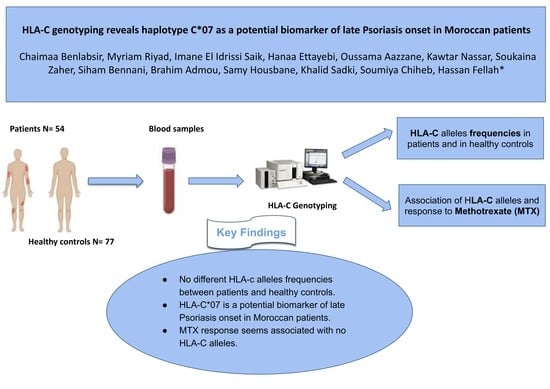
HLA-C Genotyping Reveals Haplotype C*07 as a Potential Biomarker of Late Psoriasis Onset in Moroccan Patients
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethical Considerations
2.2. Patients and Controls
2.3. DNA Extraction
2.4. HLA-C Genotyping
2.5. Efficacy Evaluation of Methotrexate Treatment
2.6. Data Statistics and Analysis
3. Results
3.1. Demographic and Clinical Features of Psoriatic Patients
3.2. HLA-C Alleles Expression
3.2.1. Allelic Distribution of HLA-C Frequencies in Patients and Healthy Controls
3.2.2. Allelic Distribution of HLA-C Frequencies According to the Age of Disease Onset
3.2.3. Allelic Distribution of HLA-C Frequencies According to Psoriasis Clinical Phenotypes
3.2.4. Allelic Distribution of HLA-C Frequencies in Responders and Non-Responders to MTX
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rachakonda, T.D.; Schupp, C.W.; Armstrong, A.W. Psoriasis prevalence among adults in the United States. J. Am. Acad. Dermatol. 2014, 70, 512–516. [Google Scholar] [CrossRef] [PubMed]
- Ammar-Khodja, A.; Benkaidali, I.; Bouadjar, B.; Serradj, A.; Titi, A.; Benchikhi, H.; Amal, S.; Hassam, B.; Sekkat, A.; Mernissi, F.Z.; et al. EPIMAG: International Cross-Sectional Epidemiological Psoriasis Study in the Maghreb. Dermatology 2015, 231, 134–144. [Google Scholar] [CrossRef] [PubMed]
- Benchikhi, H.; Amal, S.; Ammar-Khodja, A.; Benkaidali, I.; Bouadjar, B.; Dhaoui, M.; Denguezli, M.; Doss, N.; Hassam, B.; Mernissi, F.; et al. Étude PSOMAG: Prévalence des cas de psoriasis au Maghreb. Ann. Dermatol. Venereol. 2012, 139, B162–B163. [Google Scholar] [CrossRef]
- Boehncke, W.-H.; Schön, M.P. Psoriasis. Lancet 2015, 386, 983–994. [Google Scholar] [CrossRef] [PubMed]
- Mabuchi, T.; Hirayama, N. Binding Affinity and Interaction of LL-37 with HLA-C*06:02 in Psoriasis. J. Investig. Dermatol. 2016, 136, 1901–1903. [Google Scholar] [CrossRef] [Green Version]
- Ammar, M.; Souissi-Bouchlaka, C.; Gati, A.; Zaraa, I.; Bouhaha, R.; Kouidhi, S.; Ben Ammar-Gaied, A.; Doss, N.; Mokni, M.; Marrakchi, R. Le psoriasis: Physiopathologie et immunogénétique [Psoriasis: Physiopathology and immunogenetics]. Pathol. Biol. 2014, 62, 10–23. [Google Scholar] [CrossRef]
- Sagoo, G.S.; Tazi-Ahnini, R.; Barker, J.W.; Elder, J.T.; Nair, R.P.; Samuelsson, L.; Traupe, H.; Trembath, R.C.; Robinson, D.A.; Iles, M.M. Meta-Analysis of Genome-Wide Studies of Psoriasis Susceptibility Reveals Linkage to Chromosomes 6p21 and 4q28–q31 in Caucasian and Chinese Hans Population. J. Investig. Dermatol. 2004, 122, 1401–1405. [Google Scholar] [CrossRef] [Green Version]
- Stuart, P.E.; Nair, R.P.; Tsoi, L.C.; Tejasvi, T.; Das, S.; Kang, H.M.; Ellinghaus, E.; Chandran, V.; Callis-Duffin, K.; Ike, R.; et al. Genome-wide Association Analysis of Psoriatic Arthritis and Cutaneous Psoriasis Reveals Differences in Their Genetic Architecture. Am. J. Hum. Genet. 2015, 97, 816–836. [Google Scholar] [CrossRef] [Green Version]
- Nair, R.P.; Duffin, K.C.; Helms, C.; Ding, J.; Stuart, P.E.; Goldgar, D.; Gudjonsson, J.E.; Li, Y.; Tejasvi, T.; Feng, B.J.; et al. Genome-wide scan reveals association of psoriasis with IL-23 and NF-κB pathways. Nat. Genet. 2009, 41, 199–204. [Google Scholar] [CrossRef] [Green Version]
- Genetic Analysis of Psoriasis Consortium & the Wellcome Trust Case Control Consortium 2. A genome-wide association study identifies new psoriasis susceptibility loci and an interaction between HLA-C and ERAP1. Nat. Genet. 2010, 42, 985–990. [Google Scholar] [CrossRef]
- Chen, L.; Tsai, T. HLA-Cw6and psoriasis. Br. J. Dermatol. 2018, 178, 854–862. [Google Scholar] [CrossRef]
- Henseler, T.; Christophers, E. Psoriasis of early and late onset: Characterization of two types of psoriasis vulgaris. J. Am. Acad. Dermatol. 1985, 13, 450–456. [Google Scholar] [CrossRef]
- Puig, L.; Julià, A.; Marsal, S. The pathogenesis and genetics of psoriasis. Actas Dermo-Sifiliogr. 2014, 105, 535–545. [Google Scholar] [CrossRef]
- Huang, Y.-W.; Tsai, T.-F. HLA-Cw1 and Psoriasis. Am. J. Clin. Dermatol. 2021, 22, 339–347. [Google Scholar] [CrossRef]
- Li, J.; Li, X.; He, F.; Zhao, X.; Hou, R.; Lin, H.; Shen, J.; Wu, X.; Liao, Q.; Xing, J.; et al. Cross-sectional study reveals that HLA -C*07:02 is a potential biomarker of early onset/lesion severity of psoriasis. Exp. Dermatol. 2020, 29, 639–646. [Google Scholar] [CrossRef]
- Brick, C.; Atouf, O.; Bouayad, A.; Essakalli, M. Moroccan study of HLA (-A, -B, -C, -DR, -DQ) polymorphism in 647 unrelated controls: Updating data. Mol. Cell. Probes 2015, 29, 197–207. [Google Scholar] [CrossRef]
- World Health Organization. Global Report on Psoriasis; World Health Organization: Geneva, Switzerland, 2016; Available online: https://apps.who.int/iris/handle/10665/204417 (accessed on 25 July 2022).
- Puig, L. Methotrexate: New therapeutic approaches. Actas Dermo-Sifiliogr. 2014, 105, 583–589. [Google Scholar] [CrossRef]
- Edmundson, W.F.; Guy, W.B. Treatment of Psoriasis with Folic Acid Antagonists. Arch. Dermatol. 1958, 78, 200–203. [Google Scholar] [CrossRef]
- Menter, A.; Gelfand, J.M.; Connor, C.; Armstrong, A.W.; Cordoro, K.M.; Davis, D.M.; Elewski, B.E.; Gordon, K.B.; Gottlieb, A.B.; Kaplan, D.H.; et al. Joint American Academy of Dermatology–National Psoriasis Foundation guidelines of care for the management of psoriasis with systemic nonbiologic therapies. J. Am. Acad. Dermatol. 2020, 82, 1445–1486. [Google Scholar] [CrossRef]
- Hider, S.L.; Bruce, I.N.; Thomson, W. The pharmacogenetics of methotrexate. Rheumatology 2007, 46, 1520–1524. [Google Scholar] [CrossRef]
- Ogdie, A.; Weiss, P. The Epidemiology of Psoriatic Arthritis. Rheum. Dis. Clin. N. Am. 2015, 41, 545–568. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, K.; Lv, Y.-M.; Yin, X.-Y.; Wang, Z.-X.; Sun, L.-D.; He, S.-M.; Cheng, H.; Hu, D.-Y.; Zhang, Z.; Li, Y.; et al. Psoriasis Regression Analysis of MHC Loci Identifies Shared Genetic Variants with Vitiligo. PLoS ONE 2011, 6, e23089. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gelfand, J.M.; Weinstein, R.; Porter, S.B.; Neimann, A.L.; Berlin, J.A.; Margolis, D.J. Prevalence and Treatment of Psoriasis in the United Kingdom: A population-based study. Arch. Dermatol. 2005, 141, 1537–1541. [Google Scholar] [CrossRef] [PubMed]
- Alamri, A.; Alqahtani, R.; Alshareef, I.; Alshehri, A.; Balkhy, A. Psoriasis in Saudi Population: Gender Differences in Clinical Characteristics and Quality of Life. Cureus 2022, 14, e22892. [Google Scholar] [CrossRef] [PubMed]
- Ayanlowo, O.; Akinkugbe, A. Clinical pattern of psoriasis in patients seen at a tertiary hospital in Nigeria. J. Clin. Sci. 2016, 13, 137–142. [Google Scholar] [CrossRef]
- Schön, M.P. Adaptive and Innate Immunity in Psoriasis and Other Inflammatory Disorders. Front. Immunol. 2019, 10, 1764. [Google Scholar] [CrossRef] [Green Version]
- Fan, X.; Yang, S.; Huang, W.; Wang, Z.-M.; Sun, L.-D.; Liang, Y.-H.; Gao, M.; Ren, Y.-Q.; Zhang, K.-Y.; Du, W.-H.; et al. Fine Mapping of the Psoriasis Susceptibility Locus PSORS1 Supports HLA-C as the Susceptibility Gene in the Han Chinese Population. PLoS Genet. 2008, 4, e1000038. [Google Scholar] [CrossRef] [Green Version]
- Romphruk, A.; Oka, A.; Tomizawa, M.; Choonhakarn, C.; Naruse, T.K.; Puapairoj, C.; Tamiya, G.; Leelayuwat, C.; Inoko, H. Corneodesmosin gene: No evidence for PSORS 1 gene in North-eastern Thai psoriasis patients. Tissue Antigens 2003, 62, 217–224. [Google Scholar] [CrossRef]
- Mallon, E.; Bunce, M.; Wojnarowska, F.; Welsh, K. HLA-CW*0602 Is a Susceptibility Factor in Type I Psoriasis, and Evidence Ala-73 Is Increased in Male Type I Psoriatics. J. Investig. Dermatol. 1997, 109, 183–186. [Google Scholar] [CrossRef] [Green Version]
- Ikäheimo, I.; Tiilikainen, A.; Karvonen, J.; Silvennoinen-Kassinen, S. HLA risk haplotype Cw6,DR7, DQA1*0201 and HLA-Cw6 with reference to the clinical picture of psoriasis vulgaris. Arch. Dermatol. Res. 1996, 288, 363–365. [Google Scholar] [CrossRef]
- Gudjonsson, J.E.; Elder, J.T. Psoriasis: Epidemiology. Clin. Dermatol. 2007, 25, 535–546. [Google Scholar] [CrossRef]
- West, J.; Ogston, S.; Berg, J.; Palmer, C.; Fleming, C.; Kumar, V.; Foerster, J. HLA-Cw6-positive patients with psoriasis show improved response to methotrexate treatment. Clin. Exp. Dermatol. 2017, 42, 651–655. [Google Scholar] [CrossRef] [Green Version]
- Ferrándiz, C.; Pujol, R.M.; García-Patos, V.; Bordas, X.; Smandía, J.A. Psoriasis of early and late onset: A clinical and epidemiologic study from Spain. J. Am. Acad. Dermatol. 2002, 46, 867–873. [Google Scholar] [CrossRef]
- Blanco, E.A.; Bejerano, C.; Pinto-Tasende, J.; Pértega, S.; Rego, I.; Fernandez, C.; Freire, M.; De Toro, J.; Blanco, F.J.; Fernández-Sueiro, J.L. AB0575 Prevalence of hla-cw * 06 and * 07 and its relationship with psoriatic arthritis in northwestern spain. Ann. Rheum. Dis. 2013, 72, A965–A966. [Google Scholar] [CrossRef]
- Chandran, V.; Bull, S.B.; Pellett, F.J.; Ayearst, R.; Rahman, P.; Gladman, D.D. Human leukocyte antigen alleles and susceptibility to psoriatic arthritis. Hum. Immunol. 2013, 74, 1333–1338. [Google Scholar] [CrossRef]
- Queiro, R.; Gonzalez, S.; López-Larrea, C.; Alperi, M.; Sarasqueta, C.; Riestra, J.L.; Ballina, J. HLA-C locus alleles may modulate the clinical expression of psoriatic arthritis. Arthritis Res. Ther. 2006, 8, R185. [Google Scholar] [CrossRef] [Green Version]
- Sin, C.-Z.; Wang, T.-S.; Chiu, H.-Y.; Tsai, T.-F. Human leukocyte antigen and demographic characteristics in Chinese patients with active peripheral type psoriatic arthritis who had inadequate response to conventional disease-modifying antirheumatic drugs in a single dermatologic clinic. PLoS ONE 2019, 14, e0210076. [Google Scholar] [CrossRef] [PubMed]
- Emmungil, H.; Ilgen, U.; Direskeneli, R.H. Autoimmunity in psoriatic arthritis: Pathophysiological and clinical aspects. Turk. J. Med. Sci. 2021, 51, 1601–1614. [Google Scholar] [CrossRef] [PubMed]
- Yan, K.-L.; Huang, W.; Zhang, X.-J.; Yang, S.; Chen, Y.-M.; Xiao, F.-L.; Fan, X.; Gao, M.; Cui, Y.; Zhang, G.-L.; et al. Follow-Up Analysis of PSORS9 in 151 Chinese Families Confirmed the Linkage to 4q31–32 and Refined the Evidence to the Families of Early-Onset Psoriasis. J. Investig. Dermatol. 2007, 127, 312–318. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Siow, K.Y.; Safdar, N.A.M.; Chong, K.H.; Chua, K.B. A clinical appraisal of patients with psoriasis treated in Seremban General Hospital, Malaysia. Med. J. Malays. 2004, 59, 330–334. [Google Scholar]
- Talamonti, M.; Botti, E.; Galluzzo, M.; Teoli, M.; Spallone, G.; Bavetta, M.; Chimenti, S.; Costanzo, A. Pharmacogenetics of psoriasis:HLA-Cw6but notLCE3B/3Cdeletion norTNFAIP3polymorphism predisposes to clinical response to interleukin 12/23 blocker ustekinumab. Br. J. Dermatol. 2013, 169, 458–463. [Google Scholar] [CrossRef]
- Inani, K.; Meziane, M.; Mernissi, F. Méthotrexate et psoriasis: À propos de 46 cas [Methotrexate and psoriasis: About 46 cases]. Pan Afr. Med. J. 2014, 19, 84. [Google Scholar] [CrossRef]
- Indhumathi, S.; Rajappa, M.; Chandrashekar, L.; Ananthanarayanan, P.H.; Thappa, D.M.; Negi, V.S. Pharmacogenetic markers to predict the clinical response to methotrexate in south Indian Tamil patients with psoriasis. Eur. J. Clin. Pharmacol. 2017, 73, 965–971. [Google Scholar] [CrossRef]
- Gupta, R.; Debbaneh, M.G.; Liao, W. Genetic Epidemiology of Psoriasis. Curr. Dermatol. Rep. 2014, 3, 61–78. [Google Scholar] [CrossRef] [Green Version]
- Fry, L.; Baker, B.S. Triggering psoriasis: The role of infections and medications. Clin. Dermatol. 2007, 25, 606–615. [Google Scholar] [CrossRef]
- Elfatoiki, F.Z.; El Azhari, M.; El Kettani, A.; Serhier, Z.; Othmani, M.B.; Timinouni, M.; Benchikhi, H.; Chiheb, S.; Fellah, H. Psoriasis and staphylococcus aureus skin colonization in Moroccan patients. Pan Afr. Med. J. 2016, 23, 33. [Google Scholar] [CrossRef]




| Variables | Patients (N = 54) |
|---|---|
| Demographic features | |
| - Mean age, years old (SD) | 42.28 (16.98) |
| - Gender | |
| Male | 29 (53.70%) |
| Female | 25 (46.30%) |
| Sex ratio (M/F) | 1.16 |
| Clinical features | |
| - Age of disease onset, years old (SD) | 30.94 (19.13) |
| Early disease onset (≤30 Years) | 30 (55.56%) |
| Late disease onset (>30 Years) | 24 (44.44%) |
| - Clinical phenotypes of psoriasis | |
| Chronic plaque psoriasis (psoriasis vulgaris) | 34 (62.97%) |
| Erythrodermic psoriasis | 14 (25.92%) |
| Pustular psoriasis | 6 (11.11%) |
| - Nail involvement | 18 (33.33%) |
| - Concomitant psoriasis arthritis | 13 (24.07%) |
| - Comorbidities | |
| Diabetes | 7 (12.96%) |
| Obesity | 5 (9.25%) |
| Hypertension | 3 (5.50%) |
| - Risk factors | |
| Stress | 37 (68.51%) |
| Smoking | 15 (27.77%) |
| Alcohol intake | 5 (9.25%) |
| - Baseline PASI | |
| Mean baseline PASI (SD) | 30.31 (12.63) |
| Moderate Psoriasis (PASI 5–10) | 1 (1.85%) |
| Severe Psoriasis (PASI > 10) | 53 (98.14%) |
| - PASI Score at week 12 of MTX | |
| Mean PASI at week 12 of MTX (SD) | 8.17 (12.97) |
| No improvement | 8 (16.32%) |
| PASI 50 | 7 (14.28%) |
| PASI 75 | 10 (20.40%) |
| PASI 90 | 9 (18.36%) |
| PASI 100 | 15 (30.61%) |
| - Tolerable side effects due to MTX | 13 (24.07%) |
| - MTX interruption due to liver and/or digestive toxicity | 4 (7.40%) |
| Variables | Responders (N = 34) | Non-Responders (N = 15) | p-Value |
|---|---|---|---|
| Demographic features | |||
| - Mean age, years old (SD) | 40.23 (17.80) | 44 (16.46) | 0.485 |
| - Gender | |||
| Male | 18 | 8 | 0.980 |
| Female | 16 | 7 | |
| Sex ratio (M/F) | 1.125 | 1.14 | |
| Clinical features | |||
| - Clinical phenotypes of psoriasis | |||
| Chronic plaque psoriasis (psoriasis vulgaris) | 23 (67.60%) | 7 (46.70%) | 0.345 |
| Erythrodermic psoriasis | 8 (23.50%) | 5 (33.33%) | |
| Pustular psoriasis | 3 (8.90%) | 3 (20%) | |
| - Nail involvement | 15 (44.11%) | 1 (6.67%) | 0.081 |
| - Concomitant psoriasis arthritis | 6 (17.60%) | 7 (46.70%) | 0.077 |
| - Age of disease onset, years old (SD) | 28 (18.05) | 33.27 (12.29) | |
| Early disease onset (≤30 Years) | 22 (64.70%) | 7 (46.70%) | 0.235 |
| Late disease onset (>30 Years) | 12 (35.30%) | 8 (53.30%) | |
| - Family history of psoriasis | 8 (23.50%) | 1 (6.67%) | 0.315 |
| - PASI score | |||
| Mean baseline PASI (SD) | 29.74 (13.03) | 33.33 (12.29) | 0.765 |
| Mean PASI at week 12 of MTX (SD) | 2.75 (2.85) | 23.55 (17.66) | 0.002 |
| - Side effects due to MTX | 6 (17.64%) | 7 (46.70%) | 0.077 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Benlabsir, C.; Riyad, M.; Saik, I.E.I.; Ettayebi, H.; Aazzane, O.; Nassar, K.; Zaher, S.; Bennani, S.; Admou, B.; Housbane, S.; et al. HLA-C Genotyping Reveals Haplotype C*07 as a Potential Biomarker of Late Psoriasis Onset in Moroccan Patients. Curr. Issues Mol. Biol. 2023, 45, 1012-1023. https://doi.org/10.3390/cimb45020066
Benlabsir C, Riyad M, Saik IEI, Ettayebi H, Aazzane O, Nassar K, Zaher S, Bennani S, Admou B, Housbane S, et al. HLA-C Genotyping Reveals Haplotype C*07 as a Potential Biomarker of Late Psoriasis Onset in Moroccan Patients. Current Issues in Molecular Biology. 2023; 45(2):1012-1023. https://doi.org/10.3390/cimb45020066
Chicago/Turabian StyleBenlabsir, Chaimaa, Myriam Riyad, Imane El Idrissi Saik, Hanaa Ettayebi, Oussama Aazzane, Kawtar Nassar, Soukaina Zaher, Siham Bennani, Brahim Admou, Samy Housbane, and et al. 2023. "HLA-C Genotyping Reveals Haplotype C*07 as a Potential Biomarker of Late Psoriasis Onset in Moroccan Patients" Current Issues in Molecular Biology 45, no. 2: 1012-1023. https://doi.org/10.3390/cimb45020066
APA StyleBenlabsir, C., Riyad, M., Saik, I. E. I., Ettayebi, H., Aazzane, O., Nassar, K., Zaher, S., Bennani, S., Admou, B., Housbane, S., Sadki, K., Chiheb, S., & Fellah, H. (2023). HLA-C Genotyping Reveals Haplotype C*07 as a Potential Biomarker of Late Psoriasis Onset in Moroccan Patients. Current Issues in Molecular Biology, 45(2), 1012-1023. https://doi.org/10.3390/cimb45020066