Deliv. 2013, 10, 421–435. [Google Scholar] [CrossRef] [PubMed]
- Siepmann, F.; Siepmann, J.; Walther, M.; MacRae, R.J.; Bodmeier, R. Polymer blends for controlled release coatings. J. Control Release Off. J. Control. Release Soc. 2008, 125, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Rhodes, C.T.; Porter, S.C. Coatings for controlled-release drug delivery systems. Drug Dev. Ind. Pharm. 1998, 24, 1139–1154. [Google Scholar] [CrossRef] [PubMed]
- Stubbe, B.G.; De Smedt, S.C.; Demeester, J. “Programmed Polymeric Devices” for Pulsed Drug Delivery. Pharm. Res. 2004, 21, 1732–1740. [Google Scholar] [CrossRef] [PubMed]
- Maderuelo, C.; Lanao, J.M.; Zarzuelo, A. Enteric coating of oral solid dosage forms as a tool to improve drug bioavailability. Eur. J. Pharm. Sci. 2019, 138, 105019. [Google Scholar] [CrossRef] [PubMed]
- Franc, A.; Vetchý, D.; Fülöpová, N. Commercially Available Enteric Empty Hard Capsules, Production Technology and Application. Pharmaceuticals 2022, 15, 1398. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, J.A.C.; Al-Kauraishi, M.M.; Smith, A.M.; Conway, B.R.; Merchant, H.A. Achieving gastroresistance without coating: Formulation of capsule shells from enteric polymers. Eur. J. Pharm. Biopharm. Off. J. Arbeitsgemeinschaft Fur Pharm. Verfahrenstechnik E.V 2019, 144, 174–179. [Google Scholar] [CrossRef] [PubMed]
- Zema, L.; Loreti, G.; Melocchi, A.; Maroni, A.; Palugan, L.; Gazzaniga, A. Gastroresistant capsular device prepared by injection molding. Int. J. Pharm. 2013, 440, 264–272. [Google Scholar] [CrossRef]
- Moghrabi, F.S.; Fadda, H.M. Drug Physicochemical Properties and Capsule Fill Determine Extent of Premature Gastric Release from Enteric Capsules. Pharmaceutics 2022, 14, 2505. [Google Scholar] [CrossRef] [PubMed]
- Torpac Inc. PRO COATER Enteric/Film Coat Capsules & Caplets. Available online: https://torpac.com/procoater/coater-main.htm (accessed on 5 February 2024).
- Moghimipour, E.; Rezaei, M.; Kouchak, M.; Fatahiasl, J.; Angali, K.A.; Ramezani, Z.; Amini, M.; Dorkoosh, F.A.; Handali, S. Effects of coating layer and release medium on release profile from coated capsules with Eudragit FS 30D: An in vitro and in vivo study. Drug Dev. Ind. Pharm. 2018, 44, 861–867. [Google Scholar] [CrossRef] [PubMed]
- Fülöpová, N.; Pavloková, S.; DeBono, I.; Vetchý, D.; Franc, A. Development and Comparison of Various Coated Hard Capsules Suitable for Enteric Administration to Small Patient Cohorts. Pharmaceutics 2022, 14, 1577. [Google Scholar] [CrossRef]
- National Center for Biotechnology. Information PubChem Compound Summary for CID 1983, Acetaminophen. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Acetaminophen. (accessed on 4 November 2023).
- Smyj, R.; Wang, X.-P.; Han, F. Chapter Eleven—Tramadol Hydrochloride. In Profiles of Drug Substances, Excipients, and Related Methodology; Brittain, H.G., Ed.; Academic Press: New York, NY, USA, 2013; Volume 38, pp. 463–494. ISBN 1871-5125. [Google Scholar]
- Cespi, M.; Bonacucina, G.; Mencarelli, G.; Casettari, L.; Palmieri, G.F. Dynamic mechanical thermal analysis of hypromellose 2910 free films. Eur. J. Pharm. Biopharm. 2011, 79, 458–463. [Google Scholar] [CrossRef]
- Fu, M.; Blechar, J.A.; Sauer, A.; Al-Gousous, J.; Langguth, P. In Vitro Evaluation of Enteric-Coated HPMC Capsules—Effect of Formulation Factors on Product Performance. Pharmaceutics 2020, 12, 696. [Google Scholar] [CrossRef] [PubMed]
- Obara, S.; Kokubo, H. Application of HPMC and HPMCAS to Aqueous Film Coating of Pharmaceutical Dosage Forms. In Aqueous Polymeric Coatings for Pharmaceutical Dosage Forms; McGinity, J.W., Felton, L., Eds.; Informa Healthcare USA, Inc.: New York, NY, USA, 2008. [Google Scholar]
- Li, T.; Wan, B.; Jog, R.; Costa, A.; Burgess, D.J. Pectin microparticles for peptide delivery: Optimization of spray drying processing. Int. J. Pharm. 2022, 613, 121384. [Google Scholar] [CrossRef]
- Monschke, M.; Kayser, K.; Wagner, K.G. Influence of Particle Size and Drug Load on Amorphous Solid Dispersions Containing pH-Dependent Soluble Polymers and the Weak Base Ketoconazole. AAPS PharmSciTech 2021, 22, 44. [Google Scholar] [CrossRef] [PubMed]
- Evonik Industries. AG Ready-to-Fill Coated Capsules for Fast Drug Development. Available online: https://healthcare.evonik.com/en/drugdelivery/oral-drug-delivery/oral-excipients/eudracap-portfolio (accessed on 17 March 2024).
- European Directorate for the Quality of Medicines & HealthCare. Chapter 2.9.3, Dissolution Test for Solid Dosage Form. In European Pharmacopoeia 10.0; European Directorate for the Quality of Medicines & HealthCare: Strasbourg, France, 2020. [Google Scholar]







| Component | 5% Polymer Formulation | 10% Polymer Formulation |
|---|---|---|
| Amount (%) * | Amount (%) * | |
| Polymer ** | 5 | 10 |
| TEC | 0.5 | 1 |
| Yellow Eosin | 0.05 | 0.05 |
| Iso–water (92:8) mixture | 94.45 | 88.95 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sabbatini, B.; Perinelli, D.R.; Palmieri, G.F.; Cespi, M.; Bonacucina, G. Exploring Immersion Coating as a Cost-Effective Method for Small-Scale Production of Enteric-Coated Gelatin Capsules. Pharmaceuticals 2024, 17, 433. https://doi.org/10.3390/ph17040433
Sabbatini B, Perinelli DR, Palmieri GF, Cespi M, Bonacucina G. Exploring Immersion Coating as a Cost-Effective Method for Small-Scale Production of Enteric-Coated Gelatin Capsules. Pharmaceuticals. 2024; 17(4):433. https://doi.org/10.3390/ph17040433
Chicago/Turabian StyleSabbatini, Beatrice, Diego Romano Perinelli, Giovanni Filippo Palmieri, Marco Cespi, and Giulia Bonacucina. 2024. "Exploring Immersion Coating as a Cost-Effective Method for Small-Scale Production of Enteric-Coated Gelatin Capsules" Pharmaceuticals 17, no. 4: 433. https://doi.org/10.3390/ph17040433
APA StyleSabbatini, B., Perinelli, D. R., Palmieri, G. F., Cespi, M., & Bonacucina, G. (2024). Exploring Immersion Coating as a Cost-Effective Method for Small-Scale Production of Enteric-Coated Gelatin Capsules. Pharmaceuticals, 17(4), 433. https://doi.org/10.3390/ph17040433